Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2141
Peer-review started: December 13, 2016
First decision: January 10, 2017
Revised: January 21, 2017
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 28, 2017
Processing time: 105 Days and 11.5 Hours
To assess the insulating effect of a poloxamer 407 (P407)-based gel during microwave ablation of liver adjacent to the diaphragm.
We prepared serial dilutions of P407, and 22.5% (w/w) concentration was identified as suitable for ablation procedures. Subsequently, microwave ablations were performed on the livers of 24 rabbits (gel, saline, control groups, n = 8 in each). The P407 solution and 0.9% normal saline were injected into the potential space between the diaphragm and liver in experimental groups. No barriers were applied to the controls. After microwave ablations, the frequency, size and degree of thermal injury were compared histologically among the three groups. Subsequently, another 8 rabbits were injected with the P407 solution and microwave ablation was performed. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and creatinine (Cr) in serum were tested at 1 d before microwave ablation and 3 and 7 d after operation.
In vivo ablation thermal injury to the adjacent diaphragm was evaluated in the control, saline and 22.5% P407 gel groups (P = 0.001-0.040). However, there was no significant difference in the volume of ablation zone among the three groups (P > 0.05). Moreover, there were no statistical differences among the preoperative and postoperative gel groups according to the levels of
ALT, AST, BUN and Cr in serum (all P > 0.05).
Twenty-two point five percent P407 gel could be a more effective choice during microwave ablation of hepatic tumors adjacent to the diaphragm. Further studies for clinical translation are warranted.
Core tip: Collateral thermal damage is the most common complication of microwave ablation. Conventional liquids can move away and be absorbed quickly, proving difficult to get a good separation effect. This study aimed to assess the insulating effect of a poloxamer 407 (P407)-based thermosensitive gel during microwave ablation of the liver adjacent to the diaphragm. We prepared serial dilutions of P407, and 22.5% (w/w) concentration was identified as suitable for ablation procedures. The 22.5% P407 effectively protected the diaphragm during microwave ablation of the liver, and was superior to 5% dextrose in water and 0.9% saline.
- Citation: Zhang LL, Xia GM, Liu YJ, Dou R, Eisenbrey J, Liu JB, Wang XW, Qian LX. Effect of a poloxamer 407-based thermosensitive gel on minimization of thermal injury to diaphragm during microwave ablation of the liver. World J Gastroenterol 2017; 23(12): 2141-2148
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2141
Percutaneous thermal ablation has been widely used over the past 20 years as a minimally invasive procedure for treating liver tumors, especially hepatocellular carcinoma (HCC)[1]. Over the years, different types of ablation applicators have become widely accepted, such as microwave (MW), radiofrequency (RF) electrical current, laser and cryoablation[2,3]. Percutaneous thermal ablation has been credited with almost equivalent survival rates and a rapid return to normal status as compared to surgical resection[4,5]. Several studies have noted that MW ablation could create larger ablation zones compared to RF ablation[6,7]. A MW ablation at 60 °C has been found to immediately induce coagulative necrosis of the tumors[8].
In the treatment of HCC, a low energy can result in incomplete ablation and local progression. However, high-power MW ablations often result in thermal injury to non-target organs, including the gallbladder, diaphragm and so on[9]. This poses a challenge to the interventional doctors. As previous studies have reported, about 15% of liver tumors deemed as high risk are not suitable for thermal ablation[10,11].
To reduce such harmful effects, several methods have been suggested during the ablation of subcapsular hepatic lesions. Hydrodissection is the most commonly applied technique to insulate adjacent structures such as 5% dextrose in water (D5W) and 0.9% normal saline (NS)[12,13]. That has been found to be effective at decreasing unintended thermal injury, however D5W and NS tend to move away quickly from target sites, thereby reducing the insulating effect.
P407 is a nonionic surfactant composed of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymers[14]. It is currently used in clinical therapy as a drug carrier[15,16]. P407 has an attractive property that it can, from being in liquid state at low temperatures, transform into a semisolid gel state at elevated temperatures (gelation temperature), which depends on heat conduction of the surroundings[15,17]. This indicated that P407 gel may be useful in MW ablation of the liver. The aim of our study was to evaluate in vivo the insulating properties of a P407-based thermosensitive gel during MW ablation of the liver adjacent to the diaphragm.
The subjects included in this study were 32 male and female healthy New Zealand white rabbits (weight range: 1.5-2.5 kg). The study protocol was approved by the Animal Care and Use Committee of our research institution. The treatment of animals was according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The sol-gel transformation of the injectable thermosensitive solution is expected to occur at slightly below room temperature. A series of dilutions ranging from 15%-30% (w/w) P407 (Batch No. WPWJ554C; BASF, Germany) in deionized water were prepared and their gelation temperatures were determined using a rotor equipped with a controlled heating system[18] (magnetic rotor size of 1.5 cm × 0.6 cm, stirring rate of 300 rotations/min). The samples were slowly heated at a rate of 0.5 °C/min from the initial temperature of 15 °C. The gelation temperatures were defined as when the magnetic rotor completely stopped rotating. Each concentration was tested in triplicate simultaneously. Eventually, an optimal concentration of the P407 solution was obtained. Then, the viscosity of 22.5% P407 gel was tested with a Brookfield R/S+ rheometer (Stoughton, MA, United States) with a circulating water bath. The sample was heated from 15 °C to 30 °C at a constant shear rate of 5/s. The rheological behavior of 22.5% P407 was investigated.
A water-cooled MW ablation system was used in this study (KY-2000; Kangyou Medical Instruments, Nanjing, China). The generator can produce 1-100 W of power at 2450 MHz. We used a Model T11 (outer diameter of 15 G) MW ablation needle, with distance of 11 mm from the front end of the gap to the tip. An output setting of 40 W for 300 s was usually used for ablation sessions. However, since rabbit liver is small and fragile, such high MW power could easily penetrate the liver; therefore, ablation was performed for 180 s at 30 W in this study.
An iron/constantan thermocouple was used to monitor temperature in real time. The system had 21-gauge thermocouple needles, which were percutaneously placed at a designated location. For data acquisition, HP 34970A (Hewlett-Packard, Palo Alto, CA, United States) with a 16-bit analog output function was used.
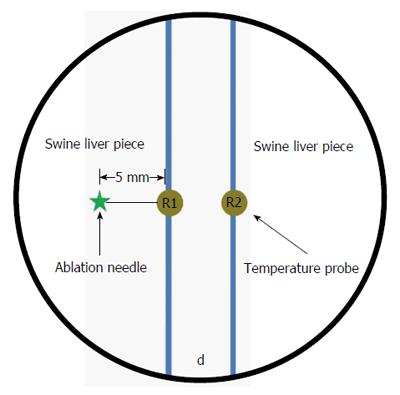
Insulation effectiveness during MW ablation was evaluated ex vivo as described in Figure 1. Two swine liver pieces were placed in a six-well plate (diameter 2 cm and depth 2 cm) which was positioned in a water bath at 37 °C. To obtain a 5 mm or 10 mm barrier between the liver pieces, 22.5% P407 gel was used as a hydrodissection. The ablation needles were placed vertically into the livers at a depth of 1.5 cm, 5 mm away from the barrier. The ablation needle and temperature probes were positioned in parallel, maintaining a distance of 5 mm between the ablation needle and the primary needle (R1). We compared the insulation effects of a 5 mm-thick barrier against a 10 mm-thick barrier. MWs were applied three times at 30 W for 3 min. The temperature differences between probes R1 and R2 were recorded every 30 s and mapped. Moreover, whenever R1 reached 60 °C, the temperature at R2 was measured.
Of the 32 rabbits used in this study, 8 were employed to study the safety of the P407 gel, as described later. The other 24 rabbits were randomly assigned to three experimental groups. Two experimental groups were injected with 5 mL P407 and 5 mL 0.9% NS, respectively, between the diaphragm and the liver. Such volume enabled the presence of a 5 mm barrier by ultrasonic examination. For control animals, no protective technique was used. Before each ablation procedure, the rabbits were anesthetized with 30 mg/kg intravenous pentobarbital sodium (Sigma, St Louis, MO, United States). The abdomen was shaved, disinfected routinely, and the animals were placed in a supine position for MW ablation.
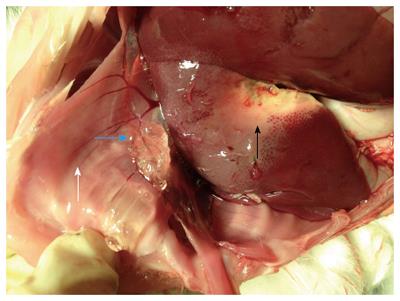
All of the procedures described in this study were performed by two interventional clinicians. The animals were ultrasonically scanned to choose the best puncture sites (avoiding important blood vessels and ribs). A 2 mm incision was made at the edge of the skin with a sharp knife. The MW antennas were placed 5 mm away from the liver surface. The ablation applicator was used for 3 min at an output power of 30 W. During MW ablations, the thickness of the hydrodissection barrier was observed for each experimental group. All interventional procedures were monitored and guided by ultrasound examination.
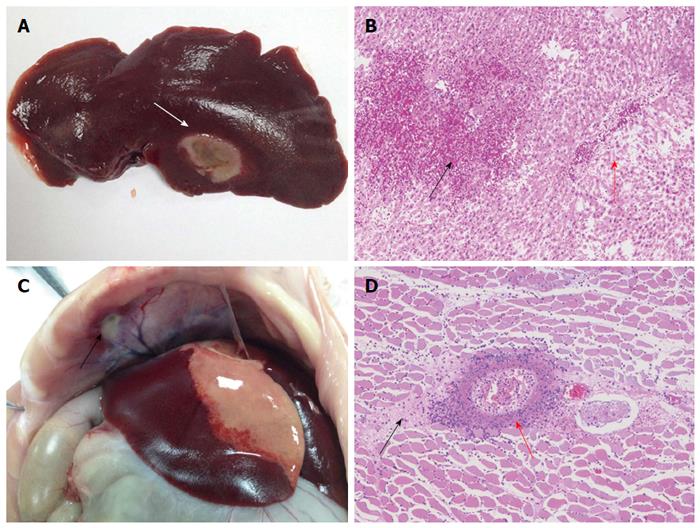
The 24 rabbits were sacrificed and dissected immediately after MW ablation. The liver ablation zones and adjacent diaphragms were photographed and the ablation effects compared. Subsequently, the diaphragm and liver samples were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. An experienced pathologist evaluated thermal injury to the diaphragm histologically. The volumes of ablation zones were calculated and compared using the following formula.
Volume (V) = π/6×a b c
where a is dimension 1, b is dimension 2, and c is dimension 3. (a is the largest diameter, and b and c are the other mutually perpendicular diameters).
Thermal injury to the diaphragm was expressed as a diameter of injured lesions. In addition, we graded the degree of thermal injury to the diaphragm according to a 4-point scoring system (none, 0; mild, 1; moderate, 2; severe, 3) based on a consensus of two of the contributing authors. If a diaphragm was seen discolored and having a thickened pale area that extended toward the pleural margin, it was considered seriously injured. The suspected injured diaphragms were sectioned and graded on a scale of 0-3 (0, no injury; 1, mild injury up to one-third thickness; 2, moderate injury to two-thirds thickness; 3, severe injury)[19].
Eight rabbits were injected with the P407 solution at a dose of 5 mL into the potential space between the diaphragm and liver under ultrasonic guidance and MW ablation was performed at 30 W for 3 min. Using 2 mL of ear vein blood, the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (Cr) in serum were tested 1 d before and 3 and 7 d after the procedures, so as to check liver and renal functions.
The experimental data were analyzed using SPSS software, version 16.0. Quantitative data were described as mean ± SD and were evaluated using one-way analysis of variance. The levels of thermal injury were compared using the Mann-Whitney test (Kruskal-Wallis test). P < 0.05 was considered statistically significant.
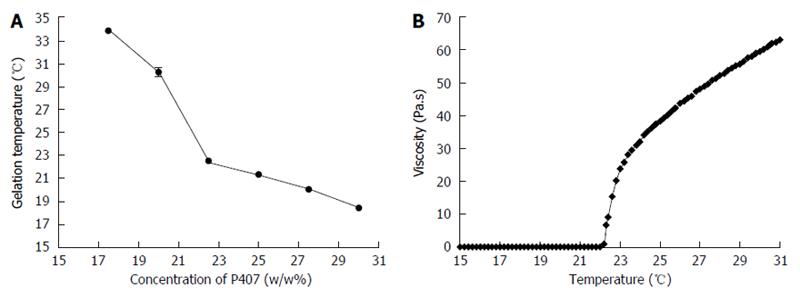
The sol-gel transformation temperature decreased as P407 concentration increased (Figure 2A). Finally, gelation temperature of 22.5% P407 (BASF) solution was 22 °C. For clinical purposes, the thermosensitive gel should have a relatively lower gelation temperature in order to gelate rapidly in target site and facilitate our operation smoothly. Our results show that 22.5% P407 solution gelled at about 1.5 min in a water bath at 37 °C but took 16 min at room temperature. Such short interval is beneficial to operate the surgery rapidly and smoothly. So, we propose that 22.5% P407 gel could be an ideal choice for ablation procedures.
The rheological behavior of P407 is also presented as a flow curve (Figure 2B). The sample exhibited low viscosity and characterized fluidic behavior below 18 °C. It is feasible to be injected. When the gelation temperature was reached, the viscosity sharply increased. By this time, the sample had transformed into a semi-solid state.
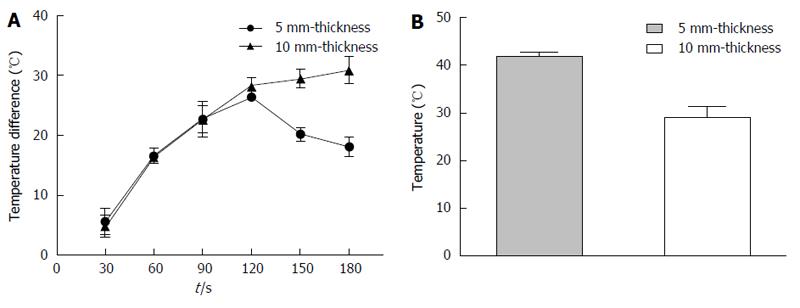
After MW ablation for 120 s, the maximum temperature difference of 26.4 ± 0.5 °C was observed between R1 and R2 with a P407 gel thickness of 5 mm (Figure 3A). When the mean temperature of R1 reached 60 °C at 180 s, the temperature of R2 was 41.9 ± 1.1 °C (Figure 3B) and the temperature difference was 18.1 ± 1.5 °C (Figure 3A). However, the maximum temperature difference of 30.9 ± 2.2 °C (Figure 3A) was observed after MW ablation for 3 min with a P407 gel thickness of 10 mm; the mean temperature of R1 was 60 °C and that of R2 was 29.1 ± 2.4 °C (Figure 3B). Our results demonstrate that a 5 mm P407 gel is adequate to insulate the surrounding tissue from thermal damage.
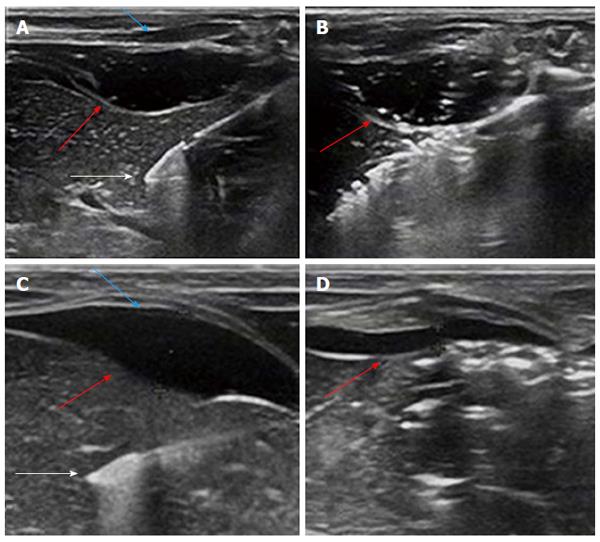
When monitored ultrasonically, no changes in gel thickness were observed during MW ablation (Figure 4A and B). After MW ablation, laparotomy was performed on the experimental animals immediately and the in situ gel and liver ablation zones were observed (Figure 5). Similarly, for the NS group, the initial barrier thickness was 5 mm (Figure 4C). The distance between ablation needle tip and the edge of the liver was approximately 5 mm. However, the thickness had become reduced to 1.3 mm at the end of the ablation procedure (Figure 4D). After several hours, the 22.5% P407 gel was undetectable by ultrasound.
The effects of ablations extended into the surrounding diaphragm in all of the control animals (n = 8), in 5 of the NS-protected animals and none of the gel-protected animals. Table 1 shows that thermal damage to the diaphragm differed significantly in size and severity among the three groups (P < 0.05). However, no difference in the volume of ablation zone was detected among the three experimental groups (P = 0.353; Table 2). Representative photographs of gross specimens of ablated liver and injured diaphragm are shown in Figure 6.
| Gel group | Saline group | Control group | P value | |
| Dimension 1 in cm | 2.06 ± 0.38 | 2.14 ± 0.15 | 2.19 ± 0.14 | |
| Dimension 2 in cm | 1.28 ± 0.18 | 1.26 ± 0.22 | 1.38 ± 0.14 | |
| Dimension 3 in cm | 1.24 ± 0.18 | 1.23 ± 0.22 | 1.34 ± 0.16 | |
| Volume in cm3 | 1.76 ± 0.66 | 1.75 ± 0.54 | 2.11 ± 0.43 | 0.353 |
The levels of ALT, AST, BUN and Cr in serum were assayed before and after MW ablation (Table 3) as indicators of liver and renal functions. Our statistical analysis showed that there was no significant difference among the groups pre- and postoperatively (P > 0.05; Table 3).
| Indicator | Baseline | Postablation | P value | |
| Day 3 | Day 7 | |||
| ALT in U/L | 45.38 ± 5.24 | 41.57 ± 3.96 | 41.50 ± 4.14 | 0.166 |
| AST in U/L | 50.79 ± 3.95 | 47.25 ± 3.28 | 45.63 ± 5.10 | 0.062 |
| BUN in mmol/L | 7.50 ± 0.90 | 7.14 ± 1.10 | 7.12 ± 1.05 | 0.708 |
| Cr in μmol/L | 66.24 ± 4.14 | 62.77 ± 7.16 | 62.04 ± 3.39 | 0.244 |
MW ablation is considered an effective treatment for small HCCs[20,21]. However, several complications may occur, including hemorrhage, pleural effusion and thermal injury[1]. Among these, thermal injury to non-target tissue is the most common side effect, in particular when the tumor is close to vital organs, thereby resulting in poor prognosis. Therefore, ablation is not recommended for large tumors located close to the diaphragm or the gastrointestinal tract.
Many investigators have attempted to reduce collateral thermal damage by means of hydrodissection[13,22]. Although several conventional thermoprotective fluids are known, low viscosities as a result of their high mobility pose a challenge. In some cases, a continuous infusion of the fluid needs to be maintained during the entire ablation procedure, which can lead to fluid overload and patient discomfort[23,24]. Thus, we optimized the fluids to replace conventional hydrodissection applied for MW ablation of the liver.
In the present study, 22.5% P407 solution exhibited potential as a thermoprotective barrier during MW ablations. It exhibited low viscosity at below 18 °C as D5W and 0.9% NS, which allows for injectability without resistance through small needles. However, 22.5% P407 transformed into a semi-solid state rapidly at 37 °C, providing a stable gel barrier at the injection site. It was not detected by ultrasound after several hours. Therefore, performance of more critical ablations, such as for high-risk liver cancers, is possible when using P407 as a thermoprotective agent, although MW ablation is generally not the preferred method for treating such cases. It is well known that conventional hydrodissection fluids flow away from target sites due to heat convection, thereby dissipating heat from the ablation site. Nevertheless, this appears to play little role in the mechanism of P407 gel. Instead, it appears to work mainly through heat conduction. Further studies are needed to establish the mechanisms of thermoprotection by P407.
According to ex vivo temperature studies, 22.5% P407 gel of 5 mm thickness can result in a temperature difference of about 18 °C between both sides of the gel. During ablation, the temperature was 29.1 ± 2.4 °C on the other side of the gel (corresponding to the one side of tissue necrosis temperature, 60 °C). This insulation effect is enough to protect the surrounding tissues adjacent to the ablation zones, as well as to reduce postoperative complications. Besides, since the volume of the fluid required to be injected into the body is significant low, it will be much more easily accepted by patients in a clinical setting.
Most important of all, none of the animals in the gel group experienced diaphragmatic injury, even when MW ablation was performed at the subcapsular region of the liver. This is partly due to the fact that the gel had been placed into a preset position and remained stable during the MW ablation. In contrast, thermal damage in the NS group was serious, due in part to the tendency of saline to flow away from the injection site, thereby providing partial protection to the diaphragm during liver ablations. In many cases, continuous infusion is unavoidable with saline, which is considered not suitable for use in clinical practice especially for patients susceptible to volume overload.
In addition, 22.5% P407 was found to be safe on our experimental animals, as demonstrated through in vivo safety studies involving liver and renal function tests. Yet, 3 and 7 d postoperatively are still in acute the timeframe and further studies over a longer period of time are necessary to establish the safety of P407 gel.
In spite of the accomplishments of the present study, there are a few limitations. Firstly, the sample size was relatively small (n = 24 ablations), yet the insulation effect showed statistical significance. Further comprehensive studies are required to prove the safety and effectiveness of 22.5% P407 gel during MW ablation for small HCC. Secondly, healthy rabbits were included rather than tumor models; however, this may not affect our study findings, because the study aim was to assess thermoprotection properties rather than treatment effectiveness.
In conclusion, 22.5% P407 gel could be a more effective choice during MW ablation of subcapsular hepatic tumors adjacent to the diaphragm. Further studies for clinical translation are warranted.
Percutaneous thermal ablation has been a widely used method for treating liver tumors. However, about 15% of liver tumors deemed as high risk are not suitable for thermal ablation due to collateral thermal damage. Several methods, especially hydrodissection, have been suggested for ablation of subcapsular hepatic lesions.
Hydrodissection, such as 5% dextrose in water (D5W) and 0.9% normal saline (NS), that has been found to be effective at decreasing unintended thermal injury; however, D5W and NS tend to move away quickly from target sites, thereby reducing the insulating effect.
In the study, the authors utilized the thermosensitivity of poloxamer 407 (P407) as novel hydrodissection to protect the surrounding tissues during microwave (MW) ablations.
In medical practice, percutaneous MW ablation has been credited with almost equivalent survival rates as surgical resection. As the results of this study suggest, critical ablations, such as for high-risk liver cancers, are possible to be performed when using P407 as a thermoprotective agent, although MW ablation is generally not recommended for treating such cases. In addition, since the volume of the fluid required to be injected into the body is significantly low, it would be more easily accepted by patients in a clinical setting. It should be noted that this material would be much-needed in most clinical situations, such as high-risk liver tumors.
The study material is thermosensitive in nature. This means that it behaves like a liquid at low temperature and transforms into a get state at an increased temperature.
This is an interesting manuscript about the effect of a P407-based thermosensitive gel on minimization of thermal injury to diaphragm during MW ablation of the liver.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gilbert MR, Mayer RJ S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Mertyna P, Goldberg W, Yang W, Goldberg SN. Thermal ablation a comparison of thermal dose required for radiofrequency-, microwave-, and laser-induced coagulation in an ex vivo bovine liver model. Acad Radiol. 2009;16:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases--why cryoablation? Skeletal Radiol. 2009;38:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192-S203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 6. | Laeseke PF, Lee FT, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Laeseke PF, Lee FT, van der Weide DW, Brace CL. Multiple-Antenna Microwave Ablation: Spatially Distributing Power Improves Thermal Profiles and Reduces Invasiveness. J Interv Oncol. 2009;2:65-72. [PubMed] |
| 8. | Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg. 2009;13:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123-134; discussion 134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol. 2008;190:W187-W195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Hinshaw JL, Laeseke PF, Winter TC, Kliewer MA, Fine JP, Lee FT. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol. 2006;186:S306-S310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia. 2014;30:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 854] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 15. | Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull (Tokyo). 1984;32:4205-4208. [PubMed] |
| 16. | Ricci EJ, Lunardi LO, Nanclares DM, Marchetti JM. Sustained release of lidocaine from Poloxamer 407 gels. Int J Pharm. 2005;288:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Johnson A, Sprangers A, Cassidy P, Heyrman S, Hinshaw JL, Lubner M, Puccinelli J, Brace C. Design and validation of a thermoreversible material for percutaneous tissue hydrodissection. J Biomed Mater Res B Appl Biomater. 2013;101:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Oh PS, Choi HG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226:195-205. [PubMed] |
| 19. | Kim YS, Rhim H, Paik SS. Radiofrequency ablation of the liver in a rabbit model: creation of artificial ascites to minimize collateral thermal injury to the diaphragm and stomach. J Vasc Interv Radiol. 2006;17:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Martin RC, Scoggins CR, McMasters KM. Microwave hepatic ablation: initial experience of safety and efficacy. J Surg Oncol. 2007;96:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S104-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Chen EA, Neeman Z, Lee FT, Kam A, Wood B. Thermal protection with 5% dextrose solution blanket during radiofrequency ablation. Cardiovasc Intervent Radiol. 2006;29:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology. 2002;222:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Bodily KD, Atwell TD, Mandrekar JN, Farrell MA, Callstrom MR, Schmit GD, Charboneau JW. Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol. 2010;194:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |