INTRODUCTION
Wound healing is a complex mechanism which is essential for tissue regeneration. The wound healing cascade includes three main phases, which partly overlap[1-5]. First of all there is the inflammatory phase, which lasts the first few days after wounding. In this phase, neutrophils and macrophages are attracted to the wound and produce among others proinflammatory cytokines and growth factors[6]. Thereafter, angiogenesis starts[7-11] and new collagen fibres are synthesized in the wounded area as main components of the granulation tissue representing the proliferation phase[12-17]. Simultaneously, old collagen fibres are degraded by collagenases namely the matrix metalloproteinases 2 and 9 (MMP-2/-9). Finally, there is the phase of remodeling and maturation of the formatted scar. As the proliferation phase is crucial for the stability of the wounds, impairment of the inflammatory or proliferation phases may result in severe conditions as anastomotic insufficiency.
The mammalian target of rapamycin (mTOR) is a protein kinase that regulates among others protein synthesis and cell proliferation. Its effects are mediated via two different complexes, the mTOR complex 1 (mTOR1) and the mTOR complex 2 (mTOR2). There are inhibitors of mTOR namely sirolimus and its derivate everolimus. Via inhibition of protein synthesis and cell proliferation they act as potent immunosuppressive drugs and are widely used both after bone marrow and solid organ transplantation to prevent graft rejection. However, impairment of wound healing is one major side effect of immunosuppressive drugs like tacrolimus, sirolimus or steroids[18-30]. Especially the mTOR-inhibitors have been shown to impair not only cutaneous wound healing but also have significant effects on the healing of enteral anastomoses[20,21,25-27]. These effects limit the use of the mTOR-inhibitors in the immediate postoperative period after solid organ transplantation as well as they force surgeons to switch immunosuppressive medication from mTOR-inhibitors to another, mTOR-inhibitor-free regimen prior to elective surgery. Recently, it has been demonstrated that the negative effects of everolimus on intestinal wound healing are characterized by a prolongation of the inflammatory phase of the wound healing cascade[31]. This prolongation leads to a delayed initiation of the proliferation phase of the wound healing cascade resulting in a decreased mechanical stability of the anastomoses.
On the other hand, human Growth Hormone (hGH) is an anabolic hormone produced in the pituitary gland. It has been shown previously that hGH improves intestinal wound healing[32-37]. Administration of hGH results in a 30% increase of wound collagen as well as an improved generation of granulation tissue in intestinal wounds[35]. These biochemical findings result in an enhanced intestinal wound healing under medication with hGH[32].
Aim of this animal study was to investigate whether simultaneous administration of hGH and the mTOR-inhibitor everolimus results in an improved intestinal wound healing.
MATERIALS AND METHODS
Animals
Fourty-eight male Sprague-Dawley rats (300-380 g; Harlan Winkelmann, Borchem, Germany) were kept under controlled conditions with constant temperature at 23 °C, constant humidity of 50% and a 12 h light/12 h dark cycle. The animals were acclimatized to the laboratory conditions for at least to weeks prior to the beginning of the experiments. Water and standard rodent laboratory chow (Provimi Kliba SA, Kaiseraugst, Switzerland) were supplied ad libitum. The institutional guidelines of the University of Tübingen for care and use of laboratory animals were followed throughout the study.
Surgical procedure
Rats were anesthetized by an intraperitoneal injection of ketamine (Ketanest, 100 mg/kg; Curamed Pharma, Karlsruhe, Germany) and xylazine (Rompun, 15 mg/kg; Bayer, Leverkusen, Germany). A midline laparotomy was performed, and the descending colon was divided under protection of the mesenteric vessels. Continuity was restored with an end-to-end-anastomosis by using 10 all-layer single-stitch sutures (6/0-Prolene; Ethicon, Norderstedt, Germany). The abdomen was closed in two layers by running sutures (3/0-Ethilon; Ethicon). Postoperatively, all animals had free access to water and chow. All operations were performed by the same surgeon. Postoperative analgesia with carprofen sc twice daily (Rimadyl, 5 mg/kg; Pfizer Animal Health, New York, NY) was administered until the third postoperative day.
Study design
The study protocol was designed to minimize pain or discomfort to the animals. Animals were randomized in three groups with 16 animals each. Group I received placebo, group II received everolimus (RAD001; Novartis Pharma, Basel, Switzerland) in a dosage of 3.0 mg/kg alone and group III received everolimus (3.0 mg/kg) plus human Growth Hormone (hGH, Somatropin, Novo Nordisk Pharma, Mainz, Germany) in a dosage of 2.5 mg/kg. Everolimus was administered daily by gastric gavage, while hGH was administered daily subcutaneously. The first dose was given 7 d prior to surgery, and the medication was continued over a period of 14 d. At day 7 after surgery, rats were sacrificed by intracardiac puncture under isoflurane anesthesia. Relaparotomy was performed and the anastomotic region was resected over a length of 40 mm with the suture line in the middle. Surrounding tissues or adhesions were resected with the anastomosis. The following parameters were assessed in the anastomotic region: anastomotic bursting pressure, histology [hematoxylin and eosin (HE) and Azan staining], quantification of hydroxyproline, immunohistochemical staining for proliferating cell nuclear antigen (PCNA) and for myeloperoxidase (MPO), and zymography for quantification of the matrixmetalloproteinases MMP-2 and MMP-9.
Anastomotic bursting pressure
The resected colon segment with the anastomosis in the middle was cleared carefully of mesenteric fat. Feces were removed, and the segment was washed gently in sterile saline. One end of the bowel was connected to an infusion pump; the other end was connected to a manometer, which registered the increasing intraluminal pressure graphically and numerically. The bowel lumen was then infused with sterile isotonic saline solution at an infusion rate of 1 mL/h. The bursting pressure was defined as the highest pressure that was resisted by the bowel segment[38].
Quantification of hydroxyproline
After the assessment of bursting pressure, the anastomotic segment was bisected longitudinally into two-third and one-third segments as previously described by Agren et al[39]. Punch biopsies (4 mm diameter) were taken from the sutured area of the two-third longitudinal segment and stored in liquid nitrogen or dried for 48 h at 37 °C. Tissue dry weight (DW) was measured, and the content of hydroxyproline was then determined by the method of Woessner, modified by Stegemann and Stalder[40].
Histology and immunohistochemistry
For the histopathologic and immunohistochemical analyses, the one-third longitudinal segment was pinned to a cork plate and immersed into 4% paraformaldehyde overnight at 4 °C, dehydrated in alcohol, and embedded in paraffin. Tissue was cut in 1mm serial cross sections, deparaffinized, and stained with H and E. Cell proliferation was assessed by proliferating cell nuclear antigen antibody (PCNA, Oncogene Science, Uniondale, NY) and inflammatory activity of neutrophil granulocytes by myeloperoxidase antibody (MPO, Dianova, Germany). These assays use the avidin-biotin complex method with 3,3’diaminobenzidine serving as chromagen. Tissue sections were incubated overnight at 4 °C with PCNA or MPO antibody (1:75) followed by incubation with biotinylated secondary antibody as previously described[41].
The analysis of PCNA and MPO positive cells was performed in the granulation tissue of the anastomosis. In total, four high power fields (× 400) of granulation tissue (two at each margin) were examined in every anastomosis. Stainings were evaluated by positive stained cells per area using the Quantimet system (Leica, Jena, Germany). Anastomotic morphology was assessed in H and E-stained slides by standard light microscopy. Analysis was performed semiquantitatively (grade 0-3 for each parameter) under a binocular light microscope (× 400). Histologic parameters were defined as cell density and the amount of granulation tissue in the anastomotic region. Angiogenesis was quantified by the extent of vessel growth at the anastomotic site. Samples were analyzed by an independent blinded investigator.
For the visualization of the collagen fibers, samples from the anastomosis were stained with the Azan method[42]. These samples were also analyzed semiquantitatively by an independent blinded investigator.
Zymography
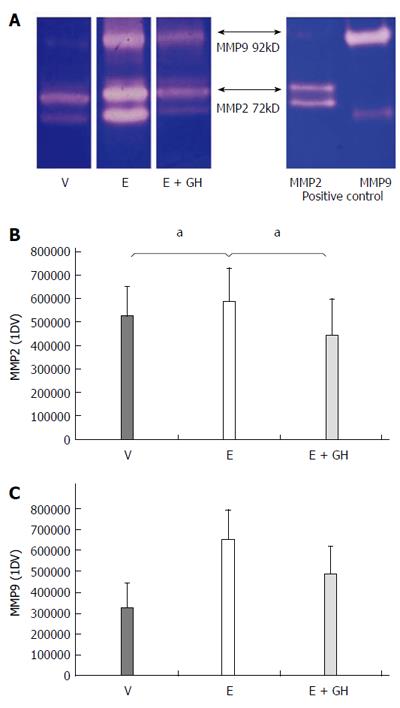
Zymography was performed with equal amounts of pooled anastomotic protein samples from all animals of each group. The presence of MMP-2 and -9 activities was demonstrated by the use of 10% SDS-PAGE containing 10% gelatine (Ready Gel Zymogram; Bio-Rad Laboratories GmbH, Munich, Germany) under nonreducing conditions. Gels were washed twice with 2.5% Triton X-100 (Sigma-Aldrich, Seelze, Germany), incubated for 48 h at 37 °C in incubation buffer (50 mmol/L TRIS pH 7.0, 5 mmol/L CaCl2, 200 mmol/L NaCl, 1 mmol/L ZnCl2, 0.05% Brij35, 0.05% NaN3), and stained with 0.1% Coomassie brilliant blue. Proteolytic activities were visualized by clear zones indicating the lysis of gelatine. The lytic zones on the zymograms were defined as MMP-2 or MMP-9 according to the size standard.
Statistical analysis
Data are expressed as mean ± SD unless otherwise stated. Differences between the groups were calculated by the Mann-Whitney-U test. For multiple comparisons, values were adjusted according to Bonferroni. A P-value < 0.05 was considered significant.
The statistical methods of this study were reviewed by the Institute of clinical epidemiology and applied biostatistics from the University of Tübingen.
RESULTS
All animals survived the procedure and no anastomotic dehiscence or peritonitis were found during re-laparotomy.
Bursting pressure
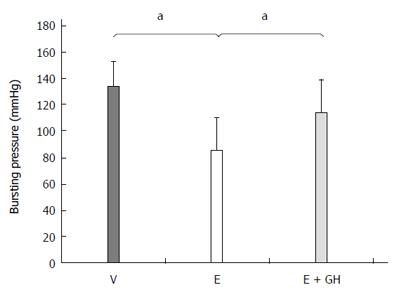
The anastomotic bursting pressure was significantly reduced by everolimus (I: 134 ± 19 mmHg, II: 85 ± 25 mmHg, P < 0.05) (Figure 1). Simultaneous treatment with hGH resulted in a higher bursting pressure (III: 114 ± 25 mmHg). However, this increase was not statistically significantly both compared to groups I and II (P = 0.09, III vs I and III vs II each).
Figure 1 Bursting pressure of the colonic anastomosis (mmHg).
Everolimus treatment decreased the bursting pressure significantly. Additional treatment with human growth hormone (hGH) partial antagonized this effect. V: Vehicle, E: Everolimus 3.0 mg/kg, E + GH: Everolimus 3.0 mg/kg + hGH 2.5 mg/kg; aP < 0.05, V vs E, E vs E + GH.
Histology
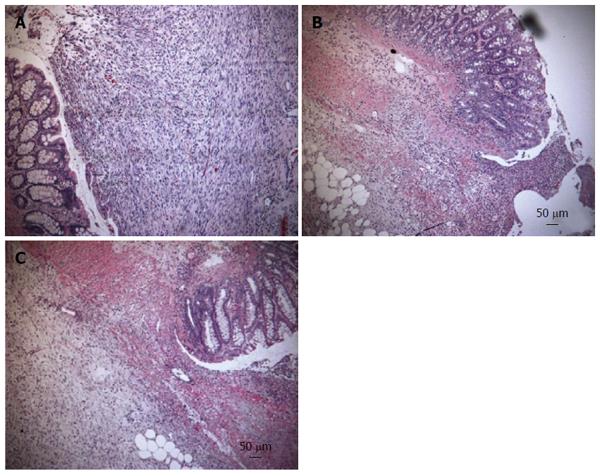
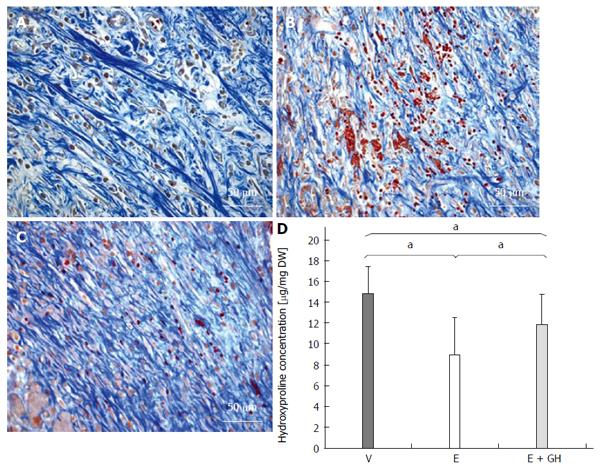
In the H and E stained samples there were significant changes in anastomotical architecture in everolimus-treated animals, which were reduced by simultaneous treatment with hGH (Figure 2A-C). Azan staining revealed a decreased arrangement of the collagen fibres under treatment with only everolimus compared to placebo and simultaneous treatment with hGH (Figure 3A-C).
Figure 2 Hematoxylin and eosin staining from the anastomotic region.
In the hematoxylin and eosin stained samples there were significant changes in anastomotical architecture in everolimus-treated animals, indicated by decreased cell density and reduced angiogenesis (B) which are indicators for granulation tissue. Simultaneous treatment with human growth hormone (hGH) showed more granulation tissue (C). A: Vehicle; B: Everolimus 3.0 mg/kg; C: Everolimus 3.0 mg/kg + hGH 2.5 mg/kg (magnification × 100).
Figure 3 Azan staining from the anastomotic region.
The collagen fibres appear blue. Everolimus treated animals show a disturbed arrangement of collagen fibres, which is antagonized by additional human growth hormone (hGH)-treatment (A-C). A: Vehicle; B: Everolimus 3.0 mg/kg; C: Everolimus 3.0 mg/kg + hGH 2.5 mg/kg. Hydroxyproline concentration in the anastomotic region in mg per mg dry weight (DW). The decrease of hydroxyproline under everolimus-treatment is reduced by additional hGH-treatment (D) (magnification × 400). V: Vehicle, E: Everolimus 3.0 mg/kg, E + GH: Everolimus 3.0 mg/kg + hGH 2.5 mg/kg; aP < 0.05, vs E group, V vs E + GH group.
Anastomotic hydroxyproline content
Everolimus significantly reduced the hydroxyproline content in the anastomotic region, while simultaneous treatment with hGH inverted this negative effect completely (I: 14.9 ± 2.5 μg/mg DW, II: 8.9 ± 3.6 μg/mg DW, III: 11.9 ± 2.8 μg/mg DW, P < 0.05, I vs II and I vs III and II vs III; Figure 3D).
PCNA and MPO immunohistochemistry
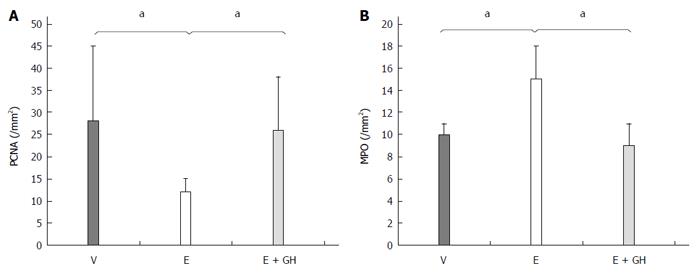
Everolimus decreased PCNA expression significantly compared to placebo treatment (I: 28 ± 3 n/mm², II: 12 ± 3 n/mm², P < 0.05), while simultaneous treatment with hGH antagonized this effect completely (III: 26 ± 12 n/mm², P < 0.05 II vs I and III; Figure 4A). On the other hand hGH lead to normalization of the number of MPO-positive cells compared to only-everolimus-treated animals (I: 10 ± 1 n/mm², II: 15 ± 3 n/mm², III: 9 ± 2 n/mm²; P < 0.05, II vs I and III; Figure 4B).
Figure 4 Immunohistochemistry for PCNA- (A) and MPO- (B) positive cells from the anastomotic region.
Treatment with everolimus alone reduced significantly the amount of PCNA-positive cells in the anastomotic region while increasing the number of MPO-positive cells, indicating a prolonged inflammation. Simultaneous treatment with hGH led to nearly normal values for PCNA- and MPO-positive cells. V: Vehicle, E: Everolimus 3.0 mg/kg, E + GH: Everolimus 3.0 mg/kg + hGH 2.5 mg/kg; V vs E, E + GH vs E, aP < 0.05.
Zymography
Everolimus-treated animals had significantly increased activity-levels of the pro-inflammatory zytokines MMP-2 and MMP-9 in the anastomotic region. Simultaneous treatment with hGH reduced these activity-levels to nearly-normal values (Figure 5A-C).
Figure 5 Zymographic activities of the pro-inflammatory matrix-metalloproteinases MMP2 and MMP9.
Zymography gel showing increased proteolytic activity under treatment with everolimus which is reduced by simultaneous treatment with human growth hormone (hGH) (A). Numeric calculation of the proteolytic activity of MMP2 (B) and MMP9 (C). V: vehicle, E: everolimus 3.0 mg/kg, E+GH: everolimus 3.0 mg/kg + hGH 2.5 mg/kg; V vs E, E + GH vs E, aP < 0.05.
DISCUSSION
Wound healing is a complex biological process which is essential for healing of injured tissue. There are different phases of wound healing finally leading to a stable scar in the wounded tissue. After initial cleaning of the wound by macrophages and neutrophil granulocytes the white cell population shifts to predominantly macrophages, which consume large amounts of oxygen by their respiratory burst which produce proinflammatory cytokines, enzymes (e.g., the matrix-metalloproteinases MMP-2 and MMP-9) and growth factors to remove destroyed collagen from the wounded tissue and to attract cells to the wound which are essential for the proliferation phase[3,43]. This particular step of the healing cascade is defined as the so-called inflammatory phase; its activity peaks in the first 3 to 5 d after wounding[2,44]. Subsequently, healing progresses to the phases of proliferation and tissue remodeling, which are mainly characterized by collagen synthesis by the fibroblasts and new vessel growth to provide enough oxygen to the wound[3,44]. In this phase fibroblasts produce mainly collagen to repair the wound. Moreover, angiogenesis is started to provide enough oxygen to the wound. If these two phases of wound healing are disturbed, it may result in severe wound healing problems which can be devastating in cases of non-healing anastomoses in the gastrointestinal tract. mTOR is a key factor in the regulation of protein synthesis and cell proliferation. Its effects are mediated via complex building with two complexes being involved [mTOR-complex 1 (mTORC1) and mTOR-complex 2 (mTORC2)]. mTORC1 is essential in the regulation of protein synthesis, while the main function of mTORC2 is regulation of the actin cytosceleton. Both, mTORC1 and mTORC2 are activated amongst others by insulin-like growth factor 1 (IGF-1), a growth factor, which is stimulated by the human growth hormone (hGH). IGF-1 acts via the Protein-Kinase B (PKB), also known as Akt, and the corresponding Akt-pathway, resulting in complex-building of mTORC1 and thus activation of protein synthesis. This has been demonstrated in animal studies previously. Christensen et al[34] investigated the effects of hGH on the healing of rodent colon anastomoses in the 1990s[35,45-49]. They found an increased bursting pressure in the hGH-treated animals which showed a significantly increased amount of collagen in the anastomotic region compared to the control groups[35,45,46]. Beckert et al[32] demonstrated in another animal study that administration of hGH leads to increased healing of gastric ulcers in rats compared to the control groups. They showed an increased cell proliferation and angogenesis, both key factors of wound healing[3,44].
On the other hand, mTOR-inhibitors have been demonstrated to significantly disturbe wound healing. Initially there were clinical reports of patients after kidney transplantation who received sirolimus as immunosuppressant drug. These patients had significantly more wound complications than patients with a mTOR-inhibitor-free immunosuppressant regime[21,22,50]. The same was demonstrated later on after liver transplantation[19,20]. van der Vliet et al[27] then showed in 2011 that the hydroxyproline-content in healing colon anastomoses in rats was significantly decreased after treatment with everolimus. With hydroxyproline acting as a progenitor of collagen, this demonstrated for the first time, that mTOR-inihibtors result in a decreased wound strength by reducing protein synthesis. A similar way of action was shown in 2011, when it was demonstrated in the same experimental setting like in this study, that everolimus resulted in a decreased hydroxyproline-content in colonic anastomotic tissue. Furthermore, cell proliferation was reduced as well as angiogenesis while the inflammatory reaction in the anastomosis was increased by means of increased myeloperoxidase (MPO)-positive cells and increased activity of the matrix-metalloproteinases 2 and 9 (MMP2/9) indicating a delayed phase of proliferation[31].
The combination of hGH and everolimus resulted in an improved wound healing compared to everolimus alone. Bursting pressure, content and organization of collagen in the anastomotic tissue, as well as histological parameters like cell density and angiogenesis were significantly improved after combination treatment with hGH and everolimus compared to everolimus alone. All of these factors contribute the proliferation phase of wound healing and were increased, whilst MPO-positive cells and activity of MMP-2 and -9, both markers of the inflammatory phase of wound healing, were reduced by combination treatment of hGH and everolimus. This indicates a partial normalization of wound healing. However, all of these factors were still reduced compared to placebo treatment. One possible explanation is the activation of the Akt-pathway by an IGF-1 synthesis, which is stimulated by hGH. This Akt-pathway leads to an increased forming of the mTOR-complex 1 (mTORC1). On the other hand, everolimus binds to this mTORC1 with high affinity resulting in reduction of the mTORC1-mediated protein-synthesis and cell proliferation. With increased amounts of mTORC1 due to the hGH-treatment, higher doses of everolimus should be needed to produce the same effects as without simultaneous hGH-treatment. However, this has to be investigated in further studies.
In conclusion we could demonstrate for the first time that simultaneous treatment of everolimus and hGH can ameliorate the negative effects of everolimus on intestinal wound healing. However, the exact mechanisms of this partial antagonization remain unclear by now and have to be further investigated.
COMMENTS
Background
Mammalian target of rapamycin (mTOR)-inhibitors are part of standard immunosuppressive medication after both solid organ and bone marrow or stem cell transplantation. The mostly used agents are sirolimus and its derivate everolimus. However, due to severe impairment of wound healing, the definitive mTOR-based immunosuppressive regime is often initiated weeks after transplantation. Moreover, in case of some medical interventions such as surgical procedures the regime has to been switched to an mTOR-inhibitor-free regime with an increased risk of adverse events like graft rejection. To reduce this risk we need to better understand the mechanisms of mTOR-inhibitor-induced impairment of wound healing. One aspect is the delayed inflammatory phase of wound healing resulting in a decreased deposition of wound collagen. So there might be a point of application to reduce this risk.
Research frontiers
The delayed inflammatory phase of wound healing probably is only one mechanism of action of impaired wound healing induced by mTOR-inhibitors as the exact working mechanisms are not known in detail yet. Moreover it is not known yet whether the immunosuppressive potential of the mTOR-inhibitors is caused by the same pathway.
Innovations and breakthroughs
This is the first study which evaluated an approach to counter the negative effects of everolimus on the wound healing and the first time that it was demonstrated that the negative effects of everolimus on wound healing can be antagonized at least partly by another agent.
Applications
The present results might be used to better understand the mechanisms of action of mTOR-inhibitors as well as the basis for further investigation of wound healing under mTOR-inhibitor medication. In the end there is the goal that changing of mTOR-inhibitor-based immunosuppressive regimes is not necessary any more in advance of a planned medical intervention or after an emergency surgical procedure.
Peer-review
The research topic has significant application for immunosuppressant medications in patients undergoing solid organ transplantation