Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4345
Peer-review started: December 7, 2015
First decision: January 28, 2016
Revised: February 25, 2016
Accepted: March 14, 2016
Article in press: March 14, 2016
Published online: May 7, 2016
Processing time: 145 Days and 6 Hours
AIM: To investigate the anti-apoptotic capability of the hepatitis B virus (HBV) in the HepG2 hepatoma cell line and the underlying mechanisms.
METHODS: Cell viability and apoptosis were measured by MTT assay and flow cytometry, respectively. Targeted knockdown of manganese superoxide dismutase (MnSOD), AMP-activated protein kinase (AMPK) and hepatitis B virus X protein (HBx) genes as well as AMPK agonist AICAR and antagonist compound C were employed to determine the correlations of expression of these genes.
RESULTS: HBV markedly protected the hepatoma cells from growth suppression and cell death in the condition of serum deprivation. A decrease of superoxide anion production accompanied with an increase of MnSOD expression and activity was found in HepG2.215 cells. Moreover, AMPK activation contributed to the up-regulation of MnSOD. HBx protein was identified to induce the expression of AMPK and MnSOD.
CONCLUSION: Our results suggest that HBV suppresses mitochondrial superoxide level and exerts an anti-apoptotic effect by activating AMPK/MnSOD signaling pathway, which may provide a novel pharmacological strategy to prevent HCC.
Core tip: Hepatitis B virus markedly protected the cells from growth suppression and cell death in the condition of serum deprivation. A decrease of superoxide anion production accompanied with an increase of manganese superoxide dismutase (MnSOD) expression and activity was found in HepG2.215 cells. Moreover, AMP-activated protein kinase activation contributed to the up-regulation of MnSOD. Hepatitis B virus X protein was identified to promote the expression of AMPK and MnSOD.
- Citation: Li L, Hong HH, Chen SP, Ma CQ, Liu HY, Yao YC. Activation of AMPK/MnSOD signaling mediates anti-apoptotic effect of hepatitis B virus in hepatoma cells. World J Gastroenterol 2016; 22(17): 4345-4353
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4345
Hepatocellular carcinoma (HCC) is one of the most frequently diagnosed malignant cancers worldwide, while 50% of cases and deaths occurred in China[1]. Chronic hepatitis B virus (HBV) infection has been internationally recognized as one of the major risk factors for the development of HCC[2]. An estimated 350 million people were chronically infected and 600000 hepatitis B-related deaths occurred every year all over the world[3]. Accumulated evidence has shown that HBV proteins, particularly hepatitis B virus X protein (HBx) and surface protein (HBs), are implicated in hepatocyte carcinogenesis[4]. However, the mechanisms underlying HBV-induced malignant transformation remain ambiguous.
Apoptosis, also named programmed cell death, plays a crucial role in the development and homeostasis in normal tissue[5]. Recently, studies have indicated that defect or insufficient apoptosis may contribute to carcinogenesis, tumor progression and resistance of tumor cells to chemo-radiotherapy[6-8]. For that reason, escape of apoptosis has been identified as one of prominent hallmarks of cancer[9]. Reactive oxygen species (ROS), as toxic products of cell metabolism, can cause cell apoptosis by leading to cellular DNA damage and subsequently activating apoptotic signaling pathways[10]. In cancer, tumor niches characterized with poor nutrient and oxygen usually possess oxidative stress with excessive ROS formation[11,12]. Mitochondrial ROS (mtROS) especially superoxide anion, a natural by-product of electron transport chain activity, is the main source of cellular ROS[13]. Thus, decreasing mtROS production to relieve oxidative stress is very important for tumor survival and progression.
Manganese superoxide dismutase (MnSOD), a key antioxidant enzyme, is responsible for scavenging superoxide anion. Liver malignant tumors have been shown to express higher protein level and activity of MnSOD than their benign counterparts[14]. Aggressive tumors possessing invasive phenotype also have a high level of MnSOD, which can facilitate them to reach distant organs[15]. Therefore, increased MnSOD expression and activity may protect cells against apoptosis and offer a growth advantage, thereby acquiring a more aggressive phenotype.
The expression of MnSOD can be modulated by many molecular factors at transcription, translation and posttranslational modifications levels, for example, p53, Sp1, and NF-κB[16-18]. AMP-activated protein kinase (AMPK) is also reported to act as a new regulator of MnSOD expression in endothelial cells[19]. Moreover, AMPK activation is associated with protection of hepatocytes against oxidative stress[20].
Based on the aforementioned studies, we investigated the effect of HBV on the growth and survival of HepG2 cells, and explored the underlying molecular mechanisms. Herein, we demonstrated that HBV protected HepG2 cells from growth suppression and apoptosis in the condition of serum deprivation. Furthermore, AMPK activation-induced up-regulation of MnSOD contributed to the resistance of HBV-integrated HepG2 cells to apoptosis caused by superoxide, which could explain in part HBV-induced hepatocellular cell malignant transformation in the context of growth factor withdrawal.
The human hepatoma cell line HepG2 was obtained from Cell Bank of Chinese Academy of Sciences where it was authenticated. HepG2.215 cell line, which was derived from HepG2 cells by integrating HBV genome and persistently produced HBV, was kindly provided by Prof. Erwei Song (Sun Yat-sen Memorial Hospital of Sun Yat-sen University, China). All of the cell lines were maintained in DMEM (Gibco, Gaithersburg, MD, United States) supplied with 10% fetal bovine serum and 1% penicillin/streptomycin, and incubated at 37 °C in a humidified incubator with 5% CO2.
The HiPerFect transfection reagent was obtained from QIAGEN (QIAGEN, Carson City, CA). Antibodies of AMPKα and phospho-AMPKα (Thr172) were purchased from Cell Signaling (Cell Signaling Technology, MA). Anti-MnSOD antibody was from BD (BD Pharmingen, San Diego, CA, United States). Antibody of HBx (anti-HBx) was obtained from Abcam (Abcam, Cambridge, UK). AICAR, Compound C and anti-β-actin were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, United States).
Cells were seeded in 24-well plates in quadruplicate. After indicated treatments, cells viability was determined with 3-[4,5-dimethylthiazol-2-yl]-2,5-dephenyl tetrazolium bromide (MTT) (Sigma, MO) following the manufacture’s protocol. Absorbance was measured at a wavelength of 570 nm.
Cells were prepared as described elsewhere[21]. AnnexinV and propidium iodide (KeyGEN BioTECH, Nanjing, China) were added for incubation in the dark for 15 min at 4 °C, and then cells were analyzed with a flow cytometer (Gallios, Beckman).
Measurements of mitochondrial superoxide anion formation in cells were performed as previously described[22]. In brief, HepG2 and HepG2.215 cells were incubated with 5 μmol/L MitoSOX (Invitrogen, Carlsbad, CA) for 20 min at 37 °C. Cells were digested using EDTA (Invitrogen), and then washed three times using HBSS with Ca/Mg (Invitrogen). Mean fluorescent intensity was measured by flow cytometry (Gallios, Beckman).
MnSOD activity was measured with a commercial SOD kit (Cayman Chemical) according to the manufacturer’s protocol. Briefly, 1 mmol/L potassium cyanide was added in order to inhibit Cu/Zn-SOD and extracellular SOD, thus only MnSOD activity was detected. O2- was generated by adding hypoxanthine/xanthine oxidase and detected with tetrazolium salt through reading the absorbance at 450 nm.
The siRNAs for silencing AMPK, MnSOD and HBx genes as well as scrambled siRNA were purchased from Ribobio (Guangzhou, China). Transfection with synthetic siRNAs was performed with HiPerFect (QIAGEN, Carson City, CA) according to the manufacturer’s instructions. The sense sequences of double-strand siRNA were as follows: siAMPK, 5’-UGCCUACCAUCUCAUAAUATT-3’; siMnSOD, 5’-GGAGAAUGUAACUGAAAGATT-3’; siHBx-1, 5’-CCGACCUUGAGGCAUACUUdTdT-3’; siHBx-2, 5’-UGUGCACUUCGCUUCACCUTT-3’.
Western blot analysis was performed as described previously[23]. Antibodies for MnSOD, AMPK, Phospho-AMPK and HBx were used at 1:1000 dilution. Antibody for β-actin was used at 1:10000 dilution. Bound antibody was visualized using HRP-conjugated secondary antibodies.
All data are expressed as mean ± standard deviation (SD). SPSS 13.0 software was used for one-way analysis of variance (ANOVA) and t-test in all statistical analyses (SPSS, Chicago, IL, United States). A P value less than 0.05 was considered statistically significant.
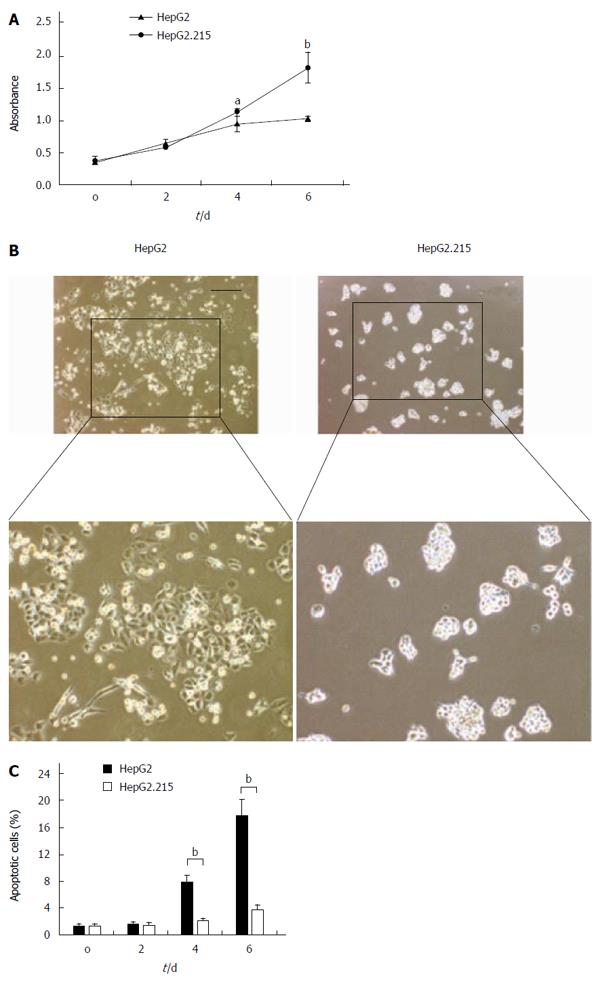
In order to assess the effect of HBV on the proliferation of HepG2 cells, we employed the HepG2.215 cell line which was derived from the HepG2 cell line and persistently produced HBV. We found that the HepG2.215 cell line showed faster growth kinetics compared with the HepG2 cell line on days 4 and 6 after serum depletion (Figure 1A). Moreover, the number of apoptotic HepG2 cells was significantly increased on days 4 and 6 compared with that of HepG2.215 cells. In contrast, the number of apoptotic HepG2.215 cells stayed at a much lower level at all testing time points (Figure 1B and C). These data suggest that HBV proteins may protect HepG2.215 cells against apoptosis induced by serum depletion.
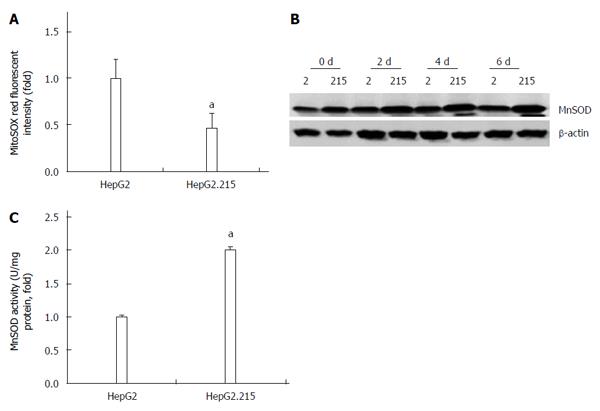
To explain the different anti-apoptotic ability of the two cell lines, we investigated the production of mitochondrial superoxide which is a well-known killer of cells[10]. Decreased mitochondrial superoxide level was found in the HepG2.215 cell line (Figure 2A). Since MnSOD is the regulator of mitochondrial superoxide, we therefore detected the expression and activity of MnSOD in the two cell lines. As shown in Figure 2B and C, both the expression and activity of MnSOD in HepG2.215 cells were higher than those of HepG2 cells.
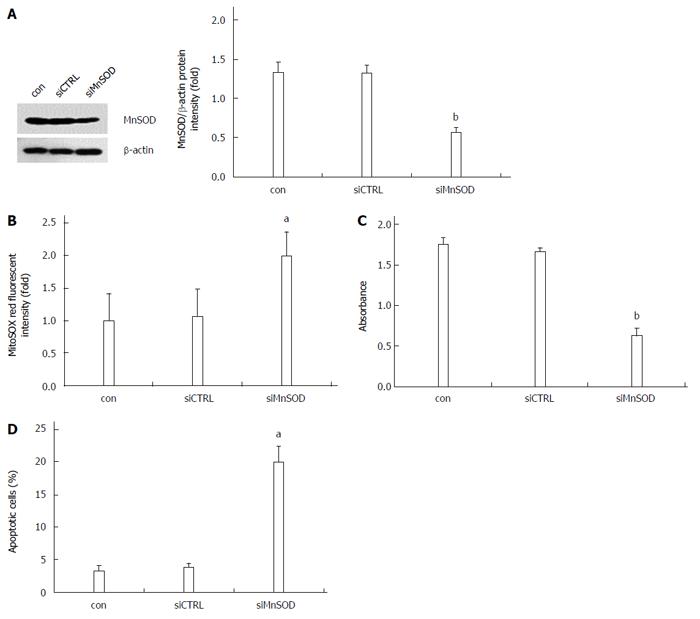
To further verify the role of MnSOD in the apoptotic resistance of HepG2.215 cells, the MnSOD siRNA was synthesized. Western blot analysis revealed that MnSOD siRNA specifically knocked down MnSOD in HepG2.215 cells (Figure 3A). Knockdown of MnSOD decreased cell viability and increased mitochondrial superoxide formation and the number of apoptotic HepG2.215 cells (Figure 3B-D), which suggests that MnSOD plays a critical role in apoptotic resistance of HepG2.215 cells.
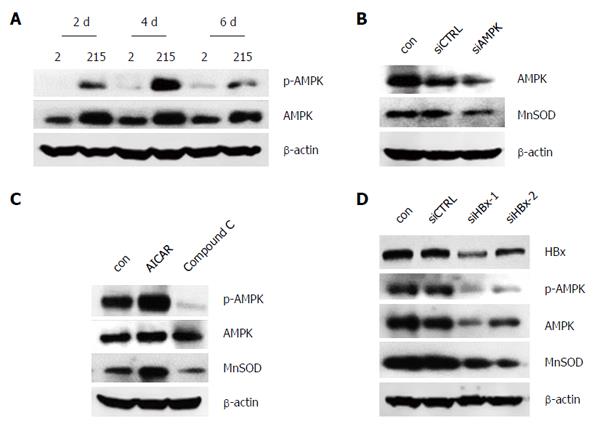
To figure out the upstream factor involving the modulation of MnSOD, AMPK was investigated. We showed the protein levels of p-AMPK and AMPK were increased in HepG2.215 cells (Figure 4A). Both knockdown of AMPK and treatment with AMPK inhibitor Compound C reduced the expression of MnSOD (Figure 4B and C). Conversely, AMPK activator AICAR increased the expression of MnSOD (Figure 4C). Furthermore, the expression of p-AMPK, AMPK and MnSOD was inhibited by HBx knockdown (Figure 4D). These results suggest that HBV up-regulates MnSOD via AMPK.
As a major cause for HCC development, HBV can promote HCC in many ways, including enhancing host chromosomal stability, inducing inflammation-mediated immune escape, regulating epigenetic modification or altering the expression of oncogenes and tumor-suppressor genes[24]. Due to these internal changes, hepatoma cells acquire the capacity of fast growth, anti-apoptosis and metastasis[25,26]. In this study, we confirmed that HBV-integrated HepG2 cells exerted survival benefit compared with its parent cell line HepG2 in the serum-deprivation condition which can to some extent mimic the adapation of tumor cells to adverse growth conditions. In line with previous studies, we also found that HBV conferred HepG2 cells resistance to apoptosis[26,27]. Our data suggest that HBV apparently acts to promote the growth and viability of hepatoma cells in growth factor-restricted conditions.
An increased level of ROS by creating a potentially toxic environment to the cells represents a critical mechanism underlying cell death[28]. Superoxide anion is the precursor of other ROS such as H2O2 and peroxynitrite, and because of that the organelles most vulnerable to oxidative stress are the mitochondria[29]. MnSOD is an essential antioxidant enzyme in the mitochondrion that acts on superoxide anion[30]. Here, we showed that HBV reduced the level of superoxide anion. Consistently, the expression and activity of MnSOD were up-regulated in HBV-integrated HepG2 cells. This result was supported by the finding of a previous study that in patients with HBV infection, there was an average 5-fold rise of serum MnSOD[31].
The expression and activity of MnSOD are not static in different tumorigenesis stages. For transformed phenotype, MnSOD levels were maintained at a low level and could directly potentiate mitochondrial defects, leading to gene mutations. For acquiring a more aggressive phenotype, enhanced MnSOD activity may protect cells against mitochondrial injury, thereby conferring a growth advantage to the cancer cells[16]. The present study demonstrated that knockdown of MnSOD increased the production of superoxide anion and the apoptosis of HepG2.215 cells, which indicated that MnSOD protected hepatoma cells against apoptosis by detoxing superoxide anion, and conferred a growth advantage to those cells. However, since the function of MnSOD is to convert diffusion-restricted and mild-toxicant superoxide anion to freely diffuse and strong-toxicant H2O2, which means that increased MnSOD may enhance the production of more toxicant H2O2, the mechanism of modulation of tumor cell survival by MnSOD seems confusing. It has been reported that HBx expressing cell line showed significantly reduced sensitivity to H2O2-induced cell death, and the level of intracellular ROS did not elevate in HBx expressing cell line after exposure to H2O2 in the medium[32]. Based on these findings, we speculate that HBV-infected cells may express relatively high amounts of catalase, they would be able to counteract the cytotoxic effects of peroxide, and thus the outcome of increased MnSOD activity would more likely reflect the capacity of MnSOD to reduce levels of oxygen radicals. Unexpectedly, the level of catalase in HBV-related hepatocellular carcinoma specimens was lower than that of surrounding non-tumor tissues[33]. Thus, further investigation is required to explain the tolerance of HBV-infected cells to H2O2-induced cell apoptosis, which will be helpful for understanding the mechanism of MnSOD-modulated tumor cell survival.
AMPK, a serine/threonine protein kinase, is well known for its role in controlling energy metabolism. Recently, it comes into focus because of its potential roles in regulating other signaling pathways, such as regulating oxidative stress[34]. Studies have reported that activation of AMPK by AICAR, or overexpression of constitutively activated AMPK suppressed O2− production in human neutrophils or HUVECs[35,36]. A similar observation was also found in HepG2 cells, which showed that AA+ iron-induced reactive oxygen species generation was inhibited by isorhamnetin through AMPK activation[20]. These studies indicate that AMPK appears to be the key factor for cellular function protection in the presence of oxidative stress. Emerging evidence suggests that AMPK inhibits oxidant production by decreasing the expression of NADPH oxidases or increasing the expression of UCP-2 as well as MnSOD[19,35,36]. In the present study, HBV-integrated HepG2 cells displayed elevated AMPK protein level, which remains consistent with the expression of MnSOD. By utilizing a specific siRNA, or a selective agonist (AICAR) and antagonist (compound C) of AMPK, we observed that knockdown of AMPK and compound C resulted in the reduction of MnSOD protein level. Moreover, activation of AMPK by AICAR up-regulated the expression of MnSOD. Taken together, these findings demonstrate that AMPK is responsible for the up-regulation of MnSOD expression in HBV-integrated HepG2 cells.
Additionally, numerous studies have shown that HBx protein serves as a transactivator in the pathogenesis of HCC through regulating cell transformation, apoptosis and cellular immune system[37-39]. In our study, HBx was identified as the active ingredient of HBV proteins to promote the expression of AMPK and MnSOD. This is consistent with previous investigation reported by Severi et al[32] that HBx expressing cell line is more resistant to ROS-induced cell apoptosis than HBsAg expressing cell line. These data suggest that HBx may alleviate oxidative stress by up-regulating AMPK/MnSOD axis to maintain “normal” live cancer cell functions.
In summary, our current study demonstrates that HBV suppresses mitochondrial superoxide level and exerts an anti-apoptotic effect by activating AMPK/MnSOD signaling pathway in HBV-infected HepG2 cells. These findings may provide a novel mechanism involved in HBV-triggered carcinogenesis, and therefore might be useful in the design of new pharmacological approaches to prevent HCC.
The authors would like to thank the members of Department of Biochemistry, Zhongshan School of Medicine, Sun Yat-sen University, for their technical support.
Chronic hepatitis B virus (HBV) infection is one of the major risk factors for the development of hepatocellular carcinoma (HCC). However, the mechanisms underlying HBV-induced HCC remain ambiguous. Recently, accumulated evidence has shown that escape of apoptosis may contribute to carcinogenesis.
Previous experiments have revealed that liver malignant tumors and patients with HBV-infection express higher protein level of manganese superoxide dismutase (MnSOD) than their counterparts. Here, the authors showed that high expression of MnSOD protected hepatoma cells against apoptosis by detoxing superoxide anion, and conferred a growth advantage to those cells. These results explain how HBV offers a survival benefit to hepatoma cells.
This is the first study to demonstrate that HBV protects hepatoma cells against apoptosis via AMPK/MnSOD signaling pathway. HBV markedly protected the cells from growth suppression and cell death in the condition of serum deprivation. A decrease of superoxide anion production accompanied with an increase of MnSOD expression and activity was found in HepG2.215 cells. Moreover, AMPK activation contributed to the up-regulation of MnSOD. HBx protein was identified to promote the expression of AMPK and MnSOD. These results provide further evidence for the role of HBV as a major cause of HCC development via an anti-apoptosis mechanism involving activation of AMPK/MnSOD signaling pathway.
The present results suggest that HBV suppresses mitochondrial superoxide level and exerts an anti-apoptotic effect by activating AMPK/MnSOD signaling pathway, which may be useful in the design of new pharmacological approaches to prevent HCC.
In this study, Li et al aimed to investigate the anti-apoptotic capability of the hepatitis B virus in the HepG2 hepatoma cell line by suppressing mitochondrial superoxide levels. Generally, their findings seem to be interesting, anyway it should be validated in different cell lines, such as HepG2.117.
P- Reviewer: Gallego-Duran R S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25533] [Article Influence: 1823.8] [Reference Citation Analysis (7)] |
| 2. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] |
| 4. | Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 624] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 5. | Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 546] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA. 2000;97:10872-10877. [PubMed] |
| 7. | Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555-562. [PubMed] |
| 8. | Makin G, Hickman JA. Apoptosis and cancer chemotherapy. Cell Tissue Res. 2000;301:143-152. [PubMed] |
| 9. | Susnow N, Zeng L, Margineantu D, Hockenbery DM. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol. 2009;19:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1269] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 11. | Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401-408. [PubMed] |
| 13. | Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 405] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Skrzycki M, Scibior D, Podsiad M, Czeczot H. Activity and protein level of CuZnSOD and MnSOD in benign and malignant liver tumors. Clin Biochem. 2008;41:91-96. [PubMed] |
| 15. | Oberley LW, Bize IB, Sahu SK, Leuthauser SW, Gruber HE. Superoxide dismutase activity of normal murine liver, regenerating liver, and H6 hepatoma. J Natl Cancer Inst. 1978;61:375-379. [PubMed] |
| 16. | Dhar SK, St Clair DK. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. 2012;52:2209-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 17. | St Clair DK, Porntadavity S, Xu Y, Kiningham K. Transcription regulation of human manganese superoxide dismutase gene. Methods Enzymol. 2002;349:306-312. [PubMed] |
| 18. | Tomita M, Katsuyama H, Okuyama T, Hidaka K, Minatogawa Y. Changes in gene expression level for defense system enzymes against oxidative stress and glutathione level in rat administered paraquat. Int J Mol Med. 2005;15:689-693. [PubMed] |
| 19. | Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120-127. [PubMed] |
| 20. | Dong GZ, Lee JH, Ki SH, Yang JH, Cho IJ, Kang SH, Zhao RJ, Kim SC, Kim YW. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur J Pharmacol. 2014;740:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Gu X, Yao Y, Cheng R, Zhang Y, Dai Z, Wan G, Yang Z, Cai W, Gao G, Yang X. Plasminogen K5 activates mitochondrial apoptosis pathway in endothelial cells by regulating Bak and Bcl-x(L) subcellular distribution. Apoptosis. 2011;16:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am J Physiol Endocrinol Metab. 2011;300:E1135-E1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Yao Y, Li L, Huang X, Gu X, Xu Z, Zhang Y, Huang L, Li S, Dai Z, Li C. SERPINA3K induces apoptosis in human colorectal cancer cells via activating the Fas/FasL/caspase-8 signaling pathway. FEBS J. 2013;280:3244-3255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20:11630-11640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 25. | Liu N, Jiao T, Huang Y, Liu W, Li Z, Ye X. Hepatitis B virus regulates apoptosis and tumorigenesis through the microRNA-15a-Smad7-transforming growth factor beta pathway. J Virol. 2015;89:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Feitelson MA, Reis HM, Tufan NL, Sun B, Pan J, Lian Z. Putative roles of hepatitis B x antigen in the pathogenesis of chronic liver disease. Cancer Lett. 2009;286:69-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Lu X, Lee M, Tran T, Block T. High level expression of apoptosis inhibitor in hepatoma cell line expressing Hepatitis B virus. Int J Med Sci. 2005;2:30-35. [PubMed] |
| 28. | Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881-1896. [PubMed] |
| 29. | Haendeler J, Dimmeler S. Inseparably tied: functional and antioxidative capacity of endothelial progenitor cells. Circ Res. 2006;98:157-158. [PubMed] |
| 30. | Chen DD, Chen AF. CuZn superoxide dismutase deficiency: culprit of accelerated vascular aging process. Hypertension. 2006;48:1026-1028. [PubMed] |
| 31. | Semrau F, Kühl RJ, Ritter S, Ritter K. Manganese superoxide dismutase (MnSOD) and autoantibodies against MnSOD in acute viral infections. J Med Virol. 1998;55:161-167. [PubMed] |
| 32. | Severi T, Vander Borght S, Libbrecht L, VanAelst L, Nevens F, Roskams T, Cassiman D, Fevery J, Verslype C, van Pelt JF. HBx or HCV core gene expression in HepG2 human liver cells results in a survival benefit against oxidative stress with possible implications for HCC development. Chem Biol Interact. 2007;168:128-134. [PubMed] |
| 33. | Cho MY, Cheong JY, Lim W, Jo S, Lee Y, Wang HJ, Han KH, Cho H. Prognostic significance of catalase expression and its regulatory effects on hepatitis B virus X protein (HBx) in HBV-related advanced hepatocellular carcinomas. Oncotarget. 2014;5:12233-12246. [PubMed] |
| 34. | Wang S, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond). 2012;122:555-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Alba G, El Bekay R, Alvarez-Maqueda M, Chacón P, Vega A, Monteseirín J, Santa María C, Pintado E, Bedoya FJ, Bartrons R. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219-225. [PubMed] |
| 36. | Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222-3230. [PubMed] |
| 37. | Zhang WY, Cai N, Ye LH, Zhang XD. Transformation of human liver L-O2 cells mediated by stable HBx transfection. Acta Pharmacol Sin. 2009;30:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58-66. [PubMed] |
| 39. | Wei C, Ni C, Song T, Liu Y, Yang X, Zheng Z, Jia Y, Yuan Y, Guan K, Xu Y. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (1)] |