Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1147
Peer-review started: October 14, 2016
First decision: December 1, 2016
Revised: December 16, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: February 21, 2017
Processing time: 129 Days and 17.1 Hours
To develop a colon-targeting bioreversible delivery system for β-boswellic acid (BBA) and explore utility of its prodrugs in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats.
Synthesis of 4 co-drugs of BBA with essential amino acids was achieved by CDI coupling, followed by their spectral characterization. In vitro kinetics were studied by HPLC in aqueous buffers, homogenates of gastrointestinal tract and fecal matter. In vivo kinetic studies were performed in Wistar rat plasma, urine and feces. The prodrugs were screened in TNBS-induced colitis modeled Wistar rats. Statistical significance was assumed at P < 0.05, P < 0.01, P < 0.001 when compared with disease controls using one-way and two-way ANOVAs.
Prodrugs were stable in 0.05 mol/L HCl buffer (pH 1.2) and stomach homogenates. Negligible hydrolysis was observed in phosphate buffer and intestinal homogenates. Substantial release (55%-72% and 68%-86%) of BBA was achieved in rat fecal matter and homogenates of colon. In vivo studies of BBA with L-tryptophan (BT) authenticated colon-specific release of BBA. But, surprisingly substantial concentration of BBA was seen to reach the systemic circulation due to probable absorption through colonic mucosa. Site-specifically enhanced bioavailability of BBA could be achieved in colon, which resulted in demonstration of significant mitigating effect on TNBS-induced colitis in rats without inducing any adverse effects on stomach, liver and pancreas. Prodrug of BT was found to be 1.7% (P < 0.001) superior than sulfasalazine in reducing the inflammation to colon among all prodrugs tested.
The outcome of this study strongly suggests that these prodrugs might have dual applicability to inflammatory bowel disease and chronotherapy of rheumatoid arthritis.
Core tip: Boswellic acids (BAs) are traditionally used in the treatment of inflammatory diseases and are effective in the treatment of inflammatory bowel disease (IBD) in particular, but they undergo extensive metabolism that results in low oral bioavailability. Colon-targeted delivery of BA was achieved by designing prodrugs that deliver BA site-specifically. The synthesized prodrugs were designed by semi-synthetic approach, wherein β-boswellic acid (BBA) was derivatized into a bioreversible delivery system by incorporation of amino acids as promoities for targeted delivery to inflamed colon in IBD. Prodrug of BBA with L-tryptophan (BT) was 1.7-times more effective than sulfasalazine (SLZ) in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in Wistar rats. In vivo behavior of prodrug BT was very interesting and similar to SLZ, which is known to treat local inflammation in IBD as well as in rheumatoid arthritis (RA). The outcome of this study strongly suggests that these prodrugs might have dual applicability to IBD and chronotherapy of RA.
- Citation: Sarkate A, Dhaneshwar SS. Investigation of mitigating effect of colon-specific prodrugs of boswellic acid on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in Wistar rats: Design, kinetics and biological evaluation. World J Gastroenterol 2017; 23(7): 1147-1162
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1147.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1147
Ulcerative colitis (UC) and Crohn’s disease (CD) are together known as inflammatory bowel disease (IBD). IBD is a chronic inflammatory state, comprised of relapsing inflammation of the gastrointestinal tract (GIT) with unknown etiology and which lasts throughout the lifetime[1-3]. It is estimated that about 1.4 million people in the United States, as well as 2.2 million people in Europe, are affected by IBD[4]. In CD, the inflammation is characteristically discontinuous, transmural and often granulomatous, and can occur anywhere in the GIT, from the mouth to the anus[3,5]. UC is limited to the colon and usually affects the superficial layers of the intestinal wall. The onset of IBD may be a result of complex and elusive interactions between genetic alterations in intestinal barrier function, inherent apoptosis, signal transduction, and immunological and environmental factors[1,6].
5-aminosalicylate (5-ASA) and corticosteroids are used as first-line therapy of IBD[7]. Azathioprine, 6-mercaptopurine, methotrexate, calcineurin inhibitors and anti-TNF-α-antibodies have an important role in the treatment of severe disease stages[8]. Minimizing drug-induced side effects and mortality are the main challenges during management of IBD. Sulfasalazine (SLZ) was the first colon-targeting prodrug of 5-ASA and sulfapyridine, representing a cornerstone of IBD therapy. Thirty percent of patients taking SLZ suffer from serious adverse effects, such as hypersensitivity, nephritis, pancreatitis, hepatitis, pneumonitis, drug-induced blood disorders and male infertility. Corticosteroid therapy induces side effects such as hypertension, hyperglycemia, osteoporosis, glaucoma, depression and development of cataracts[9]. Treatment with immunosuppressive agents is linked with an increased susceptibility to infections, malignoma, increased vulnerability for opportunistic infections and GI complaints[2,10-12]. It has been observed that use of complementary and alternative medicine is on the rise generally, and particularly for herbal medicine for IBD management.
The gum resin of Boswellia serrata (Bs) Roxb. Ex Colebr. (Family Burseraceae, Syn. B. glabra) is a traditional Ayurvedic remedy with anti-inflammatory properties and has become popular in Western countries for its usefulness in treatment of IBD[13-15]. It was in the latter part of the 20th century that the resin received scientific interest as an anti-inflammatory phytomedicine, and extracts of the resin have been applied to treat a variety of chronic inflammatory and autoimmune diseases[16]. Boswellic acids (BAs) are chemically a mixture of triterpenic acids obtained from the oleo gum resin of BS and consist of β-boswellic acid (BBA), acetyl-β-boswellic acid (ABA), 11-keto-β-boswellic acid (KBA) and acetyl-11-keto-β-boswellic acid (AKBA). BAs have been studied extensively for anti-inflammatory, immunomodulatory and anti-tumor activities. They help to preserve the structural integrity of joint cartilage, promote gastrointestinal health and maintain a healthy immune mediator cascade at the cellular level[17-28].
BAs are non-redox, non-competitive inhibitors of 5-lipoxygenase (5-LOX), human leukocyte elastase (HLE) and the nuclear factor-κB pathway, without exerting the adverse effects known for steroids[17,19,29]. The inhibition of leukotrienes is the primary and the most scientifically proven mechanism for anti-inflammatory and anti-arthritic activity of BAs[17,30]. The leukotrienes are also involved in the pathogenesis of IBD. Gerhardt et al[31] reported that alcoholic extract of BS oleo gum resin improved the quantifying parameters of UC in 34 patients. In several clinical studies, extracts of oleo gum resins of BS appeared to be effective in the treatment of chronic bowel diseases and the effects were comparable with the conventional treatment. The non-steroidal anti-inflammatory drug-induced GIT side effects were not observed with BS. The oleo gum resin has proven to be effective in dextran-induced and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis models in rodents. The in vitro assays, animal studies and numerous clinical trials have established the efficacy of BS and formulations containing BAs to be effective in the treatment of IBD[16,32]. The results obtained from the studies of BA were found to be comparable with that of standard marketed drugs SLZ and mesalazine for the treatment of CD and UC, with risk-benefit analysis findings being in favor of BAs[24,31]. BAs are reported to inhibit the intestinal motility and reduce chemically-induced edema and inflammation in intestine in rodents[33].
It has been reported that BAs improve the clinical well-being of patients with IBD. Treatment with this herbal drug is associated with improvement in a number of parameters, such as stool properties, microscopic findings of rectal biopsies, hemoglobin and other blood parameters, serum iron, calcium, phosphorus, proteins, total leukocytes and eosinophils, as well as the CD activity index[24,31,34]. BAs reduce lipid peroxidation and increase the levels of superoxide dismutase, thus ameliorating the oxidative stress associated with intestinal inflammation[17,18,35]. A clinical study conducted on patients with IBD has shown that BA reduces mucosal injury by inhibiting activity of activated leucocytes as well as their adherence to intestinal mucosal cells[36,37].
Borrelli et al[38] evaluated the effect of a Bs gum resin extract (BSE) on intestinal motility and diarrhea in a clinical study in rodents. BSE depressed electrically acetylcholine and barium chloride-induced contractions in the isolated guinea-pig ileum. BSE also prevented diarrhea and normalized intestinal motility in pathophysiological states without slowing the rate of transit in control animals. These results explain the clinical efficacy of BSE in reducing diarrhea in IBD patients.
In a study using BA in chronic UC patients, its efficacy on UC with minimal side effects was confirmed[24] and it produced an 82% remission rate, compared to 75% of those in the SLZ group. Thus, BA offered improvement of UC symptoms similar to that of SLZ[24,39]. Similar findings were reported in patients with CD[31]. As a result of the clinically established symptomatic improvement of IBD symptoms by treatment with BA, it is now considered as a potential therapeutic agent for IBD therapy[38]. Gupta et al[39] have investigated the treatment options for UC. In grade II and grade III colitis patients, the positive effect of BS has also been observed.
Bioavailability is a major hurdle in the conversion of the pre-clinical potential of many botanical extracts into remedial effects, especially for those whose active ingredients show poor water solubility and strong affinity towards self-aggregation. This is observed for many polyphenolics and triterpenoid acids[14]. Pharmacokinetic studies have demonstrated that the systemic absorption of BAs is very low, both in animals and in humans, and extensive metabolism could play a vital role in limiting their systemic availability[14,19]. A United States’ patent has utilized the extract of the gum resin of BS in a phytonutrient formulation for the relief of chronic pain resulting from inflammation[40]. A semi-synthetic form of AKBA significantly reduced the disease activity in experimental murine colitis induced by dextran sodium sulfate (DSS)[32].
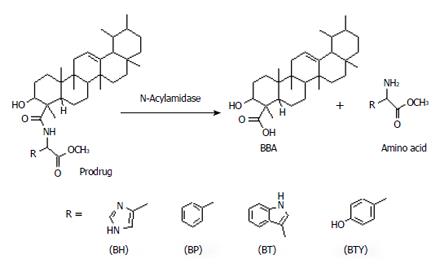
For the present work, BBA was selected as the drug candidate for developing its colon-targeting prodrugs because it is present in the highest percentage in the oleo gum resin and shows prominent anti-inflammatory activity[15,16,20-25]. Bioavailability of BAs at the site of action, i.e. colon, can be improved by enhancing hydrophilicity through designing colon-targeting prodrugs where the selected carriers will impart more hydrophilicity to the BAs so that their systemic absorption is minimized and they reach an effective concentration level in the colon. A mutual prodrug strategy was explored by conjugating BBA with various amino acids into amide co-drugs that would undergo colon-specific activation by N-acyl amidases[41]. This would ensure attainment of effective concentration of BA in the colon for its local mitigating effect on colonic inflammation.
The selection of 4 amino acids, namely L-tryptophan, L-histidine, D-phenylalanine and L-tyrosine, as carriers for designing mutual prodrugs of BBA was based on the recent evidence that has indicated that amino acids may play an important role in maintaining gut health, modulate intestinal immune functions and influence inflammatory responses, and may be useful as alternative or ancillary treatment of IBD. The role of amino acids in reducing inflammation, oxidative stress and apoptosis in the gut is well documented in the literature[42,43]. L-tryptophan has been shown to reduce oxidative stress and immune suppression. It was also reported to decrease pro-inflammatory cytokine expression, thereby inhibiting Th1-mediated inflammation in DSS-induced porcine IBD[44]. Histidine significantly inhibited both hydrogen peroxide- and TNF-α-induced IL-8 secretion and mRNA expression in Caco-2 cells and HT-29 cells. Dysregulation of these cytokines’ balance plays a key role in the pathogenesis of IBD[45,46]. D-phenylalanine restores gut immune homeostasis by attenuating inflammatory responses[47,48]. D-phenylalanine, L-tryptophan and L-tyrosine were chosen as promoieties due to their marked anti-inflammatory activity[43,46,49,50]. Being the natural components of our body, these amino acids would be nontoxic and free from any side effects. N-aromatic acyl amino acid conjugates reported in the literature are stable in upper intestine and hydrolyzed when incubated with mammalian cecal content[51]. Introduction of amide linkage in the prodrug would increase aqueous solubility, thus transcellular absorption by lipid membrane permeation might be limited in the upper GIT. This would be expected to facilitate delivery of intact prodrug to the colon.
BA was purchased from Herbal Remedies Corp. (Bangalore, India). All reagents and chemicals used were of analytical reagent grade. Microwave (MW)-assisted synthesis was performed on the MW synthesizer (Discover System; CEM Corp., Matthews, NC, United States). Infra-red (FTIR) spectra were recorded using KBr on a Jasco V-530 FT/IR-4100 spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance II 400 instrument at 400 MHz. Chemical shifts (δ) are given in ppm using TMS as an internal reference. Mass spectra were recorded on an Agilent 1260 Infinity HPLC-MASS Analyzer 6460 Triple Quad LC/MS. Elemental analysis was performed using Vario Micro Cube (Elementar, Germany). The HPLC system consisted of Jasco PU model 2080, with UV detector. Thermo Scientific Syncronis C18 column (250 mm × 4.6 mm) was used for estimation of prodrugs and their active metabolites.
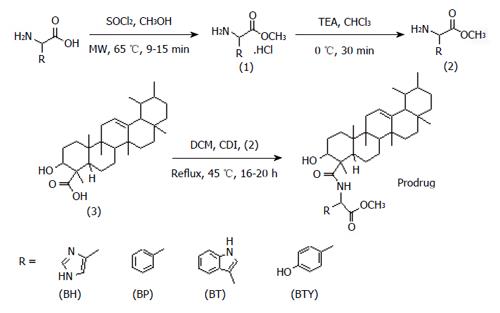
The title prodrugs were synthesized by CDI coupling[52] and purified by column chromatography or preparative TLC. The products were characterized by spectral and elemental analyses.
A mixture of amino acid (0.1 mol) and freshly distilled thionyl chloride (0.05 mol) was placed in a MW-safe glass vial. To this, methanol (5 mL) was added and the resulting solution was irradiated with 40 W, 150 psi at 65 °C for 9-15 min in the MW synthesizer. The reaction mixture was cooled at room temperature to give a clear solution and then concentrated on a rotary evaporator to yield amino acid methyl ester hydrochloride (AAME. HCl) and recrystallized with methanol[46]. TEA (0.05 mol) was added to a suspension of AAME. HCl (0.025 mol) in 30 mL chloroform at 0 °C and stirred for 30 min. The solvent was distilled off under vacuum and the dry residue of ester (AAME) was recrystallized with methanol.
BBA (0.001 mol) was dissolved in DCM (10 mL), and to this solution CDI (0.0015 mol) was added at room temperature with stirring for 2-4 h. AAME (0.001 mol) in DCM (10 mL) was then added to the above solution and refluxed at 45 °C for 16-20 h. The completion of reaction was monitored by TLC using DCM:n-hexane:TEA (0.8:0.2:0.05 v/v/v). The reaction mixture was washed with distilled water (3 × 10 mL) and saturated solution of sodium bicarbonate (2 × 10 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The residue obtained upon evaporation of the organic layer was recrystallized with ethanol. Purification of prodrugs of BBA with L-tryptophan, D-phenylalanine and L-tyrosine was achieved by column chromatography silica gel (mesh size: 60-120, for column chromatography packed in n-hexane; Merck) using ethyl acetate:hexane (80:20 v/v), while for prodrug of BBA with L-histidine preparative TLC was used (DCM:n-hexane:TEA; 80:15:5 v/v/v) for purification.
Methyl 2-(3-hydroxy-4,6b,8a,11,12,14a,14b heptamethyl 1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 14, 14a, 14b-icosahydropicene-4-carboxamido)-3-(4H-imidazol-4-yl)propanoate yield: 61% (solid); M.P 84-88 °C FTIR (KBr) cm-1: 3554 (OH str), 3327 (NH str), 3102 (aliphatic CH), 1737 (C=O str. ester), 1666 (C=O str. amide); 1H NMR (400 MHz, DMSO, δ ppm): 8.2 [s, 1H] NH, 7.3 [d, 2H] imidazole ring, 5.1 [t, 1H] CH=C, 4.4 [t, 1H] CH, 3.9 [s, 1H] OH, 3.6 [s, 3H] CH3, 3.3 [t, 2H] CH2, 2.2-1.1 [m, 25H] methylenes and methines of BA, 1.1-0.6 [m, 21H] methyls of BA. 13C NMR (400 MHz, CDCl3, δ ppm): 15.5, 17.0, 18.2, 19.9, 20.1, 21.2, 23.4, 23.7, 26.8, 26.9, 27.0, 27.2, 29.0, 32.6, 33.0, 33.9, 37.8, 39.2, 39.3, 39.4, 40.1, 40.4, 41.3, 42.3, 44.0, 47.9, 51.4, 51.8, 52.0, 59.5, 76.7, 116.8, 144.4, 153.8, 163.8, 172.2, 172.8 Mass: m/z 608.87 (M + 1). Elemental analysis: Calculated for C37H57N3O4: C, 73.11; H, 9.45; N, 6.91. Found: C, 73.23; H, 9.52; N, 6.98. Aq. Solubility: 29.45 mg/mL; log Poct: 5.1
Methyl 2-(3-hydroxy-4, 6b, 8a,11, 12, 14a, 14b-heptamethyl 1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 14, 14a, 14b-icosahydropicene-4-carboxamido)-3-phenylpropanoate Yield: 65% (solid); M.P 72-76 °C. FTIR (KBr) cm-1: 3554 (OH str), 3337 (NH str), 3034 (aliphatic CH), 1748 (C=O str. ester), 1613 (C=O str. amide); 1H NMR (400 MHz, DMSO, δ ppm): 8.2 [1H] NH, 7.3 [d, 2H] Ar. CH, 7.2 [d, 2H] Ar. CH, 7.02 [t, 1H], Ar. CH, 5.19 [t, 1H] CH=C, 4.5 [t, 1H] CH, 4.0 [s, 1H] OH, 3.6 [s, 3H] CH3, 3.3 [t, 1H] CH, 3.2 [d, 2H] CH2, 2.2-1.2 [m, 23H] methylenes and methines of BA, 1.2-0.7 [m, 21H] methyls of BA. 13C NMR (400 MHz, CDCl3, δ ppm): 15.5, 17.0, 18.2, 19.9, 20.1, 21.2, 23.4, 23.7, 26.8, 26.9, 27.0, 27.3, 29.0, 32.6, 33.9, 36.8, 37.8, 39.2, 39.3, 39.5, 40.1, 40.4, 41.3, 42.4, 44.0, 47.9, 51.9, 57.3, 59.2, 76.8, 116.5, 125.9, 127.7, 127.9, 128.6, 128.7, 136.6, 144.1, 171.6, 172.1; Mass: m/z 617.90 (M+ ion). Elemental analysis: Calculated for C40H59NO4: C, 77.75; H, 9.62; N, 2.27. Found: C, 77.82; H, 9.66; N, 2.28. Aq. Solubility: 21.08 mg/mL; log Poct: 5.7.
Methyl 2-(3-hydroxy-4, 6b, 8a, 11, 12, 14a, 14b-heptamethyl-1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 14, 14a, 14b-icosahydropicene-4-carboxamido)-3-(1H-indol-3-yl)propanoate. Yield: 68% (solid); M.P 90-94 °C. FTIR (KBr) cm-1: 3543 (OH str), 3330 (NH str), 2866 (aliphatic CH), 1735 (C=O str. ester), 1653 (C=O str. amide); 1H NMR (400 MHz, DMSO, δ ppm): 8.2 [s, 1H] NH, 7.4 [d, 1H] Ar. CH, 7.3 [d, 1H] Ar. CH, 7.08 [s, 1H] Ar. CH, 7.02 [t, 1H] Ar. CH, 6.9 [t, 1H] Ar. CH, 5.2 [t, 1H] CH=C, 4.6 [t, 1H] CH, 4.1 [s, 1H] OH, 3.6 [s, 3H] CH3, 3.32 [t, 1H] CH, 3.03 [d, 2H] CH2, 2.1-1.3 [m, 23H] methylenes and methines of BA, 1.3-0.7 [m, 21H] methyls of BA. 13C NMR (400 MHz, CDCl3, δ ppm): 15.5, 17.0, 18.2, 19.9, 20.1, 21.2, 23.4, 23.7, 26.8, 26.9, 27.0, 27.2, 28.5, 29.0, 32.6, 33.9, 37.8, 39.2, 39.3, 39.4, 40.1, 40.4, 41.3, 42.3, 44.0, 47.9, 51.9, 58.4, 59.2, 76.8, 109.8, 111.1, 116.5, 118.8, 119.9, 121.8, 123.0, 127.5, 136.6, 144.2, 171.6, 172.2; Mass: m/z 656.94 (M+ ion); Elemental analysis: Calculated for C42H60N2O4: C, 76.79; H, 9.21; N, 4.26. Found: C, 76.84; H, 9.25; N, 4.31. Aq. Solubility: 25.22 mg/mL; log Poct: 4.1.
Methyl 2-(3-hydroxy-4, 6b, 8a, 11, 12, 14a, 14b-heptamethyl-1, 2, 3, 4, 4a, 5, 6, 6a, 6b, 7, 8, 8a, 9, 10, 11, 12, 12a, 14, 14a, 14b-icosahydropicene-4-carboxamido)-3-(4-hydroxyphenyl)propanoate. Yield: 45% (solid); M.P 82-86 °C. FTIR (KBr) cm-1: 3547 (OH str), 3334 (NH str), 2856 (aliphatic CH), 1745 (C=O str. ester), 1691 (C=O str. amide); 1H NMR (400 MHz, DMSO, δ ppm): 8.2 [s, 1H] NH, 6.9 [d, 2H] CH, 6.7 [d, 2H] CH, 5.1 [t, 1H] CH=C, 4.5 [t, 1H] CH, 4.0 [s, 1H] OH, 3.6 [s, 3H] CH3, 3.3 [t, 1H] CH, 3.1 [d, 2H] CH2, 2.2-1.2 [m, 23H] methylenes and methines of BA, 1.2-0.6 [m, 21H] methyls of BA. 13C NMR (400 MHz, CDCl3, δ ppm): 15.5, 17.0, 18.2, 19.9, 20.1, 21.2, 23.4, 23.7, 26.8, 26.9, 27.0, 27.1, 29.0, 32.6, 33.9, 36.8, 37.8, 39.2, 39.3, 39.4, 40.1, 40.4, 41.3, 42.3, 44.0, 47.9, 51.9, 57.3, 59.2, 76.8, 115.7, 115.8, 116.5, 129.2, 130.1, 130.2, 144.1, 155.7, 171.5, 172.2; Mass: m/z 633.91 (M+ ion); Elemental analysis: Calculated for C40H59NO5: C, 75.79; H, 9.38; N, 2.21. Found: C, 75.83; H, 9.40; N, 2.24. Aq. Solubility: 15.22 mg/mL; log Poct: 6.3.
Partition coefficients of BBA and its prodrugs were determined in n-octanol/water system using shake flask method, whereas the aqueous solubility was determined in distilled water at 25 ± 1 °C. Estimation was carried out on UV-visible double beam spectrophotometer at predetermined λmax values.
Stability studies of prodrugs were carried out in 0.05 M HCl and phosphate buffers prepared as per IP 2007[53] (pH 1.2 and 7.4 respectively). Prodrugs in the presence of their hydrolyzed products were simultaneously estimated by RP-HPLC method using acetonitrile:water:methanol (70:25:5; v/v/v) at a flow rate of 0.5 mL/min with UV estimation wavelength of 215 nm. All-glass double-distilled water was used throughout the kinetic studies. Before analysis, the mobile phase was degassed using sonicator and filtered through a 0.45 μm membrane filter. Sample solutions were also filtered through the same. Calibration curves of prodrugs and BBA were constructed in HCl and phosphate buffers in the range of 10-100 μg/mL. Prodrug (10 mg) was introduced in 100 mL of HCl or phosphate buffer in a beaker kept in a constant temperature bath at 37 ± 1 °C, with occasional stirring. Aliquots (5 mL) were withdrawn and replaced with the fresh aqueous buffer at regular intervals of 15 min at the 1st hour and after every 30 min up to 3 h for HCl buffer and 4 h for phosphate buffer. Samples (20 μL) reconstituted with mobile phase were injected in the column. The equations generated from calibration curves were used to calculate the concentration of BBA and prodrugs. Samples were analyzed in triplicate and methods were validated as per International Conference on 1 Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines.
Wistar rats were sacrificed to remove stomach, small intestine and colon samples, which were homogenized in HCl or phosphate buffer. A stock solution of prodrug (100 μg/mL) was prepared in respective buffers. To each test tube, 0.8 mL of prodrug solution was placed and 0.2 g of stomach or small intestinal homogenate was added. In the case of colon homogenates, 0.9 mL of prodrug solution and 0.1 mL of homogenate were added. The tubes containing prodrugs and homogenates of stomach, small intestine and colon were incubated at 37 ± 1 °C for 3, 4 and 12 h respectively. The samples were removed from the incubator at predetermined time intervals, centrifuged at 10000 rpm, filtered through 0.45 μm membrane filter and analyzed by HPLC using the same mobile phase, flow rate and detection wavelength as mentioned in the above section.
Fresh rat fecal matter suspended in phosphate buffer (0.1 mL) and prodrug solution (0.9 mL) were placed in test tubes and incubated at 37 ± 1 °C. The samples were removed from the incubator at predetermined time intervals over a period of 12 h and the same procedure was followed for analysis as mentioned in the above section.
BBA and L-tryptophan (BT) was selected as a representative of the four synthesized prodrugs to investigate the in vivo behavior, which was then compared with standard BBA. Male Wistar rats (200-250 g; n = 6) were housed in metabolic cages, individually, under normal conditions (at 27 ± 0.5 °C and relative humidity of 70% ± 0.5% under natural light/dark conditions). The same HPLC system, column and mobile phase were used for this purpose as mentioned in the above section. All the kinetic studies were carried out in triplicate. Rats were starved for 24 h prior to experimentation and administered water ad libitum. Blood (0.5 mL) was withdrawn by retro-orbital puncture and the reading was considered as the 0 min reading. BT and BBA (377.5 mg and 270 mg respectively) were suspended separately in sodium CMC solution prepared in physiological saline (1 mL) and were orally administered to the animals. Blood samples were collected in EDTA-coated tubes at an interval of 15 min for the first 1 h. Then, subsequent blood collection was made on a bi-hourly basis up to the 12th h and finally at the 24th h. Blood samples were centrifuged (2 cycles of 15 min each at 5000 rpm, 0-4 °C). Supernatant was filtered through 0.45 μm membrane filter, reconstituted with methanol (0.5 mL), centrifuged and filtered again. Calibration curves generated in 80% human plasma were used to calculate the concentration of released BBA and intact prodrugs in blood, and the samples were then analyzed by HPLC. Urine and feces of the prodrug-treated rats were collected from the metabolic cages at different time intervals over a period of 24 h and then pooled together, diluted with HCl buffer (pH 1.2) and phosphate buffer (pH 7.4) by 10-fold and 100-fold respectively, and then centrifuged at 5000 rpm at 0-5 °C for 10 min. Supernatant of the centrifuged solutions of urine and feces (0.1 mL) were added to eppendorf tube (1 mL capacity) and 0.9 mL methanol was added. All the solutions were then vortexed for 2 min, re-centrifuged at 5000 rpm for 10 min at 0-5 °C (in order to precipitate other impurities), and analyzed by HPLC using the procedure described above to study the excretion pattern of prodrugs. The equations generated from the calibration curves in HCl buffer (pH 1.2) and phosphate buffers (pH 7.4) were used to calculate the concentrations of released components in urine and feces respectively.
Materials: The Institutional Animal Ethical Committee approved the experimental protocols (CPCSEA/PCH/10/2014-15), and all protocols were followed for pharmacological screening of synthesized prodrugs. The activity was performed at the CPCSEA-approved animal facilities of Poona College of Pharmacy (Pune, India). Wistar rats (males, 200-230 g) were procured from the National Institute of Biosciences (Pune, India). Animals were divided into 16 groups (n = 6 each). TNBS was purchased from Sigma Chemicals (United States). Gastric ulcers were analyzed using Adobe Photoshop and ImageJ software. Histopathological evaluation was carried out at SAI Pathology Labs (Pune, India). The tissue sections were stained with hematoxylin-eosin (H/E) and images were captured with the camera-equipped Nikon Optical Microscope Eclipse E-at 200 resolution × 40.
An average of six readings was calculated and data were expressed as mean ± SEM. Statistical evaluation was performed using one-way ANOVA followed by Dunnett’s multiple comparison test for colon-to-body weight ratio, myeloperoxidase (MPO) assay and ulcerogenic activity, and by two-way ANOVA followed by Bonferroni’s test for clinical activity score rate. Statistical significance was considered at P < 0.05, P < 0.01, P < 0.001 when compared to the disease control.
Colitis was induced by intrarectal administration of 0.25 mL of TNBS in ethanol, following a reported procedure (dose of TNBS was 100 mg/kg of body weight in 50% v/v ethanol solution)[54]. The doses of prodrugs were calculated on an equimolar basis to BBA and are presented in Table 1. The standard and the test compounds were administered orally as a suspension in 1% sodium CMC. Throughout the 11-d study, the animals were monitored for three parameters, viz weight loss, stool consistency and rectal bleeding. Colitis activity was quantified with a clinical activity score assessing these parameters as previously applied by Hartmann et al[55] (Table 2). The clinical activity score was determined by calculating the average of the above three parameters for each day, for each group and ranged from 0 (healthy) to 4 (maximal activity of colitis). On the 11th day, the animals were sacrificed. Colon-to-body weight ratio and MPO activity[56] were determined on the dissected sections of colon. Anti-colitic activity of prodrugs was compared with the standard drugs SLZ and BBA. Rat stomach, colon, liver and pancreas were removed. Gastric ulcers were scanned and ulcer index was calculated by scoring the ulcers as per the method reported by Cioli et al[57]. Specimens of colon, liver and pancreas were fixed in formalin and sent for histopathological evaluation.
| Sr. No. | Weight loss, % | Stool consistency | Rectal bleeding | Score rate |
| 1 | No loss | Well-formed pellets | No blood | 0 |
| 2 | 1-5 | - | - | 1 |
| 3 | 5-10 | Pasty and semi-formed stools, not sticking to anus | Positive finding | 2 |
| 4 | 10-20 | - | - | 3 |
| 5 | > 20 | Liquid stools, sticking to anus | Gross bleeding | 4 |
Synthesis of novel amide conjugates of BBA and L-histidine (BH), BBA and D-phenylalanine (BP), BT and BBA and L-tyrosine (BTY) was accomplished successfully using the CDI coupling method (Figure 1). MW-assisted technology was specially developed and optimized for conversion of amino acids into their methyl ester hydrochlorides[58]. Methyl esters were further coupled with BBA by refluxing with CDI at 45 °C for 16-20 h. Reactions were monitored by TLC (DCM:n-hexane:TEA, 0.8:0.2:0.01 v/v/v). Synthesized prodrugs were characterized by FTIR, 1H-NMR, 13C-NMR, mass spectroscopy and elemental analysis. Formation of amide linkage was confirmed through IR, where the characteristic amide C=O stretch and NH-stretch were observed in the range of 1653-1666 cm-1 and 3327-3439 cm-1 respectively. The other characteristic bands, such as O-H stretch of BBA between 3542 to 3554 cm-1 and C=O stretch of methyl ester of amino acid in the range of 1735-1740 cm-1, further confirmed the structures of anticipated prodrugs. Formation of amide linkage was also confirmed by 1H-NMR spectra, where the chemical shift of proton of secondary amide appeared between δ 8.1 to 8.3 as a singlet. Chemical shifts for protons of the BBA backbone: CH-OH (δ 3.9), 23 protons methylenes and methines between δ 1.1-2.2 and 21 protons of 7 methyl groups between δ 0.6-1.1, as well as relevant chemical shifts for amino acid backbone were also observed. Number of carbon atoms was confirmed by 13C-NMR. Elemental analysis and molecular ion peaks documented by mass spectroscopy for respective conjugates matched with their anticipated molecular weights.
BBA was found to be practically insoluble in water, with logPoct of 9.3 when determined experimentally. For a successful colon-targeted delivery, it is essential to restrict absorption from upper GIT so that maximum concentration is achieved at the site of action (colon). Conjugation of BBA with amino acids significantly enhanced the aqueous solubility of prodrugs (15-29 mg/mL), which was in accordance with the observed logPoct of prodrugs (4.1-6.3).
Chemical stability of colon-targeted prodrugs at physiological pH values of upper GIT is the essence of their successful design, otherwise the prodrug might revert back to the active drug prematurely. Colon-specific prodrugs face the challenge of surviving during their passage through upper GIT in an intact form, so as to reach the colon, where they are expected to become degraded (hydrolyzed) by colonic microbial enzymes. Therefore, to explore the behavior of prodrugs in acidic and basic environments, their stability was studied in HCl buffer (pH 1.2) and phosphate buffer (pH 7.4). In vitro studies confirmed the stability of prodrugs in HCl buffer and negligible hydrolysis in phosphate buffer (pH 7.4) after 4 h (Table 3).
| Prodrug | Incubation medium | |||||||||
| HCl buffer (pH 1.2) and stomach homogenates | Phosphate buffer (pH 7.4) and intestinal homogenates | Colon homogenate | Fecal matter | |||||||
| K ± SD (min-1)1 | t1/2 (min) | Prodrug hydrolyzed | BBA released | K ± SD (min-1)1 | t1/2 (min) | Prodrug hydrolyzed | BBA released | |||
| BH | Stable | Negligible | 0.0013 | 514.1 | 78.10% | 73.60% | 0.0009 | 723.1 | 62.29% | 57.37% |
| hydrolysis | ||||||||||
| BP | Stable | Negligible | 0.0014 | 488.3 | 75.08% | 71.79% | 0.0010 | 636.7 | 57.38% | 56.67% |
| hydrolysis | ||||||||||
| BT | Stable | Negligible | 0.0018 | 379.4 | 86.67% | 86.39% | 0.0010 | 662.5 | 69.75% | 72.52% |
| hydrolysis | ||||||||||
| BTY | Stable | Negligible | 0.0014 | 484.4 | 70.33% | 68.57% | 0.0010 | 677.6 | 55.95% | 55.13% |
| hydrolysis | ||||||||||
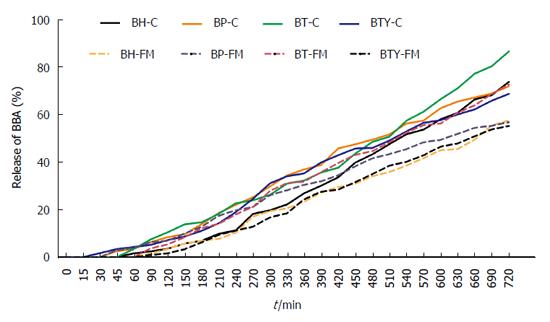
To quantify the sensitivity of the synthesized prodrugs to the enzymatic environment of the GIT, release of BBA from prodrugs was studied in homogenates of stomach, small intestine and colon of Wistar rats. Prodrugs were incubated at 37 ± 1 °C in stomach, small intestine and colon homogenates for 3 h, 4 h and 12 h respectively. All prodrugs were stable in stomach homogenate, which was in accordance with their similar behavior in HCl buffer (pH 1.2). However, negligible hydrolysis of prodrugs was observed in intestinal homogenates at the end of 4 h. In colon homogenates, 68%-86% release (Figure 2) of BBA was achieved in 12 h, following first-order kinetics that suggests anticipated colon-targeted release of BBA with minimum loss in the upper GIT. The half-lives of the prodrugs were found to be in the range of 379-514 min, whereas rate constants (K) were in the range of 0.0013-0.0018 min-1 (Table 3).
The prodrugs were incubated in fecal matter for a period of 12 h, furnishing 55%-72% BBA. The half-lives and rate constants were in the range of 636-723 min and 0.0009-0.0010 min-1 respectively. Significant release in colon homogenates and fecal matter indicates colon-specific, hydrolytic activation of prodrugs into their active metabolites, which could be mediated by N-acyl amidases secreted by the endogenous colonic microorganisms. The proposed mechanism of activation is depicted in Figure 3.
The prodrugs of BBA were designed to increase its bioavailability in colon by enhancing the hydrophilicity, so that systemic absorption of prodrugs is minimized, helping to ensure reaching the colon in intact form followed by colon-specific release of BBA for its local mitigating effect on colonic inflammation. BT was selected as a representative of the four synthesized prodrugs to study in vivo behavior, and was compared with orally administered BBA. BT and plain BBA were orally administered to the rats and their plasma concentrations were compared at pre-determined time intervals.
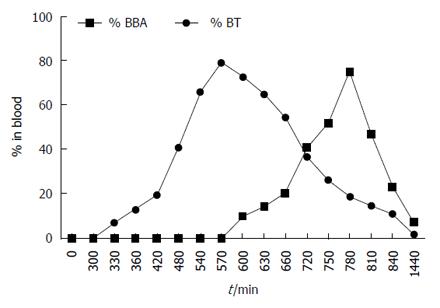
Appearance of BBA in blood started at 30 min, reaching a maximum at 240 min (4 h) and then declining gradually with disappearance at 24 h (Figure 4). This behavior indicated ready absorption of BBA in the upper GIT. On the contrary, when BT was administered orally, neither BT nor BBA was observed in blood until 300 min (5 h), indicating that prodrug did not absorb from the stomach and remained intact there. BT started appearing in blood at 330 min (5.5 h), indicating minimal absorption through the small intestine. The concentration of BT consistently increased in blood, reaching a maximum of 79% at 570 min (9.5 h), indicating its absorption from large intestine (colonic mucosa) into systemic circulation.
Interestingly, appearance of BBA was observed in blood at 600 min (10 h), indicating colon-specific hydrolysis of BT into BA in the large intestine, which - due to its high lipophilicity - might have traversed through colonic mucosa into systemic circulation. The BBA concentration reached a maximum of 75% at 780 min (13 h). The concentration of BT and BBA started declining, reaching negligible level at 24 h. Prodrug BT could restrict release of BBA throughout its passage in the upper GIT - which might be due to the introduction of an amide linkage in the prodrug - ensuring efficient delivery to the large intestine in contrast to plain BBA, which was promptly absorbed from the upper GIT with negligible fraction of administered dose available in the colon.
Incidentally, the in vivo behavior of BT seemed quite similar to that of SLZ, which has dual applicability in IBD as well as RA. Therefore, from the outcome of these in vivo studies, we hypothesize that although BT was designed for IBD, it might prove to be worthy for the treatment of inflammatory diseases affected by circadian rhythm, such as RA, due to systemic availability of BBA in high concentration at 13 h[59-62].
The synthesized prodrugs were evaluated for clinical activity score rate, colon-to-body weight ratio and MPO activity in TNBS-induced experimental colitis modeled Wistar rats[63-65]. The anti-colitic activity of prodrugs was compared with SLZ and BBA. Before induction of colitis, the animals were starved for 48 h. On the 3rd day, colitis was induced by intrarectal administration of TNBS and by 5th day colitis was fully developed. The clinical activity score increased rapidly and consistently for all TNBS-treated groups (Table 4). From day 6 to 10, standards SLZ and BBA, prodrugs, carriers and physical mixtures were orally administered to animals. All prodrugs significantly minimized the clinical activity score rate (85%-101%) as compared to BBA (79%), revealing efficient delivery of BBA to colon by the developed prodrugs. BT was 1.7% superior to SLZ in lowering the clinical activity, which might be due to significant inhibition of leukotrienes by BBA[17,30] and the inhibitory effect of L-tryptophan on Th1-mediated inflammation in the gut, thus providing a synergistic effect[44]. Overall, the positive contribution of amino acids[42-50] was obvious from the gross difference between the lowering effect of all the synthesized prodrugs and plain BBA.
| Intervention | 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | 8 d | 9 d | 10 d | 11 d |
| HC | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| DC | 0 ± 0 | 0.77 ± 1.34 | 1.00 ± 1.73 | 1.66 ± 1.52 | 1.66 ± 1.52 | 1.88 ± 1.71 | 3.11 ± 1.01 | 3.22 ± 1.07 | 3.33 ± 1.15 | 3.33 ± 1.15 | 3.33 ± 1.15 |
| BBA | 0 ± 0 | 0.66 ± 1.15 | 1.00 ± 1.73 | 1.66 ± 1.52 | 2.00 ± 1.73 | 2.77 ± 1.34 | 3.00 ± 1.00 | 2.33 ± 0.57 | 1.99 ± 0.66 | 1.33 ± 1.15 | 0.99 ± 1.10e |
| SLZ | 0 ± 0 | 0.38 ± 0.67 | 0.83 ± 0.92 | 1.88 ± 0.83 | 2.72 ± 0.63 | 2.88 ± 0.83 | 2.44 ± 0.50 | 1.61 ± 1.13 | 1.11 ± 1.01 | 0.72 ± 0.75 | 0.38 ± 0.60e |
| BH | 0 ± 0 | 0.66 ± 1.15 | 1.55 ± 1.50 | 2.11 ± 0.83 | 2.77 ± 1.01 | 3.00 ± 1.00 | 2.66 ± 0.57 | 2.11 ± 0.50 | 1.55 ± 1.07 | 1.31 ± 0.83 | 0.70 ± 1.10e |
| BP | 0 ± 0 | 0.77 ± 1.34 | 1.00 ± 1.73 | 1.50 ± 1.58 | 2.16 ± 1.09 | 2.66 ± 0.88 | 2.55 ± 0.76 | 1.88 ± 1.01 | 1.22 ± 1.17 | 0.97 ± 1.07 | 0.61 ± 0.70e |
| BT | 0 ± 0 | 0.72 ± 1.25 | 1.05 ± 1.82 | 2.16 ± 0.86 | 2.38 ± 0.67 | 2.38 ± 0.67 | 2.16 ± 0.92 | 1.44 ± 1.17 | 0.94 ± 1.10 | 0.50 ± 0.86 | 0.33 ± 0.50e |
| BTY | 0 ± 0 | 0.66 ± 1.15 | 1.44 ± 1.50 | 2.00 ± 0.88 | 2.77 ± 0.69 | 3.05 ± 1.00 | 2.88 ± 1.07 | 2.00 ± 1.20 | 1.61 ± 1.25 | 1.22 ± 1.17 | 0.83 ± 0.90e |
| H | 0 ± 0 | 0.66 ± 1.15 | 1.11 ± 1.16 | 1.33 ± 1.52 | 1.88 ± 1.01 | 2.33 ± 0.67 | 2.55 ± 0.50 | 2.88 ± 0.83 | 2.77 ± 0.69 | 2.60 ± 0.82 | 2.00 ± 1.00NS |
| P | 0 ± 0 | 0.50 ± 0.86 | 0.88 ± 1.53 | 1.22 ± 1.57 | 1.99 ± 0.88 | 2.22 ± 0.83 | 2.55 ± 0.50 | 3.16 ± 1.04 | 3.33 ± 1.15 | 2.66 ± 1.44 | 2.27 ± 1.13NS |
| T | 0 ± 0 | 0.61 ± 1.05 | 1.00 ± 1.73 | 1.66 ± 1.52 | 2.11 ± 0.83 | 2.44 ± 0.69 | 2.60 ± 0.58 | 2.94 ± 0.82 | 2.66 ± 0.66 | 2.16 ± 1.08 | 1.88 ± 1.17a |
| TY | 0 ± 0 | 0.55 ± 0.95 | 1.00 ± 1.73 | 1.00 ± 1.73 | 1.99 ± 0.88 | 2.11 ± 0.84 | 2.55 ± 0.50 | 3.22 ± 1.07 | 3.33 ± 1.15 | 2.72 ± 1.49 | 2.33 ± 1.45NS |
| BBA + H | 0 ± 0 | 0.44 ± 0.76 | 1.00 ± 1.73 | 1.88 ± 0.96 | 1.99 ± 0.88 | 2.33 ± 0.67 | 3.22 ± 1.07 | 3.33 ± 1.15 | 2.88 ± 1.92 | 2.72 ± 1.80 | 1.77 ± 1.34a |
| BBA + P | 0 ± 0 | 0.66 ± 1.15 | 1.00 ± 1.73 | 1.44 ± 1.50 | 1.99 ± 0.88 | 2.33 ± 0.88 | 3.11 ± 1.01 | 3.22 ± 1.07 | 3.22 ± 1.07 | 2.55 ± 1.64 | 1.88 ± 1.17a |
| BBA + T | 0 ± 0 | 0.38 ± 0.66 | 0.88 ± 1.53 | 1.66 ± 1.20 | 2.10 ± 0.77 | 2.44 ± 1.07 | 2.99 ± 0.87 | 3.33 ± 1.15 | 2.99 ± 1.45 | 2.55 ± 1.71 | 1.77 ± 1.34b |
| BBA + TY | 0 ± 0 | 0.55 ± 0.95 | 0.88 ± 1.53 | 1.33 ± 1.52 | 1.88 ± 1.01 | 2.55 ± 0.50 | 2.99 ± 0.87 | 3.22 ± 1.07 | 3.27 ± 1.10 | 2.44 ± 1.26 | 1.88 ± 1.17a |
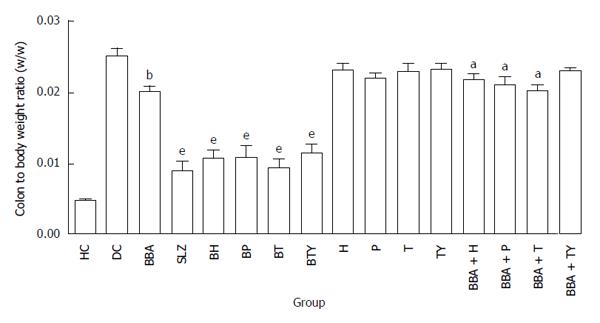
On the 11th day, all animals were sacrificed and colon-to-body weight ratio was calculated to quantify inflammation. Prodrug-treated groups showed a distinct decrease in the colon-to-body weight ratio, compared to the colitis control group (Figure 5). The extent of decrease in colon-to-body weight ratio shown by BT was highest among all prodrug-treated groups, which are in accordance with its superior lowering effect on the clinical activity score rate.
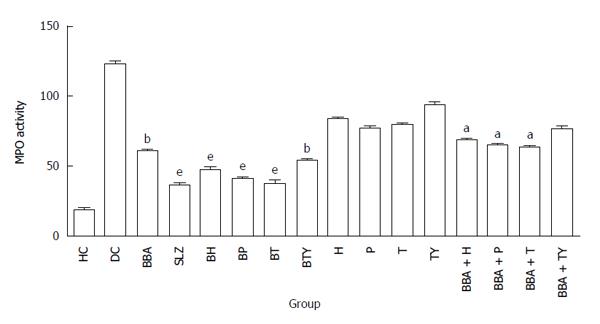
MPO activity is an important quantitative index for colonic inflammation and it was measured according to the technique described by Krawisz et al[56]. It is a peroxidase enzyme secreted by the activated neutrophils into the inflamed tissue, and is directly proportional to severity of inflammation. The results were expressed as MPO units per gram of wet tissue, and one unit of MPO activity was defined as that degrading 1 mmol min-1 of hydrogen peroxide at 25 °C. Maximum colonic MPO activity was shown by BP and BT (39.74 and 37.49 mU/100 mg tissue respectively), which was comparable to SLZ (36.21 mU/100 mg tissue), indicating significant anti-inflammatory effect on colonic inflammation (Figure 6). However, plain BBA showed significantly high MPO activity (60.71 mU/100 mg tissue), suggesting higher extent of neutrophil infiltrate in the inflamed colon, which might be due to ready absorption and considerably high concentration of BBA in upper GIT and not at the targeted site (colon).
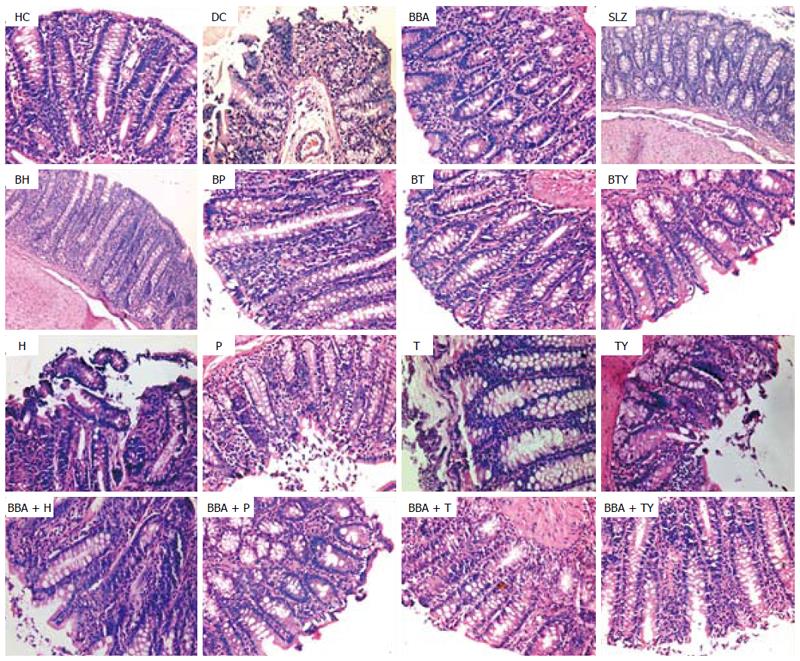
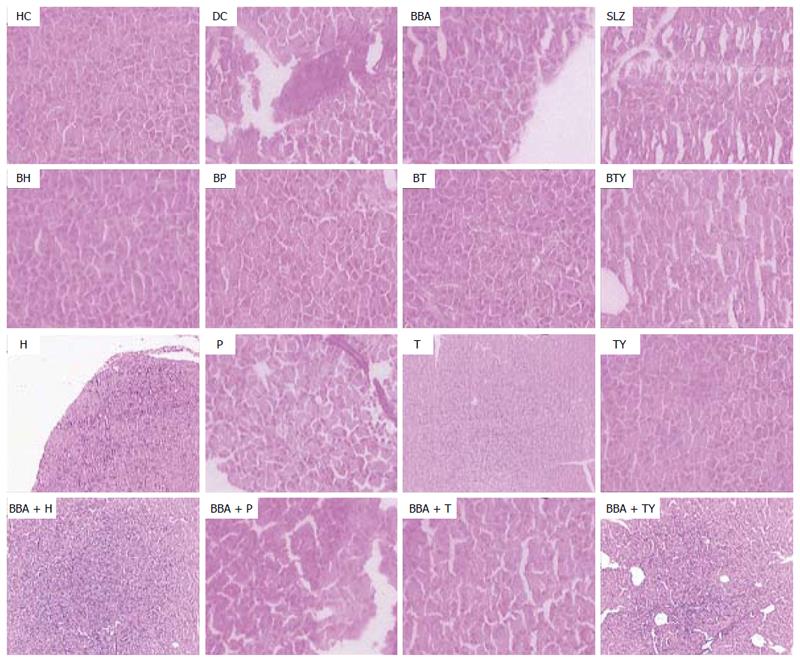
The severity of colonic inflammation and the effect of prodrugs on the recovery from TNBS-induced colitis were evaluated by examining the H/E-stained colon sections. In healthy colon, the normal colonic architecture was observed with intact mucosal layer, crypt-architecture and presence of goblet cells. The colitis control showed severe erosion with absence of mucosal layer, goblet cells’ depletion, distorted crypts’ architecture, lymphocytic infiltration, and thickening of the muscularis mucosa. The lamina propria was also infiltrated with leukocytes. Due to destruction of the crypts, the normal mucosal architecture was lost completely.
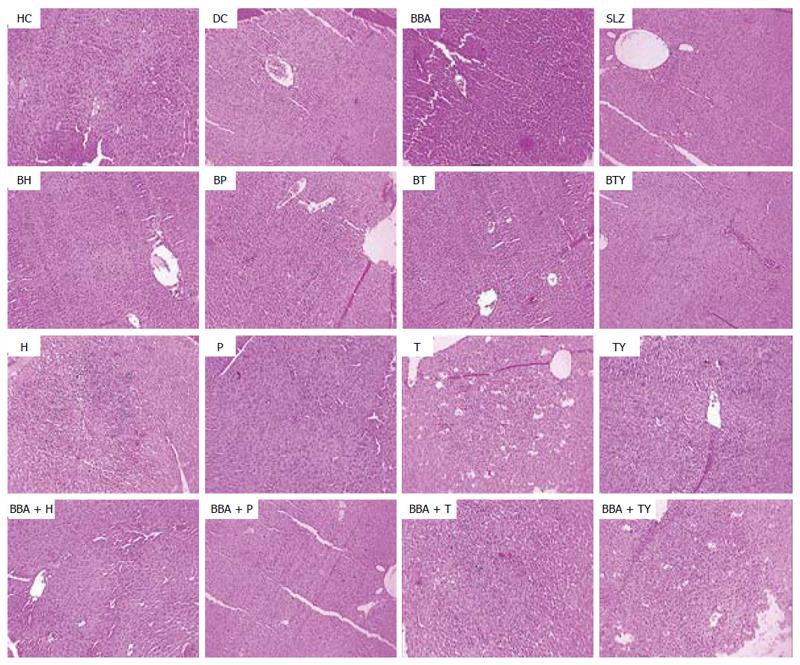
In vivo treatment with the synthesized prodrugs significantly decreased the extent and severity of colonic damage. Histopathology slides of the colon samples revealed that the inflammation and crypt damage associated with the TNBS administration were corrected by treatment with prodrugs (Figure 7). These results were comparable with those obtained for the SLZ-treated group. The prodrugs were also assessed for their probable damaging effects on pancreas and liver with the help of histopathological analysis. No adverse effects on liver and pancreas were observed (Figures 8 and 9). Ulcer indices of BBA and prodrugs were profoundly lower (2.83-3.33) than for SLZ (7.66 ± 1.21) (Table 5).
In conclusions, the present work aimed at developing novel colon-specific prodrugs of BBA with essential amino acids involved in restoration of gut immune homeostasis by attenuating inflammatory response and modulating intestinal immune functions to maintain gut health. The synthesized prodrugs were designed innovatively by applying a semi-synthetic approach, wherein one natural active constituent of BS, namely BBA, was derivatized into a bioreversible delivery system by incorporation of amino acids as promoities for targeted delivery to inflamed colon in IBD. Site-specifically enhanced bioavailability of BBA could be achieved in colon, which resulted in demonstration of significant mitigating effect on TNBS-induced colitis in rats without any adverse effects on stomach, liver and pancreas.
Interestingly, in vivo pharmacokinetic studies of BT revealed that after release of BBA in colon, its substantial concentration reached the systemic circulation due to probable absorption through colonic mucosa. This behavior was similar to that of SLZ, which is known to release 5-ASA and sulfapyridine in the colon, the former being involved in treating local inflammation in IBD. However, 30% of released 5-ASA and 100% of sulfapyridine enter circulation due to their absorption from large intestine, accounting for their usefulness in the treatment of RA. Therefore, screening of the synthesized prodrugs in animal models of RA has been undertaken and is currently under progress. The outcome of this study strongly suggests that these prodrugs might find dual applicability in IBD and chronotherapy of RA.
The need to discover effective treatment options for inflammatory bowel disease (IBD) is growing due to the incidence of treatment-related side-effects. The use of complementary and alternative medicine is also on the rise, particularly herbal medicine for IBD management. β-boswellic acid (BBA) was selected as the drug candidate for developing its colon-targeting prodrugs. 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis modeling of Wistar rats was used for investigation and to ensure attainment of an effective concentration of BBA in the colon for its local mitigating effect on colonic inflammation.
To our knowledge, this is the first study of its kind to identify the therapeutic effect of prodrugs of BBA in the TNBS-induced colitis model in Wistar rats for IBD.
This is the first study examining the potential for prodrugs of BBA for restoration of gut immune homeostasis by attenuating inflammatory response and modulating intestinal immune functions to maintain gut health. The synthesized prodrugs were designed innovatively by applying a semi-synthetic approach, wherein one natural active constituent of Boswellia serrata, namely BBA, was derivatized into a bioreversible delivery system by incorporation of amino acids as promoities for targeted delivery to inflamed colon in IBD. The prodrug BBA + L-tryptophan (BT) was found to be 1.7% superior in reducing inflammation in the colon and showed similar behavior to standard drug sulfasalazine (SLZ). Interestingly, the in vivo pharmacokinetic behavior of BT was found to be similar to SLZ in treatment of IBD, as well as for rheumatoid arthritis (RA). Therefore, screening of the synthesized prodrugs on animal models of RA has been undertaken and is currently under progress. The outcome of this study strongly suggests that these prodrugs might find dual applicability in IBD and chronotherapy of RA.
The promising findings presented in the current study indicate that the prodrugs exhibited an improvement in selected parameters of the TNBS-induced colitis model. Site-specifically enhanced bioavailability of BBA could be achieved in colon that resulted in demonstration of a significant mitigating effect on TNBS-induced colitis in rats, without any adverse effects on the stomach, liver and pancreas. These prodrugs might find dual applicability in IBD and chronotherapy of RA.
A prodrug is a pharmacologically-inactive, bioreversible derivative of a parent drug molecule that requires chemical or enzymatic activation in the biological environment to release the active drug. Physico-chemical properties can be tailored by means of changing the structure of the promoiety, and the intrinsic activity of the parent drug is assured through in vivo cleavage of the prodrugs. A mutual prodrug or co-drug strategy involves formation of a covalent linkage between drug and carrier in such a manner that upon oral administration the moiety remains intact in the stomach and small intestine but releases the active drug in the colon through enzymatic activation. This ensures attainment of effective concentration of the drug in the colon for its local mitigating effect on colonic inflammation of IBD.
The authors demonstrated the efficacy of colon-specific prodrugs of BA on TNBS-induced colitis in mice. The present study was well organized and well investigated, and will give us important information, especially in the field of IBD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Naito Y S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1880] [Article Influence: 134.3] [Reference Citation Analysis (2)] |
| 2. | Lautenschläger C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 2014;71:58-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn’s disease. Annu Rev Genomics Hum Genet. 2009;10:89-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2151] [Article Influence: 102.4] [Reference Citation Analysis (1)] |
| 5. | Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 701] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 6. | Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Taylor KM, Irving PM. Optimization of conventional therapy in patients with IBD. Nat Rev Gastroenterol Hepatol. 2011;8:646-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Isaacs KL, Lewis JD, Sandborn WJ, Sands BE, Targan SR. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11 Suppl 1:S3-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Cunliffe RN, Scott BB. Review article: monitoring for drug side-effects in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:647-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Mason M, Siegel CA. Do inflammatory bowel disease therapies cause cancer? Inflamm Bowel Dis. 2013;19:1306-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Guilliams TG. Inflammatory bowel disease and irritable bowel syndrome, understanding, distinguishing and addressing. A review of natural and nutraceutical therapies for clinical practice. Standard. 2010;10:1-20. |
| 13. | Catanzaro D, Rancan S, Orso G, Dall’Acqua S, Brun P, Giron MC, Carrara M, Castagliuolo I, Ragazzi E, Caparrotta L. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS One. 2015;10:e0125375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 520] [Reference Citation Analysis (0)] |
| 14. | Hüsch J, Bohnet J, Fricker G, Skarke C, Artaria C, Appendino G, Schubert-Zsilavecz M, Abdel-Tawab M. Enhanced absorption of boswellic acids by a lecithin delivery form (Phytosome(®)) of Boswellia extract. Fitoterapia. 2013;84:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Hüsch J, Gerbeth K, Fricker G, Setzer C, Zirkel J, Rebmann H, Schubert-Zsilavecz M, Abdel-Tawab M. Effect of phospholipid-based formulations of Boswellia serrata extract on the solubility, permeability, and absorption of the individual boswellic acid constituents present. J Nat Prod. 2012;75:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Scior T, Verhoff M, Gutierrez-Aztatzi I, Ammon HP, Laufer S, Werz O. Interference of boswellic acids with the ligand binding domain of the glucocorticoid receptor. J Chem Inf Model. 2014;54:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HP. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;261:1143-1146. [PubMed] |
| 18. | Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HP, Safayhi H. Acetyl-11-keto-beta-boswellic acid (AKBA): structure requirements for binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996;117:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Krüger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Müller WE, Karas M, Schubert-Zsilavecz M, Abdel-Tawab M. Metabolism of boswellic acids in vitro and in vivo. Drug Metab Dispos. 2008;36:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Singh GB, Singh S, Bani S. Alcoholic extract of salai-guggal ex-Boswellia serrata, a new natural source NSAID. Drugs Today. 1996;32:109-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Singh GB, Singh S, Bani S. Anti-inflammatory actions of boswellic acids. Phytomedicine. 1996;3:81-85. [PubMed] [DOI] [Full Text] |
| 22. | Singh GB, Bani S, Singh S. Toxicity and safety evaluation of boswellic acids. Phytomedicine. 1996;3:87-90. [PubMed] [DOI] [Full Text] |
| 23. | Hostanska K, Daum G, Saller R. Cytostatic and apoptosis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res. 2002;22:2853-2862. [PubMed] |
| 24. | Gupta I, Parihar A, Malhotra P, Gupta S, Lüdtke R, Safayhi H, Ammon HP. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001;67:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Johri RK, Qazi GN. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine. 2008;15:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Schweizer S, von Brocke AF, Boden SE, Bayer E, Ammon HP, Safayhi H. Workup-dependent formation of 5-lipoxygenase inhibitory boswellic acid analogues. J Nat Prod. 2000;63:1058-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995;47:1212-1216. [PubMed] |
| 28. | Shah BA, Kumar A, Gupta P, Sharma M, Sethi VK, Saxena AK, Singh J, Qazi GN, Taneja SC. Cytotoxic and apoptotic activities of novel amino analogues of boswellic acids. Bioorg Med Chem Lett. 2007;17:6411-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Poeckel D, Werz O. Boswellic acids: biological actions and molecular targets. Curr Med Chem. 2006;13:3359-3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Singh S, Khajuria A, Taneja SC, Johri RK, Singh J, Qazi GN. Boswellic acids: A leukotriene inhibitor also effective through topical application in inflammatory disorders. Phytomedicine. 2008;15:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z Gastroenterol. 2001;39:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Müller S, Senninger N, Russell J, Jauch J, Bergmann J. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1131-G1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Qurishi Y, Hamid A, Zargar MA, Singh SK, Saxena AK. Potential role of natural molecules in health and disease: Importance of boswellic acid. J Med Plants Res. 2010;4:2778-2785. |
| 34. | Rentea R. Therapeutic Advantages of highly standardized Boswellia Extracts. Original article 2008 with a review and addendum2011. Available from: http://www.koliskoinstitute.org/wp-content/uploads/2014/09/Therapeutic-Advantages-of-AKBAv2.pdf. |
| 35. | Algieri F, Rodriguez-Nogales A, Rodriguez-Cabezas ME, Risco S, Ocete MA, Galvez J. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediators Inflamm. 2015;2015:179616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Krieglstein CF, Anthoni C, Rijcken EJ, Laukötter M, Spiegel HU, Boden SE, Schweizer S, Safayhi H, Senninger N, Schürmann G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis. 2001;16:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Tabassum N, Hamdani M, Najar IH. Natural treatment for inflammatory bowel disease. Br Biomed Bull. 2014;2:85-94. |
| 38. | Borrelli F, Capasso F, Capasso R, Ascione V, Aviello G, Longo R, Izzo AA. Effect of Boswellia serrata on intestinal motility in rodents: inhibition of diarrhoea without constipation. Br J Pharmacol. 2006;148:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Gupta I, Parihar A, Malhotra P, Singh GB, Lüdtke R, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res. 1997;2:37-43. [PubMed] |
| 40. | Lemmo E, Schulman R. Phytonutrient formula for the relief of chronic pain resulting from inflammation. United States Patent. 2004;. |
| 41. | Faigle JW. Drug Metabolism in the Colon Wall and Lumen. Ed. NY: Marcel Dekker, Inc 1993; 29-54. |
| 42. | Zhang H, Hu CA, Kovacs-Nolan J, Mine Y. Bioactive dietary peptides and amino acids in inflammatory bowel disease. Amino Acids. 2015;47:2127-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Dhaneshwar SS, Chail M, Patil M, Naqvi S, Vadnerkar G. Colon-specific mutual amide prodrugs of 4-aminosalicylic acid for their mitigating effect on experimental colitis in rats. Eur J Med Chem. 2009;44:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Kim CJ, Kovacs-Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671-4677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Dhaneshwar SS, Gairola N, Kandpal M, Vadnerkar G, Bhatt L, Rathi B, Kadam SS. Synthesis, kinetic studies and pharmacological evaluation of mutual azo prodrugs of 5-aminosalicylic acid for colon-specific drug delivery in inflammatory bowel disease. Eur J Med Chem. 2009;44:3922-3929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Walsh NE, Ramamurthy S, Schoenfeld L, Hoffman J. Analgesic effectiveness of D-phenylalanine in chronic pain patients. Arch Phys Med Rehabil. 1986;67:436-439. [PubMed] |
| 48. | Balagot RC, Ehrenpreis S, Kubota K, Greenberg J. Analgesia in mice and humans by D-phenylalanine: Relation to inhibition of enkephalin degradation and enkephalin levels. Adv Pain Res Ther. 1983;5:289-293. |
| 49. | Meyers BE, Moonka DK, Davis RH. The effect of selected amino acids on gelatin-induced inflammation in adult male mice. Inflammation. 1979;3:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Takagi K, Okabe S. Prevention of gastric lesions. Available from: http://www.patentimages.storage.googleapis.com/pdfs/US3988466.pdf. |
| 51. | Lee JS, Jung YJ, Kim YM. Synthesis and evaluation of N-acyl-2-(5-fluorouracil-1-yl)-D,L-glycine as a colon-specific prodrug of 5-fluorouracil. J Pharm Sci. 2001;90:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Kashmira P, Dhaneshwar S, Shakuntala C, Poorvashree J. Design, Synthesis and In Vitro Release Studies of Co-Drugs for Rheumatoid Arthritis. Inflamm Allergy Drug Targets. 2015;14:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Indian Pharmacopoeia 2007; 1: 480-482. Available from: http://www.ajprd.com/downloadebooks_pdf/8.pdf. |
| 54. | Dhaneshwar S, Gautam H. Exploring novel colon-targeting antihistaminic prodrug for colitis. J Physiol Pharmacol. 2012;63:327-337. [PubMed] |
| 55. | Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA, Endres S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther. 2000;292:22-30. [PubMed] |
| 56. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 57. | Cioli V, Putzolu S, Rossi V, Scorza Barcellona P, Corradino C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol. 1979;50:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Sarkate A, Dhaneshwar SS. Microwave- assisted one-pot synthesis of amino acid methyl ester hydrochlorides. Int J Pharm Res. 2016;In Press. |
| 59. | Dhaneshwar SS, Vadnerkar G. Rational design and development of colon-specific prodrugs. Curr Top Med Chem. 2011;11:2318-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Taggart AJ, Neumann VC, Hill J, Astbury C, Le Gallez P, Dixon JS. 5-Aminosalicylic acid or sulphapyridine. Which is the active moiety of sulphasalazine in rheumatoid arthritis? Drugs. 1986;32 Suppl 1:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Neumann VC, Taggart AJ, Le Gallez P, Astbury C, Hill J, Bird HA. A study to determine the active moiety of sulphasalazine in rheumatoid arthritis. J Rheumatol. 1986;13:285-287. [PubMed] |
| 62. | Bird HA. Sulphasalazine, sulphapyridine or 5-aminosalicylic acid--which is the active moiety in rheumatoid arthritis? Br J Rheumatol. 1995;34 Suppl 2:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Tian L, Huang YX, Tian M, Gao W, Chang Q. Downregulation of electroacupuncture at ST36 on TNF-alpha in rats with ulcerative colitis. World J Gastroenterol. 2003;9:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 624] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 65. | Zingarelli B, Squadrito F, Graziani P, Camerini R, Caputi AP. Effects of zileuton, a new 5-lipoxygenase inhibitor, in experimentally induced colitis in rats. Agents Actions. 1993;39:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |