Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.35
Peer-review started: September 3, 2017
First decision: September 27, 2017
Revised: November 23, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 7, 2018
To investigate the effects of combined use of emodin and baicalein (CEB) at the cellular and organism levels in severe acute pancreatitis (SAP) and explore the underlying mechanism.
SAP was induced by retrograde infusion of 5% sodium taurocholate into the pancreatic duct in 48 male SD rats. Pancreatic histopathology score, serum amylase activity, and levels of tumour necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-10 were determined to assess the effects of CEB at 12 h after the surgery. The rat pancreatic acinar cells were isolated from healthy male SD rats using collagenase. The cell viability, cell ultrastructure, intracellular free Ca2+ concentration, and inositol (1,4,5)-trisphosphate receptor (IP3R) expression were investigated to assess the mechanism of CEB.
Pancreatic histopathology score (2.07 ± 1.20 vs 6.84 ± 1.13, P < 0.05) and serum amylase activity (2866.2 ± 617.7 vs 5241.3 ± 1410.0, P < 0.05) were significantly decreased in the CEB (three doses) treatment group compared with the SAP group (2.07 ± 1.20 vs 6.84 ± 1.13, P < 0.05). CEB dose-dependently reduced the levels of the pro-inflammatory cytokines IL-6 (466.82 ± 48.55 vs 603.50 ± 75.53, P < 0.05) and TNF-α (108.04 ± 16.10 vs 215.56 ± 74.67, P < 0.05) and increased the level of the anti-inflammatory cytokine IL-10 (200.96 ± 50.76 vs 54.18 ± 6.07, P < 0.05) compared with those in the SAP group. CEB increased cell viability, inhibited cytosolic Ca2+ concentration, and significantly ameliorated intracellular vacuoles and IP3 mRNA expression compared with those in the SAP group (P < 0.05). There was a trend towards decreased IP3R protein in the CEB treatment group; however, it did not reach statistical significance (P > 0.05).
These results at the cellular and organism levels reflect a preliminary mechanism of CEB in SAP and indicate that CEB is a suitable approach for SAP treatment.
Core tip: Combined use of emodin and baicalein (CEB) was found to act on key aspects of severe acute pancreatitis (SAP) pathogenesis; it regulated inflammatory factors and inhibited calcium overload, which underlie this synergy. These results at the cellular and organism levels reflect a preliminary mechanism of CEB in SAP and indicate that the development of CEB is promising for the treatment of patients with SAP.
- Citation: Li J, Zhou R, Bie BB, Huang N, Guo Y, Chen HY, Shi MJ, Yang J, Zhang J, Li ZF. Emodin and baicalein inhibit sodium taurocholate-induced vacuole formation in pancreatic acinar cells. World J Gastroenterol 2018; 24(1): 35-45
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/35.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.35
Severe acute pancreatitis (SAP) is a debilitating disease that is characterized by an acute onset, serious complications, and significant morbidity and mortality[1]. Currently, the somatostatin analogue octreotide is the most frequently used drug in SAP therapy[2,3]. However, not all patients are treated with octreotide due to its short half-life in vivo and high cost. Identifying other safe, effective, and inexpensive drugs for the treatment of SAP remains necessary.
Traditional Chinese medicines (TCMs) have shown efficacy in the management of many inflammatory diseases[4]. The most famous formula for the treatment of SAP is Qingyi decoction, which is composed of rheum, Chinese thorowax root, white peony root, baikal skullcap root, coptis chinensis, and other herbs and has been advocated for more than one hundred years in China[5,6]. Qingyi decoction has exhibited good therapeutic effects in individuals with SAP in many clinical and pre-clinical studies[7-9]. However, use of this decoction is limited because patients are not allowed access to food and water in the acute stage. Furthermore, the formulation of this TCM is difficult to standardize due to the large number of herbs in one decoction. Guided by the theories of TCM and modern medicine and the compatibility of herbs, we refined Qingyi decoction and combined emodin and baicalein into “Compound Emodin and Baicalein” (CEB, patent No. ZL200310105814.3).
Emodin and baicalein are the active ingredients in rheum and baikal skullcap root and have an identified chemical constitution[10,11]. Emodin has been reported to block the development of SAP[12]. In our previous study, we were the first to show that baicalein protected against pancreatic injury in rats with SAP and exhibited inhibitory effects on pro-inflammatory cytokine production[11]. Furthermore, CEB had a beneficial effect on SAP in rats, which is similar to the effect of octreotide[13-15]. However, the underlying mechanisms remain unknown. We aimed to investigate the above problems, which are crucial for the study and development of CEB. Here, we report that CEB exhibited a good effect on SAP rats, decreased the serum levels of pro-inflammatory cytokines, and increased the levels of anti-inflammatory factors in vivo. CEB attenuated inositol (1,4,5)-trisphosphate receptor (IP3R) mRNA expression and inhibited calcium overload in pancreatic acinar cells. Therefore, we suggest that CEB might be a novel drug for SAP treatment in the future.
Adult male Sprague-Dawley (SD) rats (weighing 220 ± 10 g) were obtained from the Experimental Animal Center of Xi’an Jiaotong University Health Science Center and were housed at 23 ± 2 °C under a 12-h light/dark cycle. The animals were fasted overnight before the start of the experiments with free access to water. All animal procedures were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University Health Science Center and performed according to the National Guide for the Care and Use of Laboratory Animals.
SAP was induced via a retrograde infusion of 5% sodium taurocholate into the pancreatic duct as previously described[16]. The animals were anaesthetized with sodium pentobarbital (50 mg/kg, Sigma-Aldrich), and the abdomen was opened via a midline incision. First, we identified the common bile duct and duodenum. The pancreatic bile duct was occluded with two microvascular clamps to prevent reflux. In total, 5% sodium taurocholate was injected into the pancreatic duct at a dose of 0.1 mL/100 g body weight (at an injection velocity of 0.1 mL/min). Then, the injection site was pressed for 3 min and the surgery was completed by abdominal stratified closing. Special attention was focused on the atraumatic surgical technique. The humane killing was performed 12 h after the operation to determine the severity of SAP.
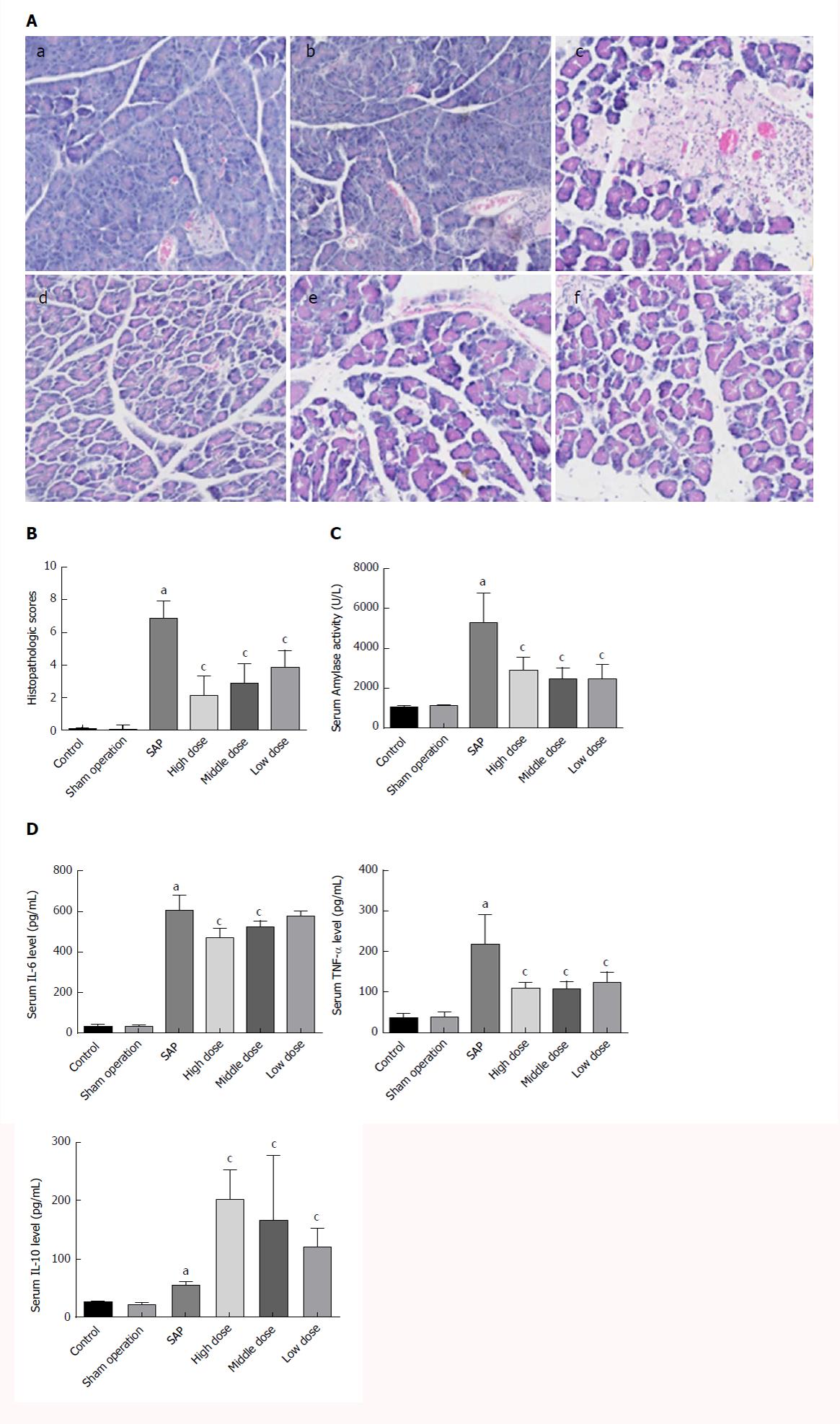
In total, SAP was induced in 48 SD rats via a retrograde injection of 5% sodium taurocholate into the common biliopancreatic duct. All SAP rats were randomly divided into four groups, including an SAP model group and three CEB groups (the low-dose group received a 1.6 mg/kg dose of emodin combined with a 3.5 mg/kg dose of baicalein; the middle-dose group received a 3.2 mg/kg dose of emodin combined with a 7 mg/kg dose of baicalein; and the high-dose group received a 6.4 mg/kg dose of emodin combined with a 14 mg/kg dose of baicalein). An additional 24 normal SD rats were separated into two groups: a normal group and a sham operation (SO) group. The normal group did not undergo the procedure. In the SO group, the incisions were closed immediately after turning over the pancreas. CEB was injected via the tail vein to animals of the three CEB treatment groups immediately after surgical operation. SO and SAP rats were given the same amount of normal saline. Pancreatic histopathology score, serum amylase activity, and levels of tumour necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-10 were determined 12 h after the surgery.
The pancreatic tissue was fixed in 4% paraformaldehyde for 24 h, dehydrated, and embedded in paraffin using a routine protocol as previously described[11]. The embedded tissue was sliced at a 4-μm thickness and stained using haematoxylin and eosin (H&E). The histology scores of the sections were independently evaluated by two experienced investigators who were blinded to the group assignment. Ten random fields per slide were examined in the histopathological analysis of each pancreas. The grading criteria were as follows: oedema, acinar necrosis, haemorrhage, and inflammatory cell infiltration (Table 1). The final score of each section was the summation of each pathological parameter[17]. Finally, the mean ± SD was calculated (2 slides/rat, ≥ 6 rats/group).
| Histological pattern | Assessment | Score |
| Edema | Mild | 1 |
| Moderate | 2 | |
| Severe | 3 | |
| Inflammatory infiltration | Mild | 1 |
| Moderate | 2 | |
| Severe | 3 | |
| Fat necrosis | < 2/section | 3 |
| 3-5/section | 5 | |
| > 5/section | 7 | |
| Parenchymal necrosis | Focal (< 5%) | 3 |
| And/or sublobular (< 20%) | 5 | |
| And/or lobular (> 20%) | 7 | |
| Hemorrhage | Mild | 3 |
| Moderate | 5 | |
| Severe | 7 |
Serum amylase activity was measured using an automatic biochemistry analyser (Roche, Basel, Switzerland). Serum IL-6, TNF-α, and IL-10 levels were determined using enzyme-linked immunosorbent assay kits (Neobioscience Biotech, Shenzhen, China and eBioscience, San Diego, USA) according to the manufacturers’ protocols. The absorbance was read at 450 nm (620 nm as the reference wavelength) using a microplate reader (BioTek Inc., Winooski, VT, United States).
Pancreatic acinar cells were isolated as described by Williams et al[18] with slight modifications. Briefly, the pancreas was incubated with 5 mL of digestion solution, consisting of collagenase (NB8 Broad Range, 0.1 g/L, Serva) and a trypsin inhibitor (0.1 g/L, Sigma). The digestion solution was injected (at multiple loci) into the pancreatic parenchyma until it was well distended. After 10 min, the pancreas was cut into 2-4-mm pieces, followed by shaking for 15 min in a 37 °C water bath (120 cycles per minute). Next, 5 mL of fresh digestion solution was added to the pancreas. The digestion period was repeated 3 times, and the liquids were then collected and combined. The tissue was then gently pipetted through the pipette tip. After the filtration and centrifugation, the resultant cell pellet was resuspended in the culture medium and preincubated at 37 °C for 30 min for the subsequent experiments. All obtained pancreatic acinar cells were identified under a transmission electron microscope.
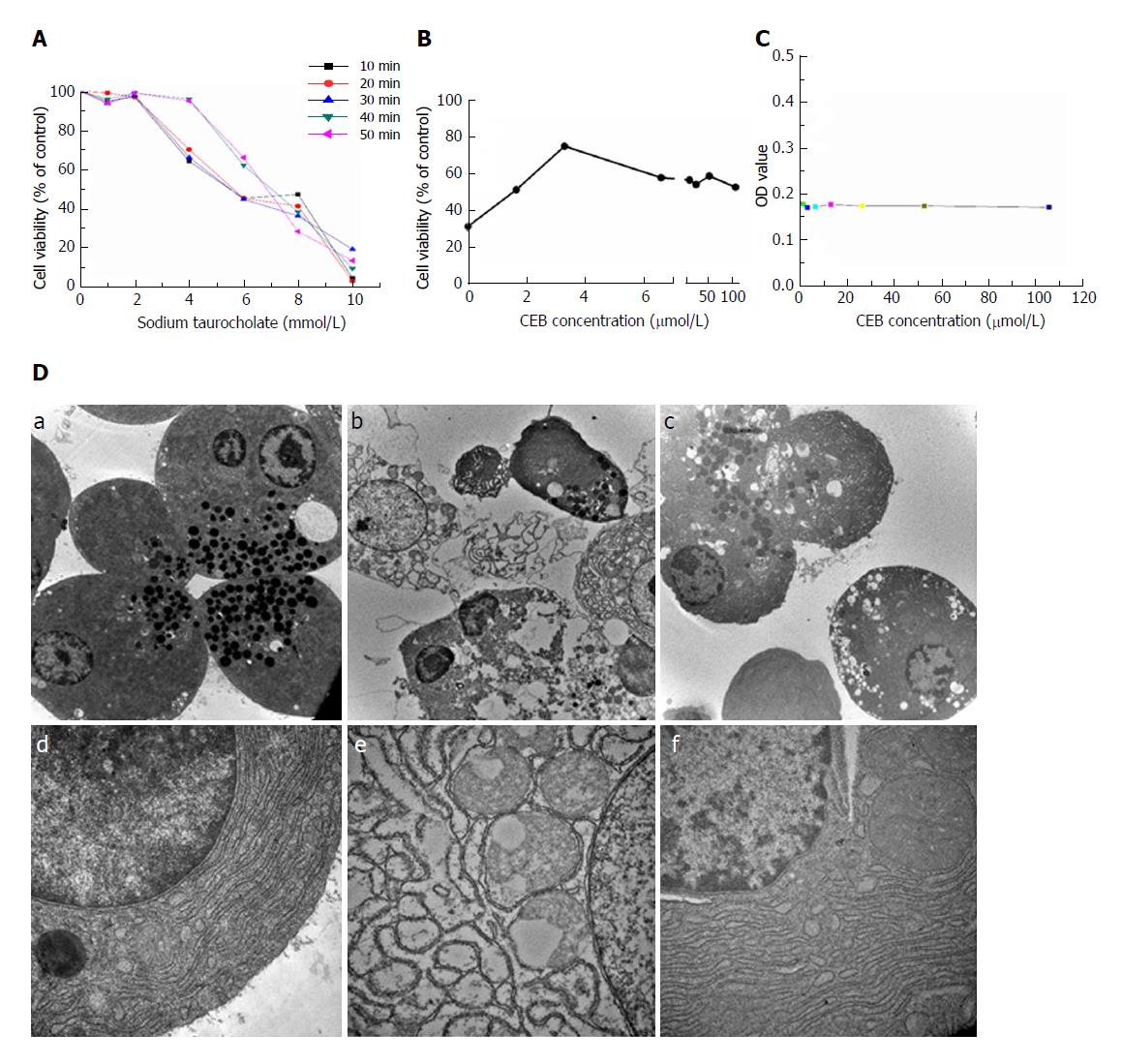
The cells were incubated with various concentrations of sodium taurocholate (final concentrations of 1, 2, 4, 6, 8, and 10 mmol/L) for 10, 20, 30, 40, and 50 min. The cell viability was determined using an MTT assay[19] to identify an appropriate concentration of sodium taurocholate for subsequent cell experiments.
The pancreatic acinar cells were divided into three groups, including a control group, an SAP model group, and a CEB group. The effects of CEB on the cell viability were determined using an MTT assay. The cells were preincubated with or without CEB (final concentration of 3.3 μmol/L) for 10 min prior to sodium taurocholate (final concentration of 8 mM) irritation. After an additional 30 min incubation with sodium taurocholate, the cell viability was measured using an MTT assay. The normal cells were treated with different concentrations of CEB (1.65, 3.3, 6.6, 13.2, 26.4, 52.8, and 105.6 μmol/L), and an MTT assay was performed. All experiments were performed in triplicate and repeated at least three times.
The cells were preincubated with various concentrations of CEB (final concentrations of 1.65, 3.3, 6.6, 13.2, 26.4, 52.8, and 105.6 μmol/L) for 30 min. The optical density value was detected using an MTT assay to assess the reliability of CEB at the cellular level.
The cells were pre-treated with or without CEB (final concentration of 3.3 μmol/L) for 10 min. Sodium taurocholate (1 mmol/L) was added to the cells as a stimulus for 1 min, immediately followed by washing with the PBS buffer, pelleting by centrifugation at 200 g for 1 min, and fixing using 2.5% prechilled glutaraldehyde at 4 °C for 24 h. The samples were then immersed in 1% osmium tetroxide at room temperature for 2 h and then uranyl acetate for the same period. The cells were dehydrated in serial solutions of ethanol and acetone before being embedded in epoxy resins. Then, 50-nm ultrathin sections were stained with lead citrate and mounted on 200-mesh copper grids. The cell ultrastructure was observed under a Hitachi H-7650 (Tokyo) transmission electron microscope.
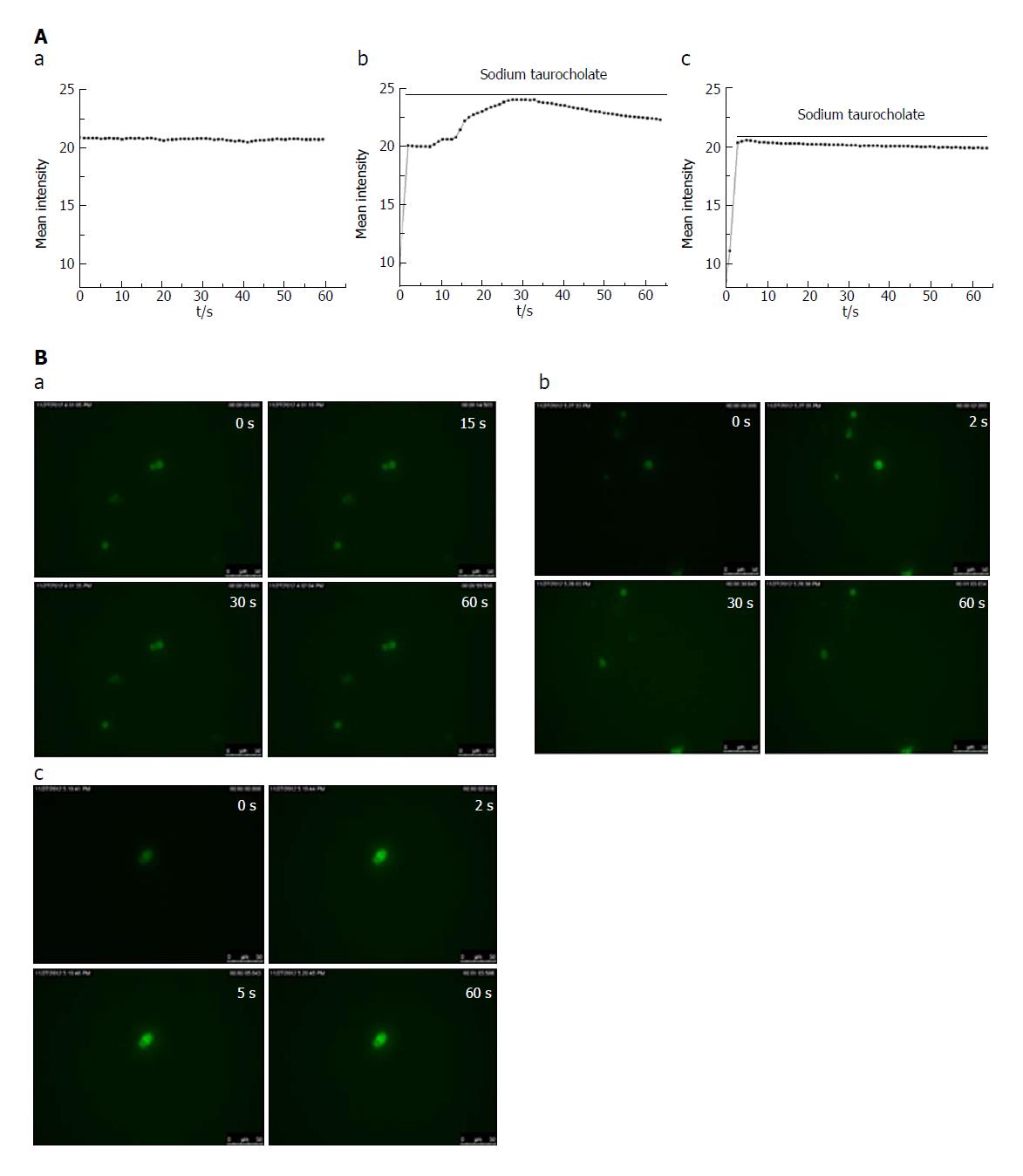
Isolated pancreatic acinar cells were pre-treated with or without CEB (final concentration of 3.3 μmol/L) for 10 min. The cell suspension was washed once with PBS and incubated with fluo-3 AM (5 μmol/L, Invitrogen) for 30 min in the dark, followed by three washes with PBS. Then, 2 mL of PBS were added to the cells and incubated for an additional 30 min. Sodium taurocholate (1 mmol/L) was added to the cells as a stimulus. The cells were imaged on a live cell imaging system to assess the cytosolic Ca2+ concentration using fluo-3 AM (488 nm) immediately after the stimulus was applied, and the recording time was maintained at 60 s. The fluorescence intensity represented the relative intracellular Ca2+ concentration.
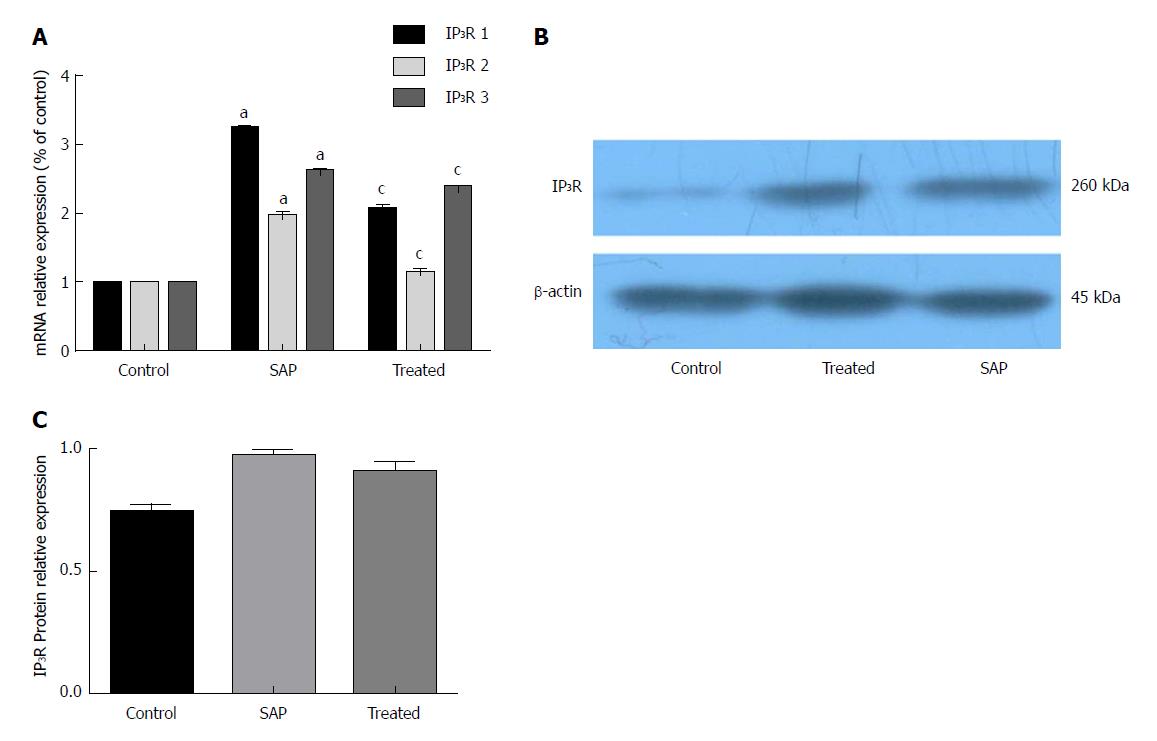
The cells were stimulated with sodium taurocholate as described above, immediately followed by washing with PBS and pelleting by centrifugation at 200 g for 1 min. Total RNA and protein were purified from the acinar cells according to the manufacturer’s instructions (Ambion, Pierce). The protein and mRNA levels of IP3R were quantified by RT-PCR and Western blot, respectively, as previously described[20].
The assays were performed in triplicate. All data are expressed as mean ± SD. Statistical comparisons were performed using t-tests or analysis of variance with SPSS 19.0 (SPSS Inc.). A P-value < 0.05 was considered statistically significant.
The SO group showed an entirely normal acinar architecture. The SAP group demonstrated pancreatic injury characterized by gross tissue oedema, sublobular haemorrhage, cell lysis associated with obvious neutrophil infiltration, and parenchymal necrosis. Treatment with CEB ameliorated pancreatic injury and decreased the histopathology score (Figure 1A and B, P < 0.05).
Serum amylase activity and levels of IL-6, TNF-α, and IL-10 in the SAP group were significantly increased compared to those in the SO group. Treatment with CEB decreased serum amylase activity and TNF-α level but increased the level of IL-10 at all the three doses (Figure 1C and D, P < 0.05). CEB decreased serum IL-6 level in SAP rats at high and middle doses (Figure 1D, P < 0.05). There was also a trend towards decreased IL-6 level at low dose; however, it did not reach statistical significance.
Different concentrations of sodium taurocholate induced a decrease in cell viability (Figure 2A, P < 0.05). Cell viability decreased in a dose- and time- dependent manner after sodium taurocholate treatment. Compared to the control group, cell viability was decreased in the SAP group. Pretreatment with CEB could increase the viability of cells treated with 8 mmol/L sodium taurocholate and showed dose-dependent protective effects at 1.65-3.3 μmol/L (Figure 2B, P < 0.05). Moreover, CEB alone had no adverse effect on the normal cells at either low or high concentration (Figure 2C, P > 0.05).
The normal cells showed characteristics that are typical of secretory polarized cells, including slick plasmalemma and a roundish nucleus in the basal region of the cell. The rough endoplasmic reticulum (ER) was abundant and arranged in the parallel cisternae in the basal region, and numerous ribosomes were present on the membrane of the ER. Many zymogen granules (ZGs) were observed in the apical region of the cell. The mitochondria exhibited a spherical shape, and well-developed cristae were observed in the basal region of the cell (Figure 2D, a and d). In the SAP group, after the 1-min stimulation with 1 mmol/L sodium taurocholate, major changes were observed in the cellular ultrastructure, including the rupture of the plasmalemma, formation of abnormal vacuoles in the cytoplasm, dilatation and degranulation of the ER, lysis of the membranes of the rough ER, and a marked reduction in the density of the normal ZGs (Figure 2D, b and e). In cells that were preincubated with 3.3 μmol/L CEB for 10 min, the vacuolization, cytolysis, and degranulation of the ER were significantly alleviated (Figure 2D, c and f).
In the control group, there was no change in the cell fluorescence intensity over time (Figure 3A and B, a). In the SAP group, the cell fluorescence intensity immediately increased within 2 s of adding the irritant sodium taurocholate to the cells compared with that before the stimulation. The cell fluorescence intensity lasted for 30 s at a high Ca2+ concentration and then began to decline (Figure 3A and B, b). In the CEB group, the cell fluorescence intensity increased within the first 2 s after the sodium taurocholate suscitation and was sustained by the high calcium concentration for 5 s before beginning to decline (Figure 3A and B, c). Thus, the duration during which the cells survived in a high Ca2+ plateau decreased in the CEB preincubated cells compared to that in the cells that were not preincubated with CEB (Table 2, P < 0.05).
Compared with the control group, the three isoforms of IP3R mRNA and IP3R protein were increased in the SAP group. CEB preincubation caused a significant decrease in the mRNA expression of the three IP3R isoforms (Figure 4A, P < 0.05). In the presence of CEB, there was also a trend towards decreased IP3R protein; however, it did not reach statistical significance (Figure 4B and C, P > 0.05).
The famous TCM Qingyi decoction has been shown to have clinical benefits in the treatment of SAP[5,7]. However, the challenges associated with its oral administration, complex components, and difficult-to-control quality limit its application and formulation. We refined Qingyi decoction and devised a compound formula with independent intellectual property rights, comprising emodin and baicalein, which we call CEB[13,14]. The components of CEB are the monomers emodin and baicalein, and the properties of CEB include clear configuration, controllable quality, and therapeutic effects following an intravenous injection in SAP rats; thus, CEB could be further developed for the treatment of patients with SAP and is worth further investigation. Our previous study has shown that the combination of emodin and baicalein exhibits good efficacy in SAP rats[13,15]. However, the specific mechanism remains unknown.
The pathogenesis of SAP remains poorly understood[21]. One hypothesis posits that inflammatory reactions are crucial for the pathogenesis of SAP[22]. Local inflammatory reactions in the pancreas lead to systemic inflammatory response syndrome (SIRS) and multiorgan failure (MOF), which is believed to be the primary cause of mortality[23]. TNF-α and IL-6 are known principal pro-inflammatory cytokines that participate in the initiation and progression of SAP and have been identified as the key cytokines mediating pancreatitis-associated lung injury[11]. IL-10 has important anti-inflammatory activities and is strongly associated with the severity of SAP[24]. IL-10 is believed to play a protective role in SAP. In our study, we similarly found that serum levels of IL-6, TNF-α, and IL-10 were considerably higher in the SAP group than in the SO group. CEB reduced the pro-inflammatory cytokines IL-6 and TNF-α and increased the anti-inflammatory cytokine IL-10 after the induction of SAP.
The pathological diagnosis is a significant index for evaluating the severity of SAP. Amylase activity is an enzymatic marker, and the serum amylase test is routinely performed for the clinical diagnosis of SAP. In this study, CEB significantly alleviated pancreatic injury and decreased pancreatic histopathology score in the SAP model. Therefore, we conclude that CEB can down-regulate the pro-inflammatory cytokines IL-6 and TNF-α, up-regulate the anti-inflammatory cytokine, and decrease the serum amylase activity, thus reducing the pathologic score and ameliorating pancreatic injury. Therefore, the therapeutic effect of CEB on SAP warrants further study.
Ca2+ overload is the earliest crucial event in the pathogenesis of many diseases[25]. In pancreatic acinar cells, pathological Ca2+ overload is a critical trigger that initiates cell injury due to sustained global Ca2+ elevations, resulting in enzyme activation, vacuolization, and necrosis, all of which are crucial for the development of pancreatitis[26]. Emodin can inhibit pancreatic enzyme levels and calcium channels[27]. Baicalein has been reported to have antibacterial, antiviral, anti-inflammatory, and calcium blocker properties in cardiomyocytes[11,28]. However, whether CEB can inhibit Ca2+ overload during the development of SAP remains unclear. We used pancreatic acinar cells prepared from healthy rats that were induced by sodium taurocholate as an SAP model at the cellular level and observed the effect of CEB. In our study, sodium taurocholate exerted a cellular Ca2+ overload, leading to decreased cell viability, intracellular vacuole formation, and cellular injury. However, CEB inverted this impairment and did not show a cytotoxic effect in normal acinar cells, revealing its safety and hinting at the mechanism of CEB action.
IP3R is a principal Ca2+-releasing channel that is concentrated in the ER membrane, and the opening of this channel is coordinated by the binding of IP3 and the activation of gated Ca2+ channels that release intracellularly stored Ca2+ from the ER to regulate the cellular Ca2+ environment[29]. In our study, at the cellular level, CEB decreased the sodium taurocholate-induced intracellular Ca2+ and IP3R mRNA expression in isolated rat pancreatic acinar cells. Thus, CEB exerts effects on the inhibition of the acinar cell calcium overload, possibly representing its underlying mechanism in SAP cells. The protein expression changes required a certain amount of time. To detect the calcium changes, we performed the observations 60 s after the sodium taurocholate stimulation, which may explain why the IP3R protein expression showed a slight decline and no statistical significance in the CEB group compared with that in the SAP group.
In summary, CEB was found to act on the key aspects of SAP pathogenesis and resulted in the regulation of inflammatory factors and the inhibition of calcium overload, which underlie this synergy. These results at the cellular and organism levels reflect a preliminary mechanism of CEB in SAP and suggest potential clinical benefits. Although research on CEB remains at the preclinical level, and data on its use in humans are lacking, CEB may be a potential future treatment for SAP, suggesting that further studies are needed.
Severe acute pancreatitis (SAP) is a debilitating disease with significant morbidity and mortality. Somatostatin analogue octreotide is the most frequently used drug but not all patients can afford its high cost and short half-life in vivo. Guided by the theories of TCM and modern medicine and the compatibility of herbs, we refined the classic prescription Qingyi decoction and combined emodin and baicalein into “Compound Emodin and Baicalein” (CEB, patent No. ZL200310105814.3). This research may provide a novel, safe, effective, and inexpensive drug for SAP treatment in the future.
The aim of this study was to explicate the effect and mechanism of CEB in SAP therapy and provides potential clinical benefits for the future treatment of SAP.
The SAP rats and the isolated pancreatic acinar cells were the main objectives in this study. Meanwhile, they also served as the main objectives of research on SAP at the cellular and organism levels.
Retrograde infusion of 5% sodium taurocholate into the pancreatic duct is the frequently used method for SAP model induction. Serum cytokine levels were determined using enzyme-linked immunosorbent assay. Tissue section were stained using haematoxylin and eosin (H&E) for histopathologic detection. Pancreatic acinar cells were isolated using collagenase digestion and the cell viability was determined using an MTT assay. Cell ultrastructure was observed using a transmission electron microscope. Intracellular Ca2+ concentration was determined using a live cell imaging system. The protein and mRNA levels of IP3R were quantified by RT-PCR and Western blot, respectively.
The results showed that CEB can regulate inflammatory factors, increase cell viability, and inhibit calcium overload, which underlie a preliminary mechanism of CEB in SAP. Although research on CEB remains at the preclinical level, and data on its use in humans are lacking, CEB may be a potential future treatment for SAP, suggesting that further studies are needed.
Emodin and baicalein display synergistic effects on SAP rats. CEB acts on the key aspects of SAP pathogenesis and regulates of inflammatory factors in SAP rats. CEB suppresses IP3R-mediated pancreatic acinar Ca2+ overload via inhibiting IP3R mRNA expression in pancreatic acinar cells. As a non-toxic natural product, CEB is the first to show a therapeutic effect at the cellular and organism levels in SAP. CEB is promising for the treatment of patients with SAP.
From this study, the characteristics of abundance, low cost, and safety of traditional Chinese medicine in SAP therapy should catch our attention. We think that the potential of traditional Chinese medicine in SAP therapy will be the direction of the future research. In our opinion, the SAP animal model and the isolated pancreatic acinar cells will always be the main avenues in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bramhall S, Tsoulfas G, Yago MD S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1151] [Cited by in F6Publishing: 1218] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 2. | Xu W, Zhou YF, Xia SH. Octreotide for primary moderate to severe acute pancreatitis: A meta-analysis. Hepatogastroenterology. 2013;60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Uhl W, Büchler MW, Malfertheiner P, Beger HG, Adler G, Gaus W. A randomised, double blind, multicentre trial of octreotide in moderate to severe acute pancreatitis. Gut. 1999;45:97-104. [PubMed] [Cited in This Article: ] |
| 4. | Liu JS, Wei XD, Lu ZB, Xie P, Zhou HL, Chen YY, Ma JM, Yu LZ. Liang-Ge-San, a classic traditional Chinese medicine formula, protects against lipopolysaccharide-induced inflammation through cholinergic anti-inflammatory pathway. Oncotarget. 2016;7:21222-21234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 2015;21:3537-3546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Li J, Zhang S, Zhou R, Zhang J, Li ZF. Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J Gastroenterol. 2017;23:3615-3623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 7. | Ji CH, Tang CW, Feng WM, Bao Y, Yao LQ. A Chinese Herbal Decoction, Huoxue Qingyi Decoction, Promotes Rehabilitation of Patients with Severe Acute Pancreatitis: A Retrospective Study. Evid Based Complement Alternat Med. 2016;2016:3456510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Zhu FS, Zhu GY, Huang DP, Shen XY, Yang CQ, Gao HJ. [Effect of Qingyi Decoction on gene expression profiles of severe acute pancreatitis rats by gene chip technique]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:51-55. [PubMed] [Cited in This Article: ] |
| 9. | Zhang XM, Chen HL, Wang ZH. [Expression of secretory type II phospholipase A2 in acute lung injury following acute pancreatitis and interventional effect of Qingyi decoction on it]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:518-521. [PubMed] [Cited in This Article: ] |
| 10. | Liu Z, Ma N, Zhong Y, Yang ZQ. Antiviral effect of emodin from Rheum palmatum against coxsakievirus B5 and human respiratory syncytial virus in vitro. J Huazhong Univ Sci Technolog Med Sci. 2015;35:916-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Li J, Wu Y, Zhang S, Zhang J, Ji F, Bo W, Guo X, Li Z. Baicalein protect pancreatic injury in rats with severe acute pancreatitis by inhibiting pro-inflammatory cytokines expression. Biochem Biophys Res Commun. 2015;466:664-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Yao WY, Zhou YF, Qian AH, Zhang YP, Qiao MM, Zhai ZK, Yuan YZ, Yang SL. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factorκB activation resulting in antioxidation. Mol Med Rep. 2015;11:1416-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Li ZF, Xia XM, Huang C, Zhang S, Zhang J, Zhang AJ. Emodin and baicalein inhibit pancreatic stromal derived factor-1 expression in rats with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:201-208. [PubMed] [Cited in This Article: ] |
| 14. | Zhang XP, Li ZF, Liu XG, Wu YT, Wang JX, Wang KM, Zhou YF. Effects of emodin and baicalein on rats with severe acute pancreatitis. World J Gastroenterol. 2005;11:2095-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Li Z, Xia X, Zhang S, Zhang A, Bo W, Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed Pharmacother. 2009;63:120-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [PubMed] [Cited in This Article: ] |
| 17. | Spormann H, Sokolowski A, Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989;184:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Williams JA. Isolation of rodent pancreatic acinar cells and acini by collagenase digestion. The Pancreapedia: Exocrine Pancreas Knowledge Base 2010; . [Cited in This Article: ] |
| 19. | Chen K, Zhang S, Ji Y, Li J, An P, Ren H, Liang R, Yang J, Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Bie B, Sun J, Li J, Guo Y, Jiang W, Huang C, Yang J, Li Z. Baicalein, a Natural Anti-Cancer Compound, Alters MicroRNA Expression Profiles in Bel-7402 Human Hepatocellular Carcinoma Cells. Cell Physiol Biochem. 2017;41:1519-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Jakkampudi A, Jangala R, Reddy BR, Mitnala S, Nageshwar Reddy D, Talukdar R. NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology. 2016;16:477-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Penido A, Coelho AM, Molan NT, Silva FP, D’Albuquerque LA, Machado MC. Do opioid receptors play a role in the pathogenesis of the inflammatory response in acute pancreatitis? Acta Cir Bras. 2012;27:600-605. [PubMed] [Cited in This Article: ] |
| 23. | Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16:3995-4002. [PubMed] [Cited in This Article: ] |
| 24. | Zhou X, Liu Z, Jang F, Xiang C, Li Y, He Y. Autocrine Sonic hedgehog attenuates inflammation in cerulein-induced acute pancreatitis in mice via upregulation of IL-10. PLoS One. 2012;7:e44121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Li J, Zhou R, Zhang J, Li ZF. Calcium signaling of pancreatic acinar cells in the pathogenesis of pancreatitis. World J Gastroenterol. 2014;20:16146-16152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Wu L, Cai B, Liu X, Cai H. Emodin attenuates calcium overload and endoplasmic reticulum stress in AR42J rat pancreatic acinar cells. Mol Med Rep. 2014;9:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Chen HM, Hsu JH, Liou SF, Chen TJ, Chen LY, Chiu CC, Yeh JL. Baicalein, an active component of Scutellaria baicalensis Georgi, prevents lysophosphatidylcholine-induced cardiac injury by reducing reactive oxygen species production, calcium overload and apoptosis via MAPK pathways. BMC Complement Altern Med. 2014;14:233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Lopez LF, Dawson SP. Luminal Ca(2+) dynamics during IP3R mediated signals. Phys Biol. 2016;13:036006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |