Published online Mar 7, 2018. doi: 10.3748/wjg.v24.i9.992
Peer-review started: November 10, 2017
First decision: December 13, 2017
Revised: December 22, 2017
Accepted: January 1, 2018
Article in press: January 1, 2018
Published online: March 7, 2018
Processing time: 116 Days and 1.2 Hours
To elucidate the potential role of autophagy and the protective effects of Jiang Zhi Granule (JZG) in metabolic stress-induced hepatocyte injury.
An in vitro and in vivo approach was used in this study. HepG2 cells were incubated in culture medium containing palmitate (PA; 0, 0.1, 0.2, 0.3, 0.4 or 0.5 mmol/L) and treated with or without JZG (100 μg/mL) for 24 h or 48 h, and the progression of autophagy was visualized by stable fluorescence-expressing cell lines LC3 and p62. Western blot analyses were performed to examine the expression of LC3-II/LC3-I, p62, mTOR and PI3K, while mitochondrial integrity and oxidative stress were observed by fluorescence staining of JC-1 and reactive oxygen species. C57BL/6 mice were divided into three groups: control group (n = 10), high fat (HF) group (n = 13) and JZG group (n = 13); and, histological staining was carried out to detect inflammation and lipid content in the liver.
The cell trauma induced by PA was aggravated in a dose- and time-dependent manner, and hepatic function was improved by JZG. PA had dual effects on autophagy by activating autophagy induction and blocking autophagic flux. The PI3K-AKT-mTOR signaling pathway and the fusion of isolated hepatic autophagosomes and lysosomes were critically involved in this process. JZG activated autophagy progression by either induction of autophagosomes or co-localization of autophagosomes and lysosomes as well as degradation of autolysosomes to protect against PA-induced hepatocyte injury, and protected mitochondrial integrity against oxidative stress in PA-induced mitochondrial dysfunction. In addition, JZG ameliorated lipid droplets and inflammation induced by HF diet in vivo, leading to improved metabolic disorder and associated liver injury in a mouse model of non-alcoholic fatty liver disease (NAFLD).
Metabolic stress-induced hepatocyte injury exhibited dual effects on autophagy and JZG activated the entire process, resulting in beneficial effects in NAFLD.
Core tip: Non-alcoholic fatty liver disease (NAFLD), which is mainly characterized by the accumulation of lipids and energy metabolism dysfunction, is now one of the most common risk factors worldwide. As studies have demonstrated that autophagy is important in the maintenance of normal hepatocyte function and in the response to pathogenic changes, we examined the potential role of autophagy in metabolic stress-induced hepatocyte injury. The results showed that metabolic stress had dual effects on autophagy, resulting in autophagy induction and autophagic flux inhibition. The Chinese herbal formula Jiang Zhi Granule activated the autophagy process to protect against metabolic stress-induced hepatocyte injury in NAFLD.
- Citation: Zheng YY, Wang M, Shu XB, Zheng PY, Ji G. Autophagy activation by Jiang Zhi Granule protects against metabolic stress-induced hepatocyte injury. World J Gastroenterol 2018; 24(9): 992-1003
- URL: https://www.wjgnet.com/1007-9327/full/v24/i9/992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i9.992
Fatty liver disease (FLD), which is mainly characterized by the accumulation of lipids in hepatocytes and energy metabolism dysfunction, is clinically classified into two broad categories: alcoholic (A)FLD and non-alcoholic (NA)FLD[1]. NAFLD is currently much more prevalent than AFLD, which accounts for 75% of all chronic liver diseases, and is increasingly recognized as one of the most common risk factors worldwide[2]. NAFLD has a wide clinical pathological spectrum, ranging from simple steatosis non-alcoholic fatty liver to non-alcoholic steatohepatitis, which includes fibrosis and cirrhosis[3]. And, in the meantime, it often occurs with other metabolic diseases, such as obesity and diabetes[4].
The pathogenesis of NAFLD is still unknown; however, the “two hits” hypothesis is now widely accepted[5]. A disturbance in metabolic homeostasis is one of the key events during the occurrence of NAFLD and typical pathological features, including steatosis, inflammation, fibrosis and cirrhosis, are considered to be related to oxidative stress resulting from lipid accumulation and reactive oxygen species (ROS) generation[6].
Oxidative stress is a stimulus for autophagy, which is important for metabolic homeostasis in the liver[7], as it can prompt nutrient recycling, remove abnormal organelles and toxic protein aggregates and alter the level of metabolic factors, thus contributing to the maintenance of normal hepatocyte function and the response to pathogenic changes in the liver[8,9]. Hepatic autophagy occurs at a basal level and can be elevated under stress conditions[10], such as oxidative stress.
Studies have shown that autophagy is a highly selective process and can modulate cellular energy stores, such as carbohydrates and lipids[11,12], and recent research has demonstrated that autophagy assists in the degradation of triglycerides in the liver[13]. Autophagy regulators, such as rapamycin and carbamazepine, have been proven effective to improve hepatic function[14]. However, no effective therapeutic approach has been accepted as the standard option for NAFLD and its complications. Thus, new treatments are still urgently needed to prevent or delay the onset as well as the progression of NAFLD.
Jiang Zhi Granule (JZG), which is composed of Herba gynostemmatis, Radix salviae, Rhizoma polygoni cuspidati, Herba artemisiae scopariae and Folium nelumbinis, is a clinically used herbal formula designed to treat patients with NAFLD[15]. JZG had a positive drug safety evaluation and has been approved for clinical trials by the State Food and Drug Administration (SFDA; Authorization Number: Z10960082). Our preliminary studies indicated that JZG had beneficial effects in improving hepatic fat accumulation in both cell lines and animals[16], and the efficacy of JZG in patients with NAFLD was also confirmed[17]. As previous studies on JZG have indicated its antisteatotic effect, we conducted this study to determine the underlying mechanism of JZG.
Male C57BL/6 mice aged 6 wk were purchased from SLAC Animal Laboratories (Shanghai, China). After 1-wk acclimatization, the mice were divided into three groups: the control group (n = 10) received a 12-wk standard diet (STD); the high fat (HF) group (n = 13) received a HF diet (HFD; consisting of 10% lard oil, 2% cholesterol and 88% STD) supplemented with 1% DSS (MP Biomedicals, Solon, OH, United States) in drinking water; and, the JZG group (n = 13) also received a HF-DSS diet, but were also given JZG which had been dissolved in saline and was administered daily by oral gavage at a dose of 994 mg/kg daily (approximately 12 times that of the standard dose used in clinical practice). DSS was given in cycles; each cycle consisted of 7 d DSS administration followed by a 10-d interval with normal drinking water[18]. At the end of the experimental period, blood samples were drawn from the heart, while the mice were under anesthesia, livers were excised, and samples were either immediately snap-frozen in liquid nitrogen or fixed in 4% PFA.
HepG2 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Saturated palmitic acid (PA) and JZG were used in this study. HepG2 cells were incubated in culture medium containing PA (0, 0.1, 0.2, 0.3, 0.4 or 0.5 mmol/L) and treated with or without JZG (100 μg/mL) for 24 h or 48 h, as described previously[16].
Western blot analyses were performed as described previously[19]. Rabbit antibodies against LC3 (monoclonal ab192890), SQSTM1/p62 (monoclonal ab109012), p-mTOR (phosphor-S2481) (monoclonal ab137133), mTOR (monoclonal ab87540), p-PI3K (phosphor-Y607) (polyclonal ab182651), PI3K (monoclonal ab40755) and actin (polyclonal ab8227) were purchased from Abcam (Cambridge, MA, United States).
HepG2 cells were cultured according to the above protocol and infected with mRFP-GFP-LC3 lentivirus at an MOI of 10 for 48 h to establish a stable mRFP-GFP-LC3-expressing HepG2 cell line, and then infected with mCherry-p62 lentivirus at an MOI of 10 for 48 h to establish a stable mCherry-p62-expressing HepG2 cell line.
Mitochondria and lysosomes were detected using the MitoTracker Green FM and LysoTracker Deep Red staining kit, respectively (Thermo Fisher Scientific, Waltham, MA, United States). Intracellular ROS generation was detected using the DCFH-DA fluorescent probe. Mitochondrial membrane potential was determined by the mitochondrial membrane potential assay kit (JC-1). Pretreated cells in 6-well plates were processed following the protocols and were observed immediately under a fluorescence microscope at an excitation wavelength of 488 nm and emission wavelength of 525 nm.
Histological staining was performed as described previously[20]. Liver samples were fixed, processed, and embedded in paraffin blocks, and then hematoxylin and eosin (H&E) staining was performed. Frozen liver sections were fixed and stained with oil Red O. Images were acquired on an Olympus BX-50 microscope.
All data were expressed as the mean ± SEM. Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, United States). The differences between groups were analyzed by Student’s t-test or ANOVA, as appropriate, and P < 0.05 was considered statistically significant.
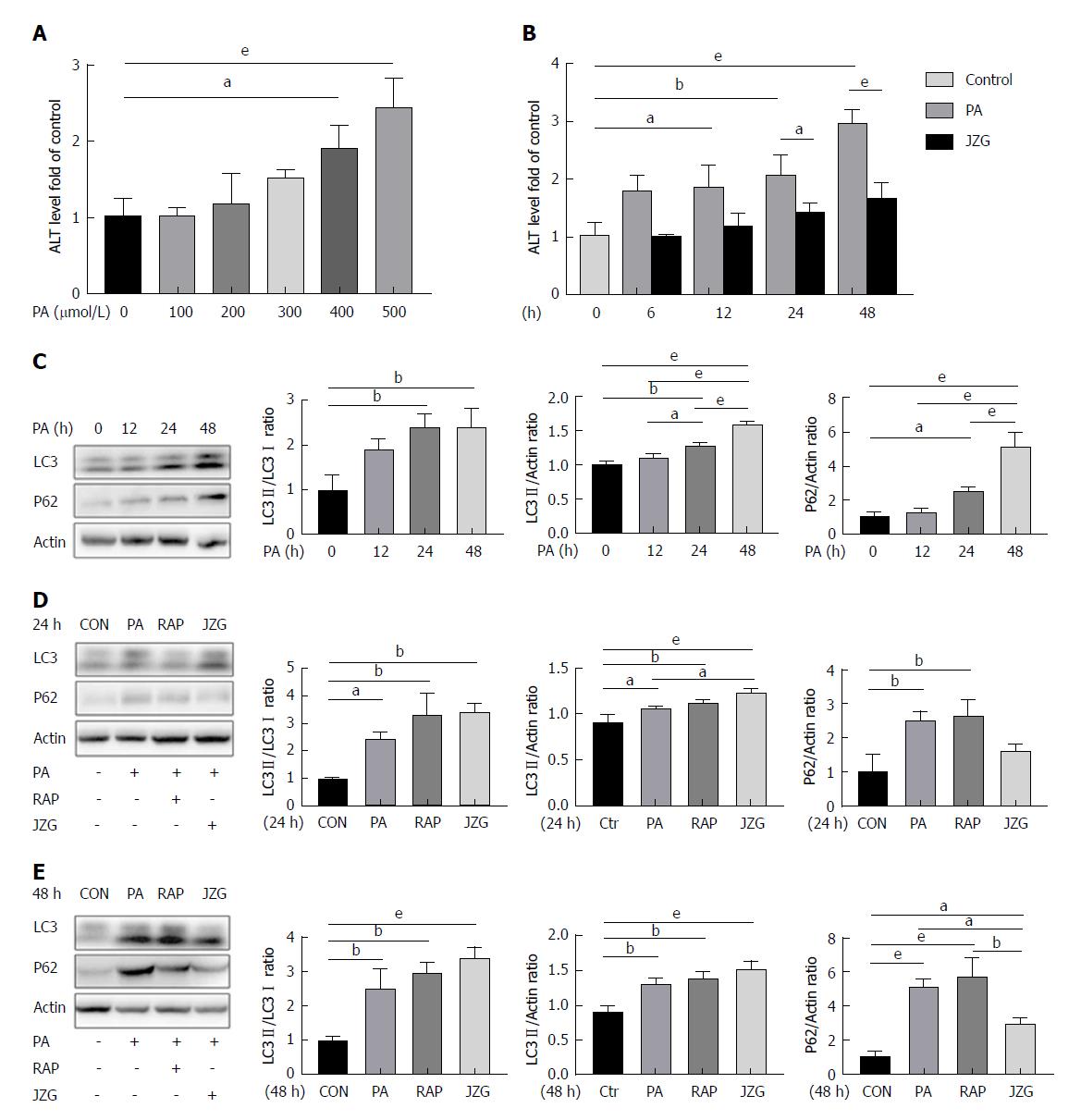
To determine whether autophagy was involved in the progression of NAFLD, HepG2 cells were treated with PA - a main type of saturated free fatty acid which is elevated in obese subjects and can induce NAFLD[21] - at various concentrations and for different time periods. Results showed that the expression of alanine aminotransferase (ALT) increased in a dose- and time-dependent manner and JZG significantly reduced high ALT levels (Figure 1A and B), suggesting that cell trauma was induced by PA and JZG improved hepatic function. These results also indicated that the dose of 0.4 mmol/L may be an appropriate concentration in subsequent experiments and the time points should include 24 h and 48 h.
Western blot analyses showed that PA increased LC3-II/actin expression (Figure 1C), indicating the activation of autophagy. However, the expression of LC3-II/LC3-I was not increased accordingly after long-term PA stimulation (Figure 1C). In the meantime, the expression of p62 was also increased (Figure 1C), suggesting the restriction of autophagic flux. Thus, it was proposed that PA exhibited dual effects on autophagy.
A recent study showed that mitochondrial dysfunction caused by elevated mitochondrial stress may block autophagic flux[18]; thus, rapamycin was used in the present study to enhance autophagy at different time points. The outcomes displayed that the expression of LC3-II/actin and p62 were both increased but the expression of LC3-II/LC3-I was not, confirming the dual effects of PA on autophagy (Figure 1D and E).
An increase in LC3-II/LC3-I expression and a reduction in p62 expression were observed following treatment with JZG for two different time periods (Figure 1D and E), indicating that JZG activated autophagy to protect against PA-induced cell injury.
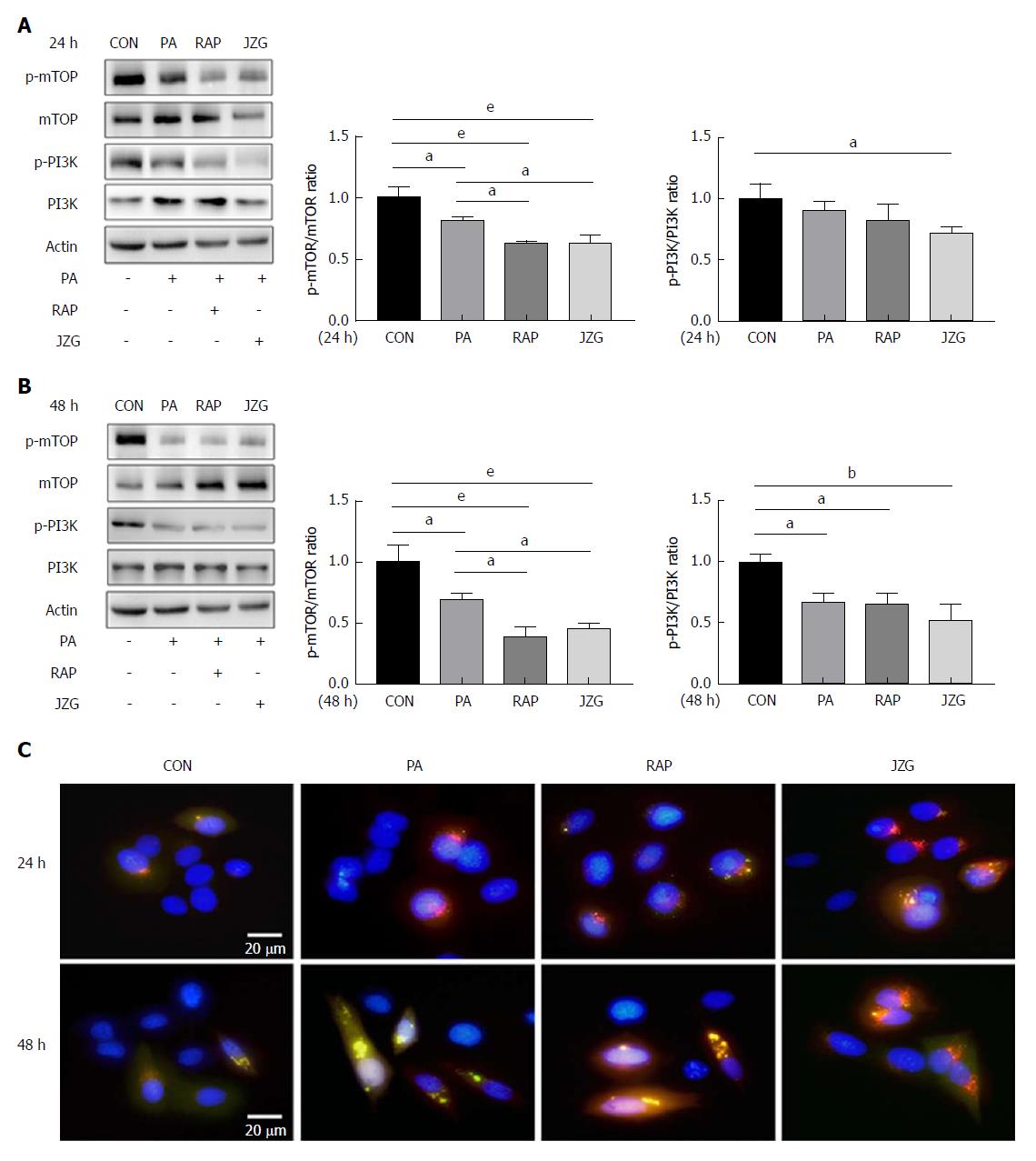
As the activation of autophagy can occur due to an increase in autophagy induction and autophagic flux, we first investigated the pathway involved in autophagy induction in JZG-treated cells. The PI3K-AKT-mTOR pathway, a classic signaling pathway that has been identified to be important in autophagy induction, was examined in this study. Western blot analyses revealed that the phosphorylation signaling processes of mTOR and PI3K were inhibited in PA-treated cells, and these results were further confirmed by the addition of rapamycin (Figure 2A and B). As an inhibitor of mTOR, rapamycin did not further depress the phosphorylation of PI3K. Conversely, an additional restraint on both phosphorylation signaling processes was observed in JZG-treated cells (Figure 2A and B), suggesting that the PI3K-AKT-mTOR pathway is critically involved in autophagy induction in response to PA challenge.
As LC3 is the major constituent of autophagosomes, a stable mRFP-GFP-LC3-expressing HepG2 cell line was established to visualize the progression of autophagosome formation in real time in liver cells. Diffuse cytoplasmic localization of mRFP-GFP-LC3 was observed in the control group, whereas punctate fluorescence was observed in PA-treated cells (Figure 2C), indicating that cytoplasmic LC3 was processed and recruited to autophagosomes. Rapamycin and JZG advanced this process, indicating increased autophagy induction, which confirmed that JZG induced the formation of autophagosomes.
To determine the effect of JZG on autophagic flux in PA-treated cells, we first observed this effect in mRFP-GFP-LC3-expressing HepG2 cells. We found that yellow fluorescence - indicating that GFP was not degraded by lysosomal enzymes - was largely seen in HepG2 cells treated with PA for 48 h, whereas red fluorescence was very rare (Figure 2C). These results led us to propose that the colocalization of autophagosomes and lysosomes was prevented and the autophagosomal degradation was blocked. Yellow punctate fluorescence was reduced in JZG-treated cells and red diffuse fluorescence was increased (Figure 2C), suggesting that JZG promoted colocalization.
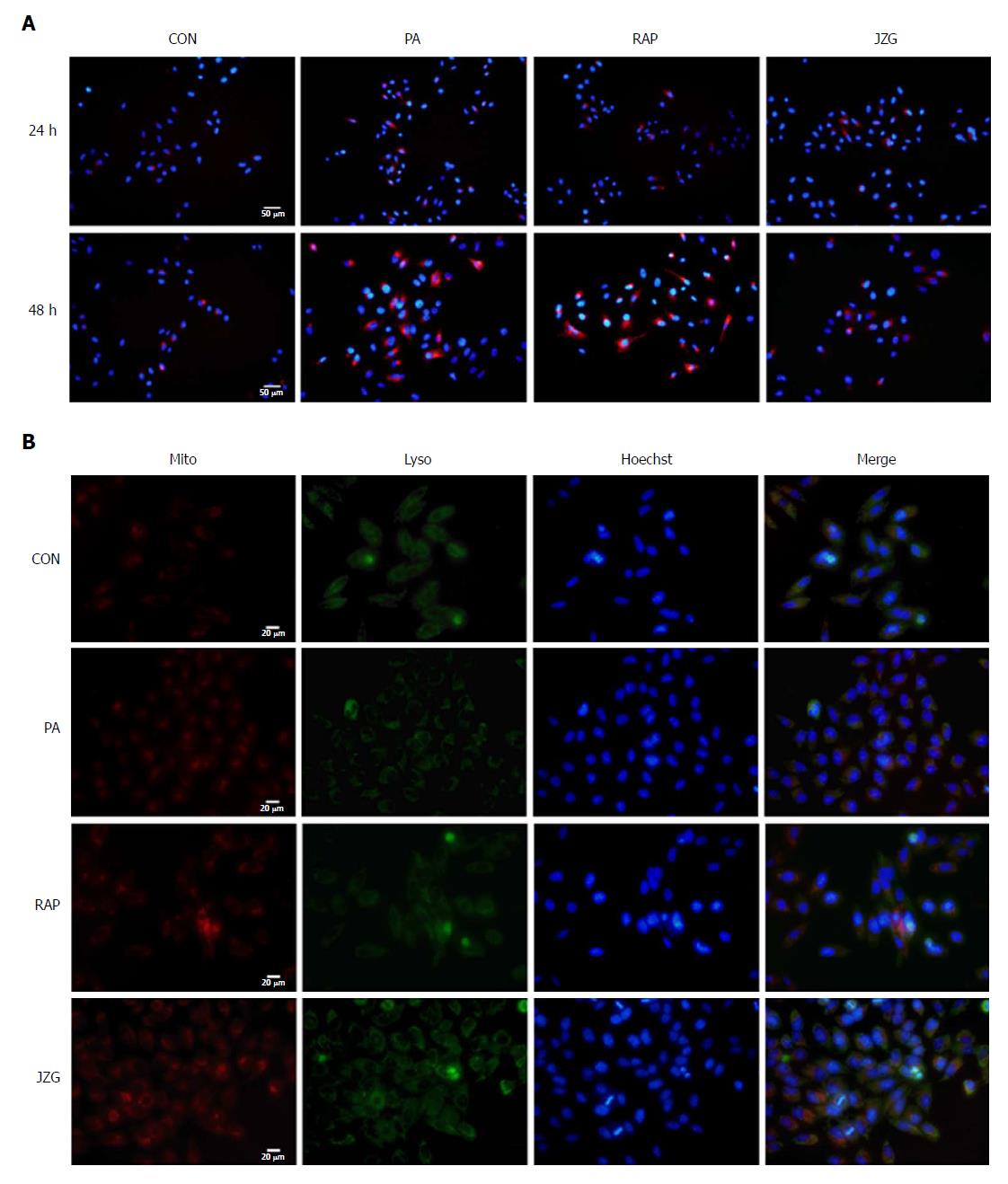
To confirm these findings, we established another stable mCherry-p62-expressing HepG2 cell line to visualize the cellular expression levels of p62, which inversely correlated with autophagic flux via selective incorporation into autophagosomes to be efficiently degraded by autophagy. We found that punctate fluorescence was enhanced in HepG2 cells treated with PA for 48 h and was weak in JZG-treated cells (Figure 3A), confirming the previous findings.
As it has been shown that mitochondrial dysfunction may block autophagic flux[22], we next examined whether the mitochondrial integrity was affected in PA-treated cells. MitoTracker and LysoTracker were used to stain the mitochondria and lysosomes, respectively. The results showed that mitochondrial dysfunction was much more serious in PA-treated cells than in control cells (Figure 3B). A protective effect of JZG on mitochondrial integrity was demonstrated and the colocalization of mitochondria and lysosomes in JZG-treated cells showed that the mitochondrial integrity was related to activation of autophagic flux. Together, these findings suggested that JZG increased the formation of both autophagosomes and autolysosomes and protected against PA-induced mitochondrial dysfunction by activating autophagy.
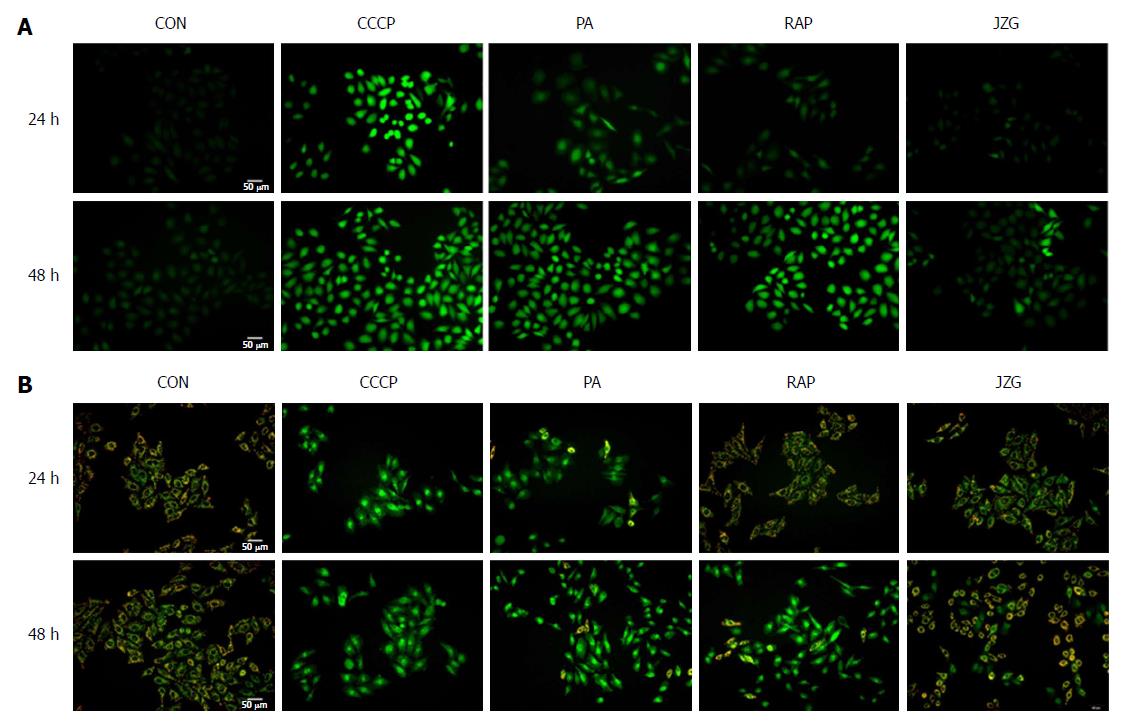
As the protective effect of JZG on mitochondrial integrity was demonstrated, we then examined whether oxidative stress was involved in this process. The ROS-sensitive fluorescent probe DCFH-DA was used to monitor cellular oxidative stress. We found that the accumulation of intracellular ROS was considerably increased in PA-treated cells, and JZG significantly reduced the PA-induced increase in ROS production (Figure 4A), indicating the potential role of oxidative stress in mitochondrial dysfunction. We also monitored mitochondrial membrane potential with JC-1 to evaluate oxidative damage, and the results revealed a reduction in mitochondrial membrane potential in PA-treated cells, and JZG prevented this PA-induced reduction (Figure 4B), confirming that JZG protected mitochondrial integrity against oxidative stress in PA-induced mitochondrial dysfunction.
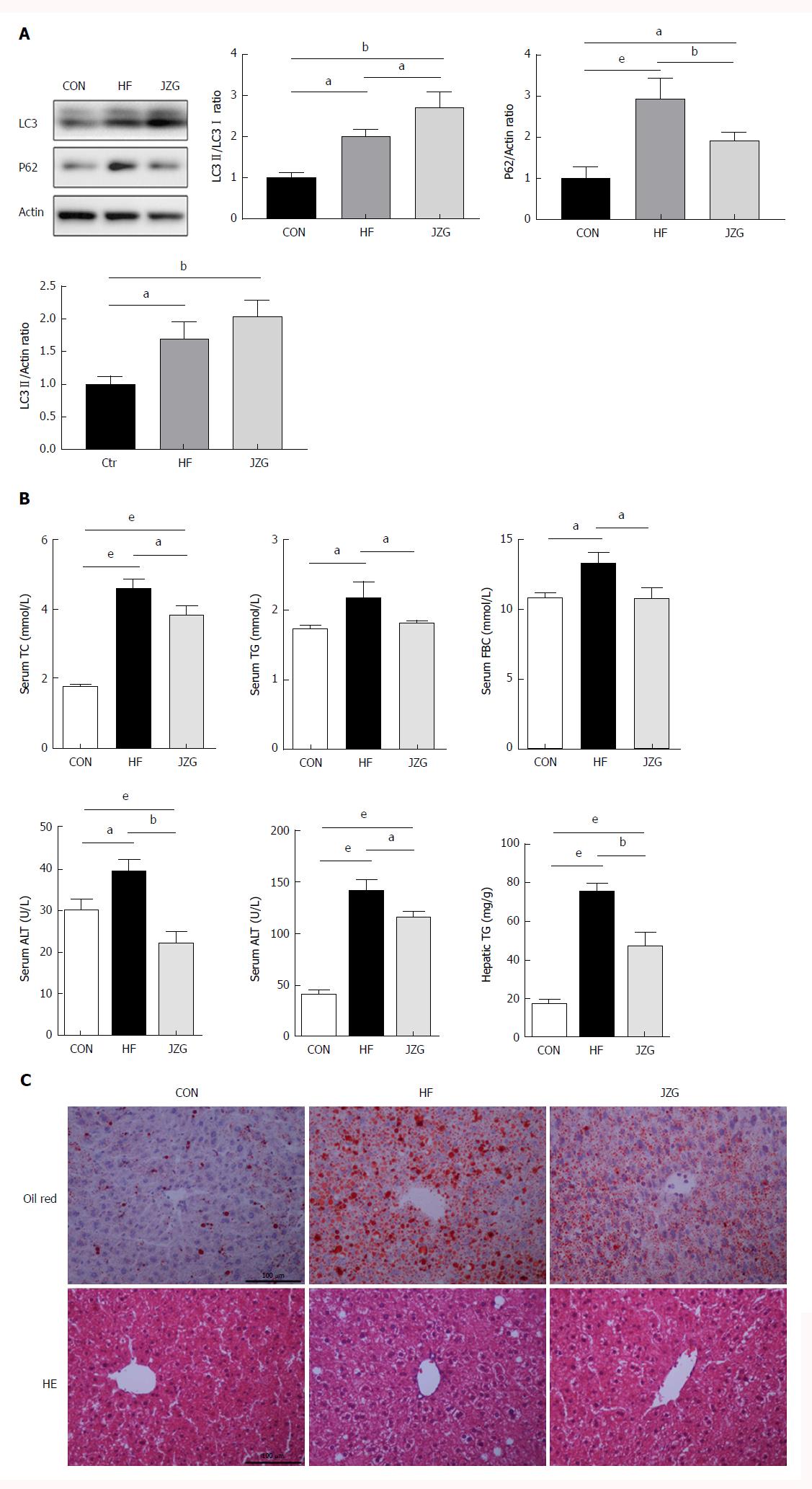
A murine model of NAFLD induced by HFD was employed to assess the potential role of autophagy in metabolic stress-induced liver injury and inflammation. As expected, the HFD increased both the expression of LC3-II/actin and p62 (Figure 5A), suggesting the activation of autophagy induction and inhibition of autophagic flux. JZG treatment induced an increase in LC3-II/LC3-I expression and led to a decrease in p62 expression (Figure 5A), indicating up-regulation of the autophagy pathway in JZG-treated mice.
Biochemical analyses showed that HFD elevated the expression of circulating ALT, aspartate aminotransferase, total cholesterol, triglycerides and fasting blood glucose as well as hepatic triglycerides, and JZG improved the metabolic disorder and associated liver injury (Figure 5B). The results of HE and oil red O staining demonstrated that lipid droplets and inflammation were induced by HFD and JZG ameliorated these conditions (Figure 5C). Together, these findings suggested that JZG had beneficial effects in improving NAFLD in vivo.
NAFLD, as the leading cause of chronic liver disease, could result in serious liver-related complications and an increase in overall mortality. In previous research, we demonstrated that the Chinese herbal formula, JZG, had beneficial effects in improving hepatic fat accumulation, and in this study, we showed that autophagy is critically involved in this process.
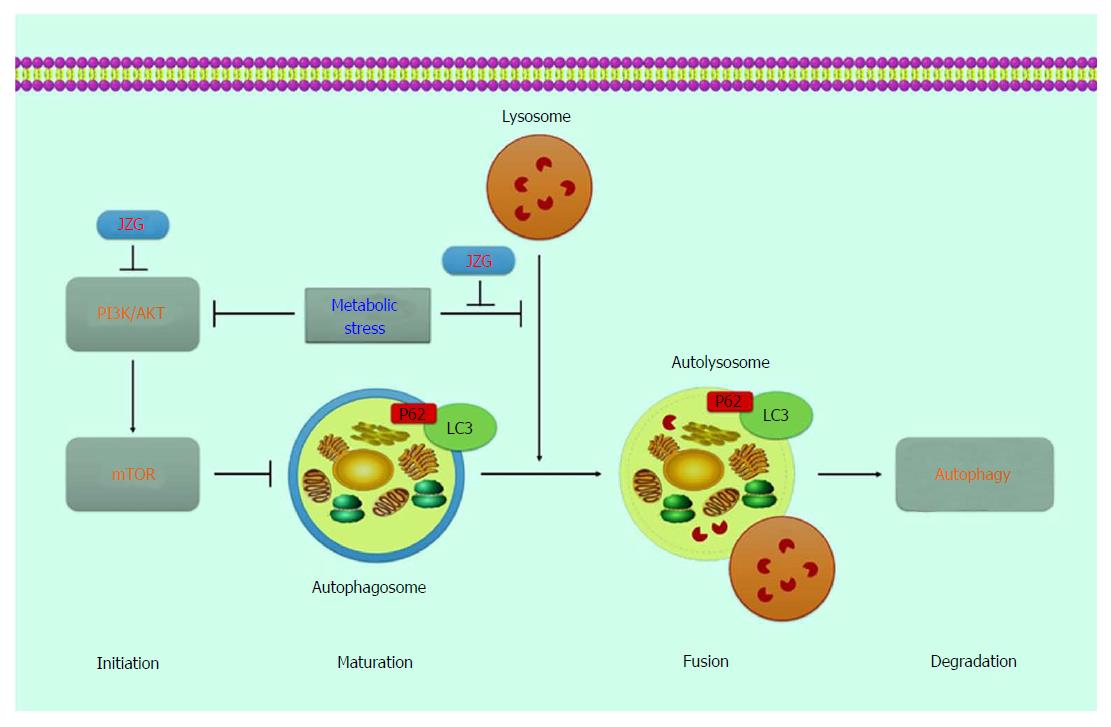
Autophagy occurs when autophagosomes are formed and autophagy induction is attributed to various origins, such as the endoplasmic reticulum, the Golgi apparatus, the mitochondria or the plasma membrane[23,24]. The autophagosomes then become autolysosomes by fusing with lysosomes and degrading the components in cytosols[25,26]. Thus, the upstream event of autophagy induction was presented by LC3-II/actin expression in this study, and the downstream event of autophagic flux was presented by the expression of LC3-II/LC3-I. Stable fluorescence-expressing cell lines of LC3 were established to visualize the whole progression of autophagic flux. Results showed that metabolic stress-induced hepatocyte injury exhibited dual effects on autophagy by activating autophagy induction and blocking autophagic flux.
A series of signaling pathways and regulators which regulate autophagy have been identified in the past decade. In this research, a classic signaling pathway, the PI3K-AKT-mTOR signaling pathway[27], was confirmed to be important in response to PA challenge. As the core target in this signaling pathway, mTOR, a master regulator of cellular metabolism, can be stimulated by multiple stimulants, such as nutritional status, hormonal factors and oxygen concentrations[28]. Under these conditions, mTOR complex 1 (mTORC1) will inhibit the ULK complex by phosphorylating Atg13 and ULK1/2, which results in autophagy suppression[29]. However, restraints on both phosphorylation signaling processes of PI3K and mTOR were observed in JZG-treated cells, indicating that the PI3K-AKT-mTOR pathway was involved in autophagy in the JZG-treated cells.
Subsequent studies have shown that SQSTM1/p62 accumulation is correlated with NAFLD and the fusion of isolated hepatic autophagosomes and lysosomes is different in NAFLD patients[30], which suggests that an excessive amount of lipids may contribute to SQSTM1/p62 accumulation by suppressing autophagosomes/lysosome fusion[31,32]. Thus, we examined the SQSTM1/p62 accumulation and the results confirmed previous findings.
Fluorescence staining was performed to connect the mitochondrial integrity with oxidative stress induced by PA. We also found that JZG could activate the autophagy process by either induction of autophagosomes or colocalization of autophagosomes and lysosomes as well as degradation of autolysosomes to protect against metabolic stress-induced hepatocyte injury in NAFLD (Figure 6).
Limitations should be acknowledged. The complex compounds contained in this prescription may have led to multitarget effects, and a series of signaling pathways and regulators were not examined. Beyond that, as p62 could also be degraded by proteasome, further studies on this aspect should be continually conducted to confirm the findings in the future. However, as the current epidemic of obesity and obesity-related NAFLD continues to increase, new approaches for prevention and treatment are urgently needed, and traditional Chinese medicine, as an alternative and complementary medicine, may be an effective addition to the current standardized intervention strategy.
Non-alcoholic fatty liver disease (NAFLD), as the leading cause of chronic liver disease, can result in serious liver-related complications and an increase in overall mortality. However, the pathogenesis of NAFLD is still unknown and no effective therapeutic strategy has been accepted as the standard treatment option. In previous studies, the authors found that JZG had beneficial effects in improving hepatic fat accumulation, metabolic disorder and associated liver injury, and its efficacy in patients with NAFLD was also confirmed.
Autophagy is important in liver diseases, and research has demonstrated that autophagy regulators can improve hepatic function. However, no effective therapeutic strategy has been accepted as the standard option for NAFLD and its complications. Thus, novel treatments are still urgently needed to prevent or delay the onset as well as the progression of NAFLD.
NAFLD, as the leading cause of chronic liver disease, could result in serious liver-related complications and an increase in overall mortality. And traditional Chinese medicine, as an alternative and complementary medicine, may be an effective addition to the current standardized intervention strategy.
The process of autophagy was detected by the expressions of LC3 and SQSTM1/p62. The upstream event of autophagy induction was presented by LC3-II/actin expression and the downstream event of autophagic flux was presented by the expressions of LC3-II/LC3-I and SQSTM1/p62. Stable fluorescence-expressing cell lines were established with mRFP-GFP-LC3 and mCherry-p62 lentivirus to visualize the whole progression of autophagic flux.
In previous research, the authors had demonstrated that the Chinese herbal formula JZG had beneficial effects in improving hepatic fat accumulation. In this study, autophagy was demonstrated to be critically involved in this process.
The authors confirmed that metabolic stress-induced hepatocyte injury exhibited dual effects on autophagy, while JZG activated the whole process to provide beneficial effects in NAFLD.
The exact compounds contained in this prescription are still unknown and the complex compounds might have led to multitarget effects. A systems pharmacology approach to determine the active compounds and action mechanisms might be a good method for the future research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Demonacos C, Marcos R, Osna NA S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8 Suppl 1:S4-S8. [PubMed] |
| 2. | López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, Méndez-Sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166-178. [PubMed] |
| 3. | Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 4. | Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 5. | Reiniers MJ, van Golen RF, van Gulik TM, Heger M. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver. Antioxid Redox Signal. 2014;21:1119-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1307] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 7. | Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65:1162-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Kim KH, Lee MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10:322-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 737] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 10. | Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279-296. [PubMed] |
| 11. | Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 940] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 12. | Christian P, Sacco J, Adeli K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta. 2013;1831:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3105] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 14. | Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Song HY, Zhang L, Pan JL, Yang LL, Ji G. Bioactivity of five components of Chinese herbal formula Jiangzhi granules against hepatocellular steatosis. J Integr Med. 2013;11:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wang M, Sun S, Wu T, Zhang L, Song H, Hao W, Zheng P, Xing L, Ji G. Inhibition of LXRα/SREBP-1c-Mediated Hepatic Steatosis by Jiang-Zhi Granule. Evid Based Complement Alternat Med. 2013;2013:584634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Pan J, Wang M, Song H, Wang L, Ji G. The efficacy and safety of traditional chinese medicine (jiang zhi granule) for nonalcoholic Fatty liver: a multicenter, randomized, placebo-controlled study. Evid Based Complement Alternat Med. 2013;2013:965723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Matsushita N, Osaka T, Haruta I, Ueshiba H, Yanagisawa N, Omori-Miyake M, Hashimoto E, Shibata N, Tokushige K, Saito K. Effect of Lipopolysaccharide on the Progression of Non-Alcoholic Fatty Liver Disease in High Caloric Diet-Fed Mice. Scand J Immunol. 2016;83:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Cui W, Wang M, Maegawa H, Teranishi Y, Kawada N. Inhibition of the activation of hepatic stellate cells by arundic acid via the induction of cytoglobin. Biochem Biophys Res Commun. 2012;425:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Shu X, Wang M, Xu H, Liu Y, Huang J, Yao Z, Zhang L. Extracts of Salvia-Nelumbinis Naturalis Ameliorate Nonalcoholic Steatohepatitis via Inhibiting Gut-Derived Endotoxin Mediated TLR4/NF-κB Activation. Evid Based Complement Alternat Med. 2017;2017:9208314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kamikubo R, Kai K, Tsuji-Naito K, Akagawa M. β-Caryophyllene attenuates palmitate-induced lipid accumulation through AMPK signaling by activating CB2 receptor in human HepG2 hepatocytes. Mol Nutr Food Res. 2016;60:2228-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z, Florens L, Li R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature. 2017;543:443-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 345] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 23. | Hamasaki M, Shibutani ST, Yoshimori T. Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol. 2013;25:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1042] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 25. | Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 748] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 26. | Madrigal-Matute J, Cuervo AM. Regulation of Liver Metabolism by Autophagy. Gastroenterology. 2016;150:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 27. | Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484-31492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5765] [Cited by in RCA: 5746] [Article Influence: 338.0] [Reference Citation Analysis (1)] |
| 29. | Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1571] [Article Influence: 157.1] [Reference Citation Analysis (0)] |
| 30. | Fukuo Y, Yamashina S, Sonoue H, Arakawa A, Nakadera E, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol Res. 2014;44:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24:3052-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 32. | Inami Y, Yamashina S, Izumi K, Ueno T, Tanida I, Ikejima K, Watanabe S. Hepatic steatosis inhibits autophagic proteolysis via impairment of autophagosomal acidification and cathepsin expression. Biochem Biophys Res Commun. 2011;412:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |