Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6540
Peer-review started: May 18, 2017
First decision: June 22, 2017
Revised: July 6, 2017
Accepted: August 8, 2017
Article in press: August 8, 2017
Published online: September 21, 2017
Processing time: 127 Days and 5 Hours
We report the first case of a patient with hepatitis C virus (HCV) infection and idiopathic thrombocytopenic purpura (ITP), who later developed acquired amegakaryocytic thrombocytopenia (AAMT), with autoantibodies to the thrombopoietin (TPO) receptor (c-Mpl). A 64-year-old woman, with chronic hepatitis C, developed severe thrombocytopenia and was diagnosed with ITP. She died of liver failure. Autopsy revealed cirrhosis and liver carcinoma. In the bone marrow, a marked reduction in the number of megakaryocytes was observed, while other cell lineages were preserved. Therefore, she was diagnosed with AAMT. Additionally, autoantibodies to c-Mpl were detected in her serum. Autoantibodies to c-Mpl are one of the causes of AAMT, acting through inhibition of TPO function, megakaryocytic maturation, and platelet formation. HCV infection induces several autoantibodies. HCV infection might also induce autoantibodies to c-Mpl, resulting in the development of AAMT. This mechanism may be one of the causes of thrombocytopenia in patients with HCV infection.
Core tip: Thrombocytopenia occurs frequently in patients with hepatitis C virus (HCV) infection. Acquired amegakaryocytic thrombocytopenia (AAMT) is one of the causes of severe thrombocytopenia. The exact mechanisms of AAMT have not been fully elucidated. However, patients with autoantibodies to thrombopoietin receptor (c-Mpl) develop AAMT. Similarly, autoantibodies are sometimes generated in patients with HCV infection. Here, we report the first case of a patient with HCV infection who later developed AAMT with autoantibodies to c-Mpl. AAMT with autoantibodies to c-Mpl may be one of the causes of thrombocytopenia in patients with HCV infection.
- Citation: Ichimata S, Kobayashi M, Honda K, Shibata S, Matsumoto A, Kanno H. Acquired amegakaryocytic thrombocytopenia previously diagnosed as idiopathic thrombocytopenic purpura in a patient with hepatitis C virus infection. World J Gastroenterol 2017; 23(35): 6540-6545
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6540
Thrombocytopenia occurs frequently in patients with chronic hepatitis C. The causes of thrombocytopenia in patients with chronic hepatitis C are multiple, such as hypersplenism, immunological processes, and decreased thrombopoietin (TPO) level[1,2].
Acquired amegakaryocytic thrombocytopenia (AAMT) is one of the causes of severe thrombocytopenia and is characterized by a marked reduction in the number of megakaryocytes, with preserved hematopoiesis of the other lineages in the bone marrow[3]. The exact mechanisms of AAMT have not been fully elucidated. However, the defect of TPO receptor (c-Mpl) expression due to c-Mpl gene mutation is the major cause of congenital amegakaryocytic thrombocytopenia[4]. Furthermore, patients with systemic lupus erythematosus (SLE) and systemic sclerosis who have autoantibodies to c-Mpl develop AAMT[5,6]. TPO, produced mainly by hepatocytes, binds to c-Mpl on hematopoietic stem cells and megakaryocytes, and promotes all stages of platelet production, from the proliferation and differentiation of megakaryocytes to megakaryocytic maturation and platelet formation[1]. Thus, autoantibodies to c-Mpl may be one of the causes of AAMT, through inhibiting TPO function. AAMT with autoantibodies to c-Mpl has not previously been reported in patients with hepatitis C virus (HCV) infection.
Here, we report the first case of a patient with HCV infection and idiopathic thrombocytopenic purpura (ITP), who later developed AAMT with autoantibodies to c-Mpl.
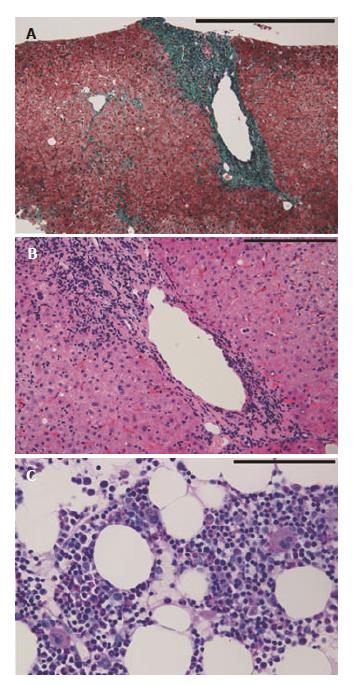
A 64-year-old woman was admitted with the chief complaint of dyspnea. She had a past history of post-transfusion hepatitis approximately forty years beforehand, and subsequently she was diagnosed with HCV infection (genotype 1b, high). At the age of 41, she developed thrombocytopenia (platelets count: 12.2 × 104/μL). At that time, she received interferon therapy, but the HCV infection persisted. At the age of 51 and 52, liver biopsy and bone marrow aspirations were performed, respectively. Liver biopsy specimens revealed periportal mild inflammatory cell infiltration and fibrosis (Modified Histological Activity Index: activity was 5/18, fibrosis was 1/6; Figure 1A and B). The clot section of the bone marrow aspirate showed no significant change, and the number of megakaryocytes was within the normal range. Although the platelet-associated IgG (PA IgG) was not measured, she was diagnosed with ITP (Figure 1C). At the age of 61, liver cancer was detected, using computed tomography and magnetic resonance imaging, and she received transcatheter arterial chemoembolization (TACE) on several occasions. On the most recent admission, her liver cancer was found to be enlarged and ascites and pleural effusion had increased. Laboratory data are shown in Table 1. Her laboratory data indicated hepatic dysfunction, remnants of liver cancer and thrombocytopenia. On day 15 of her admission, her general condition deteriorated, and she died of liver failure. An autopsy was performed.
| CBC | Chemistry | ||||
| WBC | 8.21 × 103/μL | TP | 6.4 g/dL | Na | 129 mEq/L |
| Neutrophils | 89% | Alb | 2.4 g/dL | K | 4.8 mEq/L |
| Lymphocytes | 7% | BUN | 29.5 mg/dL | Cl | 96 mEq/L |
| RBC | 3.64 × 106/μL | Cre | 1.13 mg/dL | Glu | 178 mg/dL |
| Hemoglobin | 10 g/dL | AST | 78 U/L | CRP | 1.08 mg/dL |
| HCT | 30% | ALT | 55 U/L | NH3 | 63 μg/dL |
| Platelets | 41 × 103/μL | γ-GT | 88 U/L | HCV-Ab | 12.8 COI |
| T-bil | 3.88 mg/dL | HCV (RT-PCR) | 5.2 L.IU/mL | ||
| Coagulation | D-bil | 2.72 mg/dL | T-AFP | 571.4 ng/mL | |
| PT | 17.2 s | ALP | 402 U/L | AFP L3 | 42.2 ng/mL |
| APTT | 39.3 s | LD | 273 U/L | PIVKA2 | 15 mAU/mL |
| Fibrinogen | 123 mg/dL | AMY | 63 U/L | ||
| D-dimer | 5 μg/mL | ChE | 27 U/L | ||
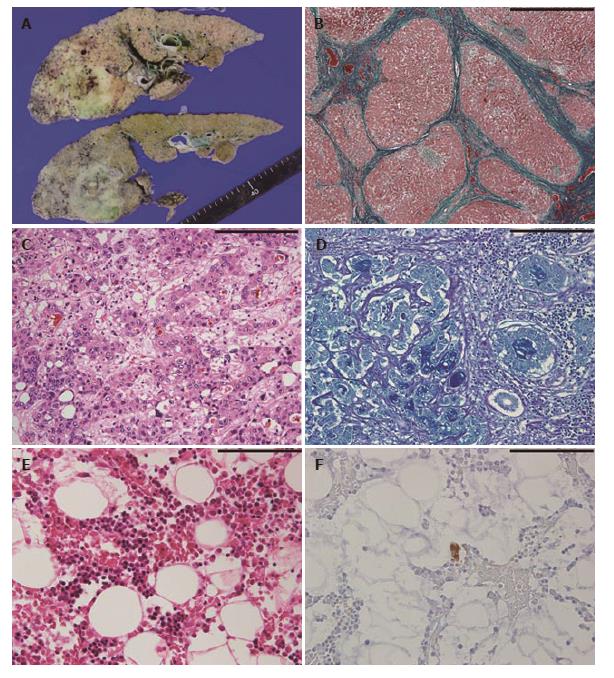
At autopsy, she showed generalized jaundice and purpura in the anterior chest wall. Ascites (2600 mL) and pleural effusion (left: 100 mL, right: 3400 mL) were observed. Liver weight was 660 g, indicating severe atrophy. The cut surface of the liver showed diffuse micronodular cirrhosis with a dark green nodule (15 mm × 15 mm) in the left lobe, and a yellow, partly reddish or green, lesion with an irregular margin (70 mm × 50 mm) in the right lobe (Figure 2A). Spleen weight was 240 g, indicating mild enlargement. Varicose veins were observed in the lower esophagus, stomach, and rectum.
Histopathologically, liver specimens showed diffuse small regenerative nodules with fibrous septum and septal mild mononuclear cell infiltration (Figure 2B). The right lobe lesion was mainly composed of two components: hepatocellular carcinoma with bile production (Figure 2C) and adenocarcinoma with mucin production (Figure 2D). Therefore, the diagnosis of combined hepatocellular-cholangiocarcinoma was made. There were no viable tumor cells in the left lobe lesion, compatible following TACE treatment for liver carcinoma. Microscopic metastases were observed in both lungs. Bone marrow specimens showed slight hypocellularity (30%-40%), with a myeloid to erythroid ratio: 3 to 1, and a marked reduction in the number of megakaryocytes, < 1 megakaryocyte/10 high-power fields (Figure 2E). Immunostaining for CD41 revealed scattered small megakaryocytes (Figure 2F). Other lineages of hematopoietic cells were preserved, and myelofibrosis, dysplasia, and metastatic lesions were not observed. Spleen specimens showed mild congestion without extramedullary hematopoiesis. Characteristic histopathological findings of the spleen in patients with ITP, such as an increase of secondary follicles with well-delineated germinal centers, an expansion of a follicular marginal zone of the white pulp and a diffuse proliferation of foamy histiocytes, were not obvious in this patient. There was no definite lesion in the thyroid.
Next, we evaluated serum TPO levels at the time of her last admission using an enzyme-linked immunosorbent assay (ELISA) kit (Quantikine, R&D Systems, Minneapolis, United States) according to the manufacturer’s protocol. The serum TPO level of the patient was 54 pg/mL, and the serum TPO levels of two healthy individuals without HCV infection were 27 pg/mL and 37 pg/mL (mean 32 pg/mL). In addition, the presence or absence of anti-TPO receptor (c-Mpl) autoantibodies was determined, using Human anti-thrombopoietin receptor (C-MPL) autoantibodies IgG ELISA kit (CUSABIO, Wuhan, China), according to the manufacturer’s protocol. The serum sample of the patient at the time of her last admission was positive for anti-c-Mpl antibodies, compared to the negative results of the sera of two healthy individuals without HCV infection.
In the current case, AAMT associated with autoantibodies to c-Mpl was considered to be one of the major causes of her severe thrombocytopenia. Hypersplenism and paraneoplastic autoimmunity were considered to be restrictive causes of her thrombocytopenia because she showed thrombocytopenia before development of her liver cirrhosis and liver cancer. In addition, her serum TPO level was preserved at the time of her last admission. Yet ITP was one of the causes of her thrombocytopenia. However, characteristic histopathological findings of the spleen in patients with ITP were not obvious in this patient at the time of autopsy.
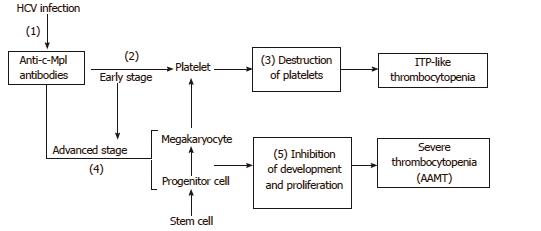
It is unclear when autoantibodies to c-Mpl started to be produced in this patient. The patient showed a normal number of megakaryocytes at least ten years before her death, that is, ten years after the onset of thrombocytopenia. This finding is compatible with ITP. Therefore, autoantibodies to c-Mpl might have developed after the diagnosis of ITP and the patient subsequently developed AAMT. It is possible that autoantibodies to c-Mpl had already been produced at the time of the ITP diagnosis. Kuwana et al[5] reported that autoantibodies to c-Mpl are detected in approximately 8% of ITP patients. Thus, patients with autoantibodies to c-Mpl might develop ITP at an early stage, and then develop AAMT during the course of the disease. This hypothesis is summarized in Figure 3. The receptor c-Mpl is expressed in the megakaryocytic lineage from late progenitors to platelets, and platelets display high-affinity receptors for TPO[7]. Therefore, even if autoantibodies to c-Mpl had been produced at an early stage, most of the autoantibodies would have been absorbed with c-Mpl on platelets, and the proliferation and differentiation of megakaryocytes would not have been severely impaired. Thus, the number of megakaryocytes in bone marrow would be relatively well preserved and the patient’s bone marrow may show a histopathology compatible with ITP. After a sufficient reduction in the number of platelets, AAMT then could then develop because autoantibodies to c-Mpl start to bind to c-Mpl on megakaryocytes and progenitor cells, inhibiting their development and maturation. Hoffman et al[8]. described a patient with ITP who later developed AAMT associated with antibodies that suppressed the colony formation of megakaryocytes[8].
HCV infection might have induced autoantibodies to c-Mpl in the current patient. Autoantibodies to c-Mpl are not detected in healthy controls[5]. In patients with HCV infection, several autoantibodies are produced, such as anti-nuclear antibodies, anti-smooth muscle antibodies, organ-specific autoantibodies, and anti-platelet antibodies[1]. In addition, anti-platelet IgG antibodies are detected in 26.3% of patients with HCV infection, showing a higher prevalence compared to healthy controls[9]. These mechanisms may play a role in the development of AAMT, with autoantibodies to c-Mpl in patients with HCV infection. However, interferon therapy induces several autoantibodies to multiple organ systems, such as anti-thyroid antibodies, auto-antibodies indicative of autoimmune hepatitis, and anti-platelet autoantibodies[10], and exhibits side effects of developing autoimmune diseases. Autoimmune thrombocytopenia sometimes occurs both during and after interferon therapy[11]. Thus, interferon therapy might have induced autoantibodies to c-Mpl in the current patient. We consider that interferon therapy did not play a significant role in the induction of autoantibodies to c-Mpl here because the patient’s bone marrow had shown a normal number of megakaryocytes for ten years at least, following interferon therapy.
The current case provides a new perspective on thrombocytopenia in patients with HCV infection. AAMT with autoantibodies to c-Mpl may be one of the causes of thrombocytopenia in these patients. Some patients with HCV infection-associated thrombocytopenia, for whom thrombopoietin receptor agonists have a weak effect, might have this condition. Further investigation will be necessary, especially concerning the relationship between AAMT with autoantibodies to c-Mpl and HCV infection.
AAMT with autoantibodies to c-Mpl can be one of the causes of thrombocytopenia in patients with chronic HCV infection.
A 64-year-old woman with hepatitis C virus (HCV) infection and idiopathic thrombocytopenic purpura presented with dyspnea.
Liver failure due to chronic hepatitis C.
Heart failure and renal failure.
Anemia, thrombocytopenia, decreased albumin, elevated bilirubin, liver dysfunction, elevated alpha-fetoprotein.
Computed tomography revealed liver cirrhosis with a right lobe mass, bilateral pleural effusions and ascites.
Liver cirrhosis, combined hepatocellular-cholangiocarcinoma in the liver and microscopic metastases in both lungs, and acquired amegakaryocytic thrombocytopenia (AAMT) in the bone marrow.
There are a limited number of reports describing AAMT with autoantibodies to thrombopoietin receptor (c-Mpl) in patients with systemic lupus erythematosus and systemic sclerosis.
AAMT is characterized by a marked reduction in the number of bone marrow megakaryocytes and occurs, in part, through autoantibodies to c-Mpl.
In patients with HCV infection, several autoantibodies are produced. Autoantibodies to c-Mpl may also be produced and AAMT may occur in patients with HCV infection. Thus, AAMT with autoantibodies to c-Mpl may be one of the causes of thrombocytopenia in patients with HCV infection.
The case record is correctly described and documented. The authors describe the first case of AAMT associated with HCV infection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez-Reimers E, Larrubia JR, Lee HC, Sergi CM
S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Weksler BB. Review article: the pathophysiology of th-rombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595-2605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 3. | Agarwal N, Spahr JE, Werner TL, Newton DL, Rodgers GM. Acquired amegakaryocytic thrombocytopenic purpura. Am J Hematol. 2006;81:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Ballmaier M, Germeshausen M, Schulze H, Cherkaoui K, Lang S, Gaudig A, Krukemeier S, Eilers M, Strauss G, Welte K. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139-146. [PubMed] |
| 5. | Kuwana M, Okazaki Y, Kajihara M, Kaburaki J, Miyazaki H, Kawakami Y, Ikeda Y. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002;46:2148-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Katsumata Y, Suzuki T, Kuwana M, Hattori Y, Akizuki S, Sugiura H, Matsuoka Y. Anti-c-Mpl (thrombopoietin receptor) autoantibody-induced amegakaryocytic thrombocytopenia in a patient with systemic sclerosis. Arthritis Rheum. 2003;48:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Broudy VC, Lin NL, Sabath DF, Papayannopoulou T, Kaushansky K. Human platelets display high-affinity receptors for thrombopoietin. Blood. 1997;89:1896-1904. [PubMed] |
| 8. | Hoffman R, Zaknoen S, Yang HH, Bruno E, LoBuglio AF, Arrowsmith JB, Prchal JT. An antibody cytotoxic to megakaryocyte progenitor cells in a patient with immune thrombocytopenic purpura. N Engl J Med. 1985;312:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Christodoulou D, Katsanos K, Zervou E, Theopistos V, Pa-pathanasopoulos A, Christou L, Tsianos EV. Platelet IgG antibodies are significantly increased in chronic liver disease. Ann Gastroenterol. 2011;24:47-52. [PubMed] |
| 10. | Sleijfer S, Bannink M, Van Gool AR, Kruit WH, Stoter G. Side effects of interferon-alpha therapy. Pharm World Sci. 2005;27:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Arena R, Cecinato P, Lisotti A, Buonfiglioli F, Calvanese C, Grande G, Montagnani M, Azzaroli F, Mazzella G. Severe immune thrombocytopenia after peg-interferon-alpha2a, ribavirin and telaprevir treatment completion: A case report and systematic review of literature. World J Hepatol. 2015;7:1718-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |