Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4136
Peer-review started: November 26, 2016
First decision: December 30, 2015
Revised: February 1, 2016
Accepted: March 1, 2016
Article in press: March 2, 2016
Published online: April 28, 2016
Processing time: 145 Days and 14.6 Hours
AIM: To investigate the protective efficacy of recombinant adenovirus containing hyper-interleukin-6 (Hyper-IL-6, HIL-6) and hepatocyte growth factor (HGF) (Ad-HGF-HIL-6) compared to that of recombinant adenovirus containing either HIL-6 or HGF (Ad-HIL-6 or Ad-HGF) in rats with acute-on-chronic liver failure (ACLF).
METHODS: The recombinant adenoviruses containing HIL-6 and/or HGF were constructed. We established an ACLF model, and rats were randomly assigned to control, model, Ad-GFP, Ad-HIL-6, Ad-HGF or Ad-HGF-HIL-6 group. We collected serum and liver tissue samples to test pathological changes, biochemical indexes and molecular biological indexes.
RESULTS: Attenuated alanine aminotransferase, prothrombin time, high-mobility group box 1 (HMGB1), endotoxin, tumour necrosis factor (TNF)-α and interferon-γ were observed in the Ad-HGF-, Ad-HIL-6- and Ad-HGF-HIL-6-treated rats with ACLF. Likewise, reduced hepatic damage and apoptotic activity, as well as reduced HMGB1 and Bax proteins, but raised expression of Ki67 and Bcl-2 proteins and Bcl-2/Bax ratio were also observed in the Ad-HGF-, Ad-HIL-6- and Ad-HGF-HIL-6-treated rats with ACLF. More significant changes were observed in the Ad-HGF-HIL-6 treatment group without obvious side effects. Furthermore, caspase-3 at the protein level decreased in the Ad-HIL-6 and Ad-HGF-HIL-6 treatment groups, more predominantly in the latter group.
CONCLUSION: This study identifies that the protective efficacy of Ad-HGF-HIL-6 is more potent than that of Ad-HGF or Ad-HIL-6 in ACLF rats, with no significant side effects.
Core tip: The purport of our study was to analyse the protective efficacy of recombinant adenovirus containing hyper-interleukin-6 (Hyper-IL-6, HIL-6) and hepatocyte growth factor (HGF) (Ad-HGF-HIL-6) compared to that of recombinant adenovirus HIL-6 or HGF (Ad-HIL-6 or Ad-HGF) in rats with acute-on-chronic liver failure (ACLF). In summary, our results suggest that Ad-HGF-HIL-6 may confer a more powerful protective effect against ACLF than do Ad-HGF or Ad-HIL-6 in rats and can restrain the secretion of diverse inflammatory cytokines and reduce the apoptosis of hepatocytes. Ad-HGF-HIL-6 is likely to be a feasible protective therapy for serious liver injury.
- Citation: Gao DD, Fu J, Qin B, Huang WX, Yang C, Jia B. Recombinant adenovirus containing hyper-interleukin-6 and hepatocyte growth factor ameliorates acute-on-chronic liver failure in rats. World J Gastroenterol 2016; 22(16): 4136-4148
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4136
Acute-on-chronic liver failure (ACLF) refers to the patients with chronic liver diseases that have the raised perils of multiple organ failure or death following one or a few precipitating incidents, such as infection or bleeding. It still lacks an effective treatment so far. Viral vectors have been engineered for gene therapy against various types of infectious diseases[1,2]. The adenovirus vector has become the ideal vehicle for liver diseases due to its hepatotropism[3].
Previous studies have reported that IL-6 plays a critical and unique role during the process of early-stage hepatic regeneration response[4]. Hyper-interleukin-6 (Hyper-IL-6, HIL-6) is an artificial protein involving IL-6 connected with a variant of glycoprotein 80 (soluble interleukin 6 receptor, sIL-6R) by an artificial short linker. HIL-6 is a steady protein expressing biological activity dozens of times or even one thousand times stronger than that of IL-6/sIL-6R complex in vitro or in vivo[5]. HGF initiates liver regeneration after liver excision or chemical injuries[6-8]. The coadministration of IL-6 and HGF most effectively raised both the weight of unoccluded lobes and the DNA synthesis of hepatocytes in animals that underwent portal branch ligation (PBL) of the median branches and left lateral[9], suggesting a possible synergistic effect of these two factors. Our study assessed the protective effect of recombinant adenovirus containing HIL-6 and HGF compared to that of recombinant adenovirus containing either HIL-6 or HGF in an ACLF rat model.
HEK (Human Embryonic Kidney) 293 is a cell line derived from human embryonic kidney cells grown in tissue culture. This particular line was initiated by the transformation and culturing of normal HEK cells with sheared adenovirus 5 DNA. HEK293 cells are used to produce viruses for biomedical research purposes. We culture the cell line in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, United States) supplemented with 10% foetal calf serum (Hyclone, Logan, UT, United States). Being cultured at 37 °C in 5% CO2 provided a favorable growth environment for HEK293 cells. Medium was renewed 2-3 times a week, and cell counting was kept between 1 × 105 and 3 × 105 cells per millilitre.
Adenovirus vector GV314 (pDC315-3FLAG-sv40-EGFP) was used in this study. The vector and the primers for amplifying the Hyper-IL-6, HGF or HGF-IRES-Hyper-IL-6 ORF were purchased from Genechem Incorporation, Shanghai, China. pDC315-3FLAG-sv40-HGF-EGFP (Ad-HGF), pDC315-3FLAG-sv40-Hyper-IL-6-EGFP (Ad-HIL-6) and pDC315-3FLAG-sv40-HGF-IRES-Hyper-IL-6-EGFP (Ad-HGF-HIL-6) were constructed as previously described[10]. An ‘‘empty’’ vector pDC315-3FLAG-sv40-EGFP (Ad-GFP) was used as a negative control. The recombinant adenoviruses were greatly propagated with HEK293 cells for enhancing titre. Viruses for animal experimentation were purified by caesium chloride gradients as previously described[11].
Female Sprague-Dawley rats weighing 150-170 g were purchased from the Experimental Animal Centre of Chongqing Medical University (Chongqing, China). Housing conditions were as detailed previously[12]. All rats were given humanitarian care in conformity to the international guidelines.
An ACLF rat model was performed as formerly depicted[13]. Briefly, human serum albumin (HAS; Octapharma m.b.H, Austria) was compounded with physiological saline in the proportion of 8 g/L and then emulsified with incomplete Freund’s adjuvant of an equal amount. The rats received 0.5 mL of the prepared solution by multipoint subcutaneous injections four times in total (The first two times had a 14-d interval, and a 10-d intervals between the latter two). After that, the rats were given 4 mg HSA by tail intravenous injection twice in one week for a total of 6 wk. Finally, we induced ACLF in rats by intraperitoneal injection of D-galactosamine (D-GalN; 400 mg/kg) with lipopolysaccharide (LPS; 100 μg/kg) (Sigma-Aldrich Co., United States).
All the rats were divided into a control group (normal rats, n = 16), a model group (ACLF model rats, n = 16), an Ad-GFP group (Ad-GFP treated ACLF rats, n = 42), an Ad-HIL-6 group (Ad-HIL-6 treated ACLF rats, n = 42), an Ad-HGF group (Ad-HGF treated ACLF rats, n = 42), and an Ad-HGF-HIL-6 group (Ad-HGF-HIL-6 treated ACLF rats, n = 42). Adenoviruses were administered by caudal vein injection at a dose of 1 × 1010 viral particles in 100 μL (diluted with physiological saline) 3 h after the ACLF model had been induced. Meanwhile, the rats of the control and model groups received physiological saline by tail intravenous injection. The time of administration of adenovirus was marked as baseline (0 time point). Rats of all of the groups were sacrificed randomly for hepatic tissue and blood collection after adenovirus or physiological saline had been given for 24 h and 48 h.
An Automatic Hitachi Analyzer (Hitachi Inc., Japan) was utilized to test serum alanine aminotransferase (ALT). We also chose to avail of plasma prothrombin time (PT) to determine liver function. Serum endotoxin was tested with a commercial kit (Houshiji, Xiamen, China) in accordance with the instructions of the kit. ELISA kits (HMGB1 ELISA kit was purchased from Westang Co., China, tumour necrosis factor (TNF)-α and interferon (IFN)-γ ELISA kits were purchased from EBioscience Co., United Kingdom, respectively) were employed to measure serum levels of HMGB1, TNF-α and IFN-γ on the basis of the manufacturer’s instructions.
We used light microscopy to assess the histopathological changes of the liver. Parts of the right lobe of liver specimens were treated with 10% neutral formalin. Paraffin-embedded specimens were cut into 5 μm sections and stained with haematoxylin and eosin (HE). The extent of injury was determined by the criteria as the literature[14] described.
Ki67-related antigen is mainly localized in the nucleus by immunohistochemistry. The proliferation of hepatocytes was evaluated by Ki67-staining. After deparaffinised, sections were incubated in a prediluted monoclonal anti-Ki67 antibody (Roche Ventana) on an automatized medical system (BenchmarkXT, Ventana) utilizing a diaminobenzidin detection kit (Ventana/VIEW 3,30) based on the manufacturer’s instructions.
A detection kit (In Situ Cell Death Kit; Roche Diagnostics GmbH, Mannheim, Germany) was employed for accurate evaluation of the typical biochemical and morphological characteristics of apoptosis. We used proteinase K to treat paraffin-embedded liver sections, and so, hydrogen peroxide hampered the endogenous peroxidase activity. The sections were incubated in a terminal TdT/nucleotide compound at 37 °C for 1 h. Following that, the slides were washed in phosphate-buffered saline. Nuclear labeling was performed with horseradish peroxidase and diaminobenzidine. We performed counterstaining using hematoxylin. The apoptotic cells were observed and photographed under an optical microscope (Positive cells were dyed brown in nuclei.). Apoptosis was determined in eight liver samples of each group by counting 1000 cells from five sections of each sample. The percentage of positive cells was used to present apoptosis rates (%).
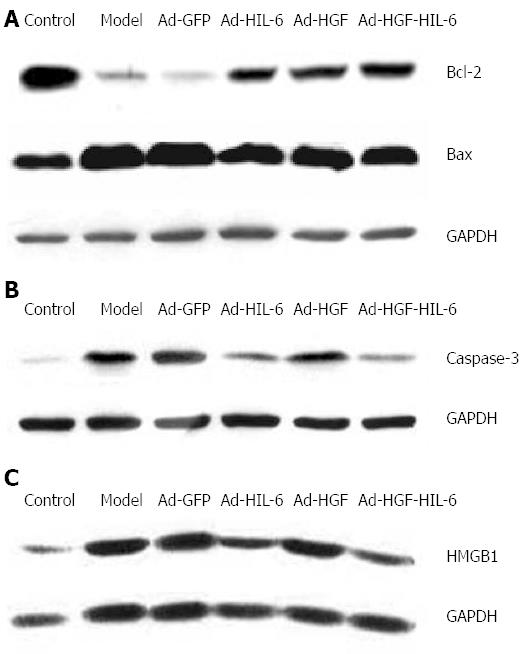
Briefly, proteins extracted from liver samples were subjected to 10% SDS-PAGE, and transferred to PVDF membranes for 2 h. Western blots were then performed in accordance with a method previously described[15]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Co., United States) was used as a control. Anti-HMGB1 antibody was purchased from Abcam Co., United Kingdom; Anti-caspase-3, -Bcl-2 and -Bax antibodies were purchased from CST Co., United States. Finally, an Odyssey infrared imaging system (LI-COR Co.) was used to detect the signals.
Trizol reagent (Invitrogen Co., United States) was used to extract total RNA from liver samples in accordance with the manufacturer’s recommendations. Total RNA was reverse-transcribed employing a reagent kit (PrimeScript RT; TaKaRa Co. Ltd., Dalian, China) in accordance with standard instructions. The expression of the target genes in the liver was detected with SYBR Premix Ex TaqTM (Takara, Otsu, Japan), and a Real-Time PCR system (ABI PRISM 7500; Applied Biosystems, United States) was employed to perform the procedure. Designing specific primers for HIL-6, HGF and β-actin originated from known human sequences (Table 1) was performed using Oligo 7.0 Software. The PCR procedure for HIL-6, HGF and β-actin was composed of 95 °C for 10 min, 40 cycles of 94 °C for 5 s, 55 °C for 30 s and 72 °C for 30 s, and then 94 °C for 15 s. The data were collected automatically using the LightCycler (Roche, Switzerland), and then the value of the threshold cycle (Ct) was analysed on the basis of the 2-ΔΔCt method[16].
| Gene | Primer sequences |
| Hyper IL-6 | Forward: 5'-GTCAGATCTATGCTGGCCGTCGGCTGC-3' |
| Reverse: 5'-CCGGAATTCCTACATTTGCCGAAGAGCCCTC-3' | |
| HGF | Forward: 5’-ATGATGTGGGGGACCAAA-3’ |
| Reverse: 5’-CAACTTGTATGTCAAAATTACTTTGTG-3’ | |
| β-actin | Forward: 5’-TGACGAGGCCCAGAGCAAGA-3’ |
| Reverse: 5’-ATGGGCACAGTGTGGGTGAC-3’ |
The Kruskal-Wallis test was used to analyse histopathological score, Ki67 proliferation index and apoptosis rates (%) in our study. Two-way analysis of variance followed by Tukey’s Honestly Significant Difference (HSD) test for independent samples was adopted to evaluate the other parameters in this study. We used SPSS 19.0 to perform statistical analysis, and regarded a P-value < 0.05 as having statistical significance.
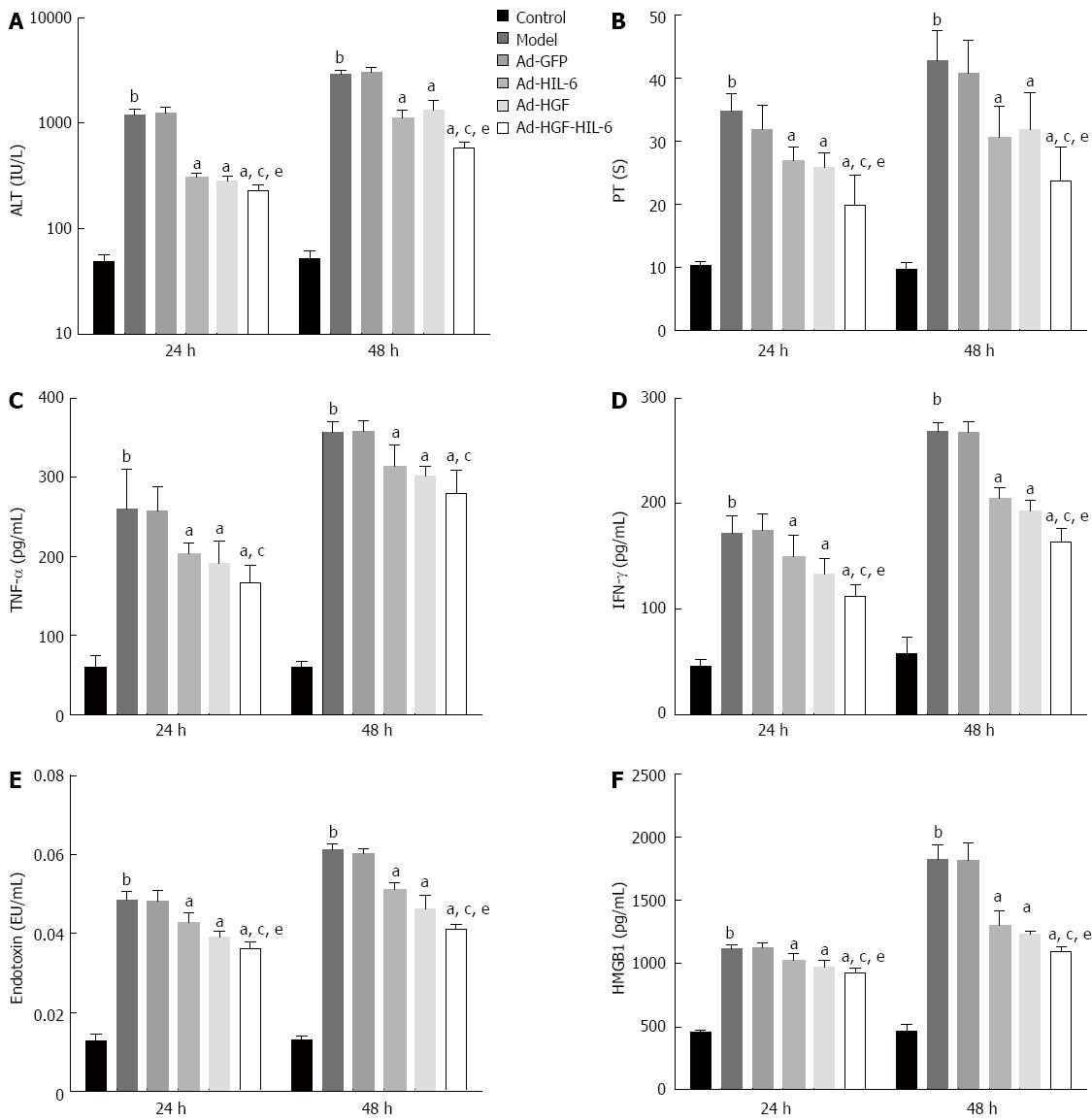
As shown in Figure 1, plasma PT, serum ALT, HMGB1, endotoxin, TNF-α and IFN-γ of the control group were 10.34 ± 0.69 s, 48.88 ± 7.29 IU/L, 454.15 ± 18.45 pg/mL, 0.013 ± 0.001 EU/mL, 59.85 ± 14.45 pg/mL and 46.21 ± 6.18 pg/mL at 24 h, and 9.69 ± 1.10 s, 51.77 ± 9.56 IU/L, 465.76 ± 46.84 pg/mL, 0.013 ± 0.001 EU/mL, 59.84 ± 6.94 pg/mL and 58.20 ± 15.04 pg/mL at 48 h, respectively. Plasma PT, serum ALT, HMGB1, endotoxin, TNF-α and IFN-γ increased significantly in the model group at 24 h and 48 h (24 h: 34.71 ± 2.79 s, 1206.13 ± 154.29 IU/L, 1115.02 ± 33.58 pg/mL, 0.049 ± 0.002 EU/mL, 257.84 ± 29.85 pg/mL and 172.17 ± 16.12 pg/mL, respectively, P < 0.01; 48 h: 42.76 ± 4.77 s, 2889.34 ± 305.46 IU/L, 1817.57 ± 121.50 pg/mL, 0.061 ± 0.002 EU/mL, 356.86 ± 14.34 pg/mL and 268.79 ± 8.34 pg/mL, respectively, P < 0.01) compared to the control group, however, the model group and the Ad-GFP group (Ad-GFP group at 24 h: 31.89 ± 3.88 s, 1255.25 ± 156.52 IU/L, 1121.35 ± 35.94 pg/mL, 0.048 ± 0.003 EU/mL, 260.07 ± 50.23 pg/mL and 174.70 ± 15.99 pg/mL, respectively; 48 h: 40.87 ± 5.13 s, 3034.15 ± 156.52 IU/L, 1810.71 ± 138.75 pg/mL, 0.051 ± 0.001 EU/mL, 357.81 ± 14.17 pg/mL and 267.53 ± 10.68 pg/mL, respectively) showed no statistical discrepancy at the two time points (P > 0.05).
Compared to the Ad-GFP group, plasma PT, serum ALT, HMGB1, endotoxin and IFN-γ were markedly lower in the Ad-HGF group (24 h: 25.95 ± 2.16 s, 285.00 ± 29.05 IU/L, 973.14 ± 46.55 pg/mL, 0.039 ± 0.001 EU/mL, 133.02 ± 15.13 pg/mL, respectively, P < 0.01; 48 h: 31.98 ± 5.79 s, 1342.87 ± 325.09 IU/L, 1233.17 ± 24.63 pg/mL, 0.046 ± 0.003 EU/mL, 193.64 ± 9.16 pg/mL, respectively, P < 0.01), Ad-HIL-6 group (24 h: 26.98 ± 2.22 s, 310.75 ± 24.38 IU/L, 1026.41 ± 49.25 pg/mL, 0.043 ± 0.002 EU/mL, 150.07 ± 20.60 pg/mL, respectively, P < 0.01; 48 h: 30.66 ± 4.87 s, 1127.54 ± 217.88 IU/L, 1303.32 ± 107.31 pg/mL, 0.051 ± 0.002 EU/mL, 205.64 ± 9.18 pg/mL, respectively, P < 0.01) and Ad-HGF-HIL-6 group (24 h: 19.88 ± 4.72 s, 227.38 ± 32.78 IU/L, 925.67 ± 32.95 pg/mL, 0.036 ± 0.002 EU/mL and 111.87 ± 11.24 pg/mL, respectively, P < 0.01; 48 h: 23.77 ± 5.31 s, 578.87 ± 87.47 IU/L, 1093.40 ± 35.86 pg/mL, 0.041 ± 0.001 EU/mL and 163.97 ± 12.89 pg/mL, respectively, P < 0.01) at 24 h and 48 h, and the changes were more predominant in the latter group at two time points (P < 0.05). Serum TNF-α also decreased in ACLF rats with the administration of Ad-HGF-HIL-6 (24 h: 166.43 ± 22.20 pg/mL; 48 h: 279.68 ± 30.31 pg/mL), Ad-HGF (24 h: 191.11 ± 28.30 pg/mL; 48 h: 302.13 ± 12.69 pg/mL) OR Ad-HIL-6 (24 h: 203.76 ± 13.73 pg/mL; 48 h: 313.52 ± 27.39 pg/mL) compared to rats in the Ad-GFP group (24 h: 257.84 ± 29.85 pg/mL, 48 h: 357.81 ± 14.17 pg/mL; P < 0.01) at 24 h and 48 h. A greater effect in reducing serum levels of TNF-α of ACLF rats was observed from the administration of Ad-HGF-HIL-6 compared to Ad-HIL-6 (P < 0.05), but serum TNF-α between the Ad-HGF-HIL-6 group and Ad-HGF group or between the model group (24 h: 260.07 ± 50.23 pg/mL, 48 h: 356.86 ± 14.34 pg/mL) and Ad-GFP group had no significant differences (P > 0.05).
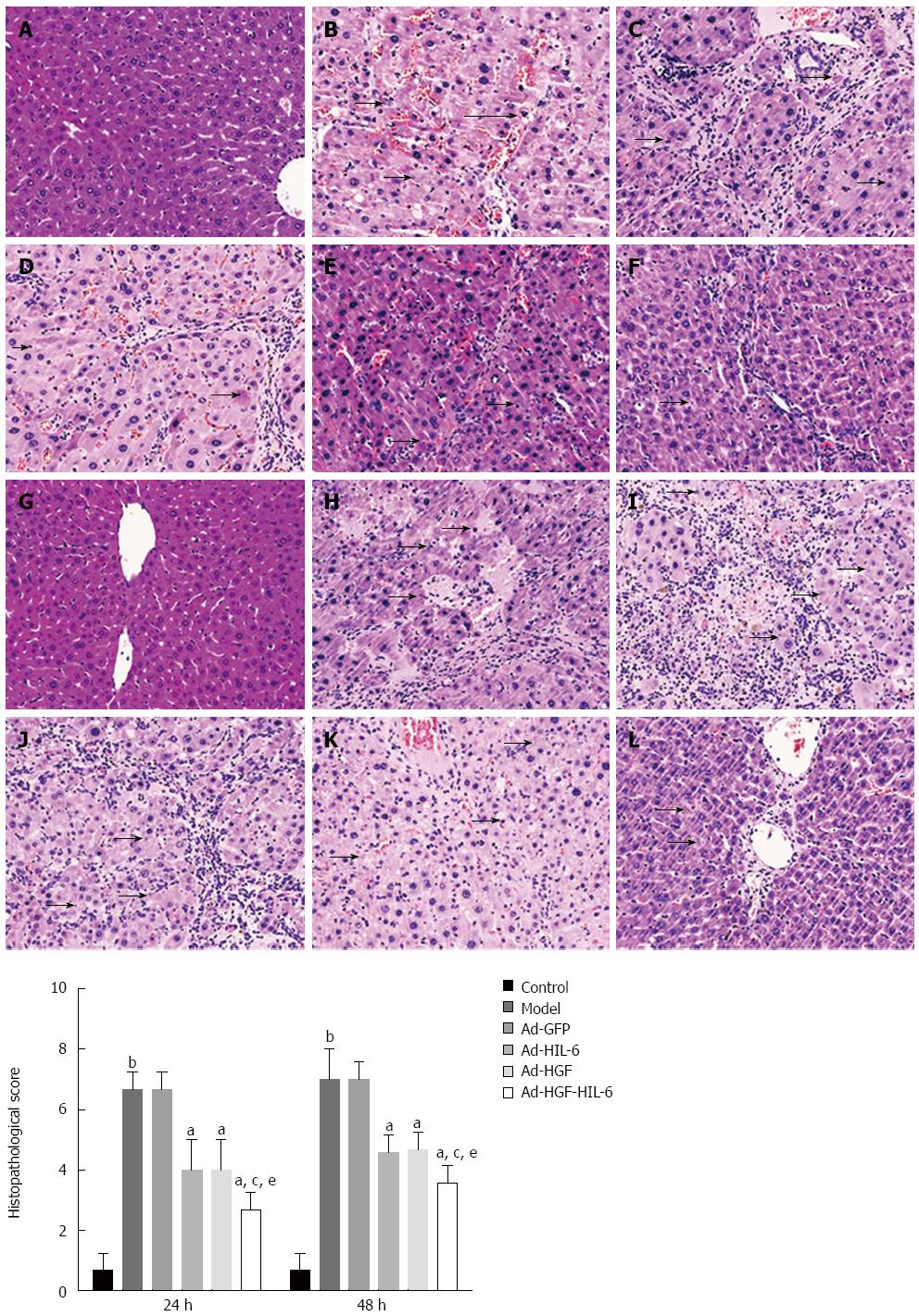
HE staining revealed neither the necrosis nor the degeneration of hepatic lobule in the liver samples of the control group (Figure 2). However, widespread necrosis was detected in the model and Ad-GFP groups, whereas histology of samples was significantly ameliorated in rats of all of the recombinant adenovirus (not including Ad-GFP) treatment groups, which displayed an obvious diminish of inflammatory cell infiltration, amelioration of liver cell swelling, and less pyrenolysis. These changes were more obvious in the Ad-HGF-HIL-6 group. The histopathological score comparison is presented in Figure 2.
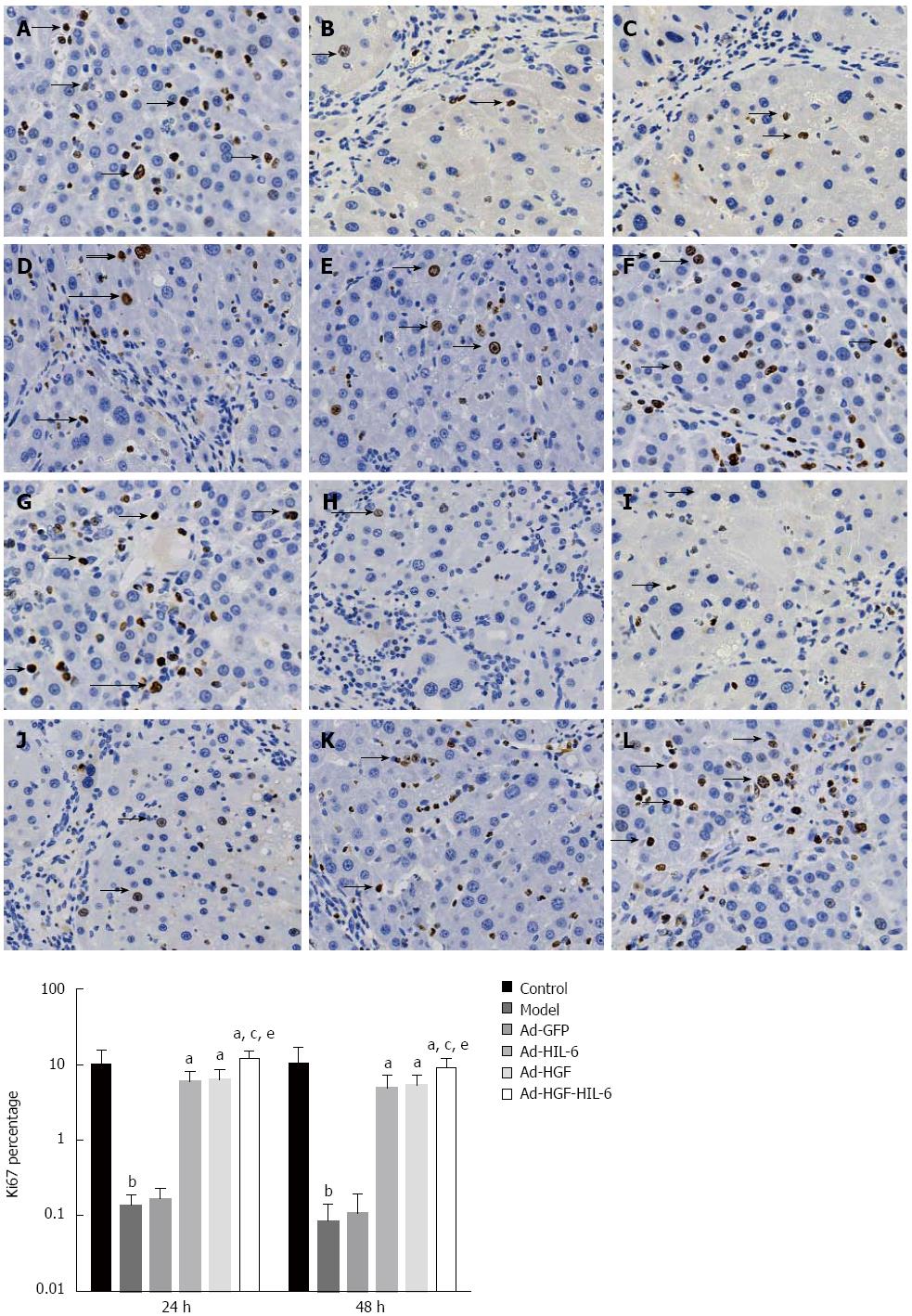
We stained the nuclear Ki67 of hepatocytes (Figure 3). As shown in Figure 3, the expression of Ki67 was higher in the Ad-HGF group (24 h: 6.27%; 48 h: 5.29%), Ad-HIL-6 group (24 h: 5.94%; 48 h: 4.90%) and Ad-HGF-HIL-6 group (24 h: 11.95%; 48 h: 9.10%) compared to the Ad-GFP group (24 h: 0.17% ; 48 h: 0.11%; P < 0.01) at 24 h and 48 h, and the expression of Ki67 was more predominant in the Ad-HGF-HIL-6 group at both two time points (P < 0.05). However, the expression of Ki67 in the model group (24 h: 0.13%; 48 h: 0.09%) had no statistical significance compared to the Ad-GFP group at the two time points (P > 0.05).
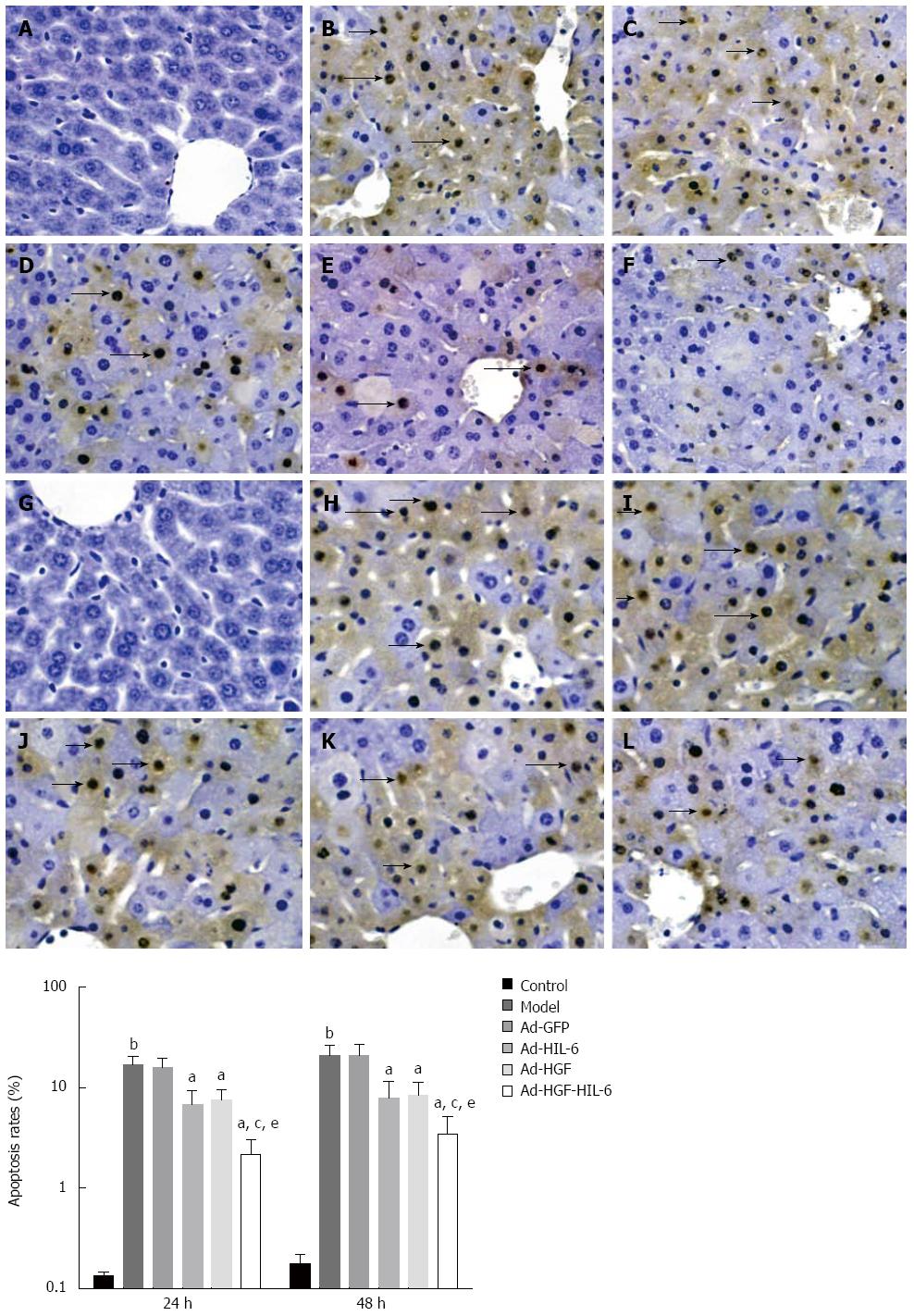
As shown in Figure 4, TUNEL assay displayed that apoptotic cells had irregular and condensed nuclei, and the nuclei was brown-stained. Apoptosis could scarcely ever be detected in the liver tissue slices of the control group, while apoptosis increased in the model and Ad-GFP groups. Recombinant adenovirus (except Ad-GFP) administration dramatically reduced apoptotic rates compared to Ad-GFP.
The percentage of apoptotic hepatocytes significantly increased in the model group (24 h: 16.8%; 48 h: 20.87%) compared to the control group (24 h: 0.14%, 48 h: 0.18%; P < 0.01). In contrast, the apoptotic index markedly decreased (P < 0.01) in the Ad-HGF-HIL-6 (24 h: 2.18%; 48 h: 3.46%), Ad-HIL-6 (24 h: 6.88%; 48 h: 7.98%) and Ad-HGF (24 h: 7.60%; 48 h: 8.35%) groups compared to the Ad-GFP group (24 h: 15.97%, 48 h: 21.13%; P < 0.01). The lowest apoptotic rates were observed in the Ad-HGF-HIL-6 group (P < 0.05). At the detected time points, there were no statistically significances (P > 0.05) in the apoptotic index between the model and Ad-GFP groups.
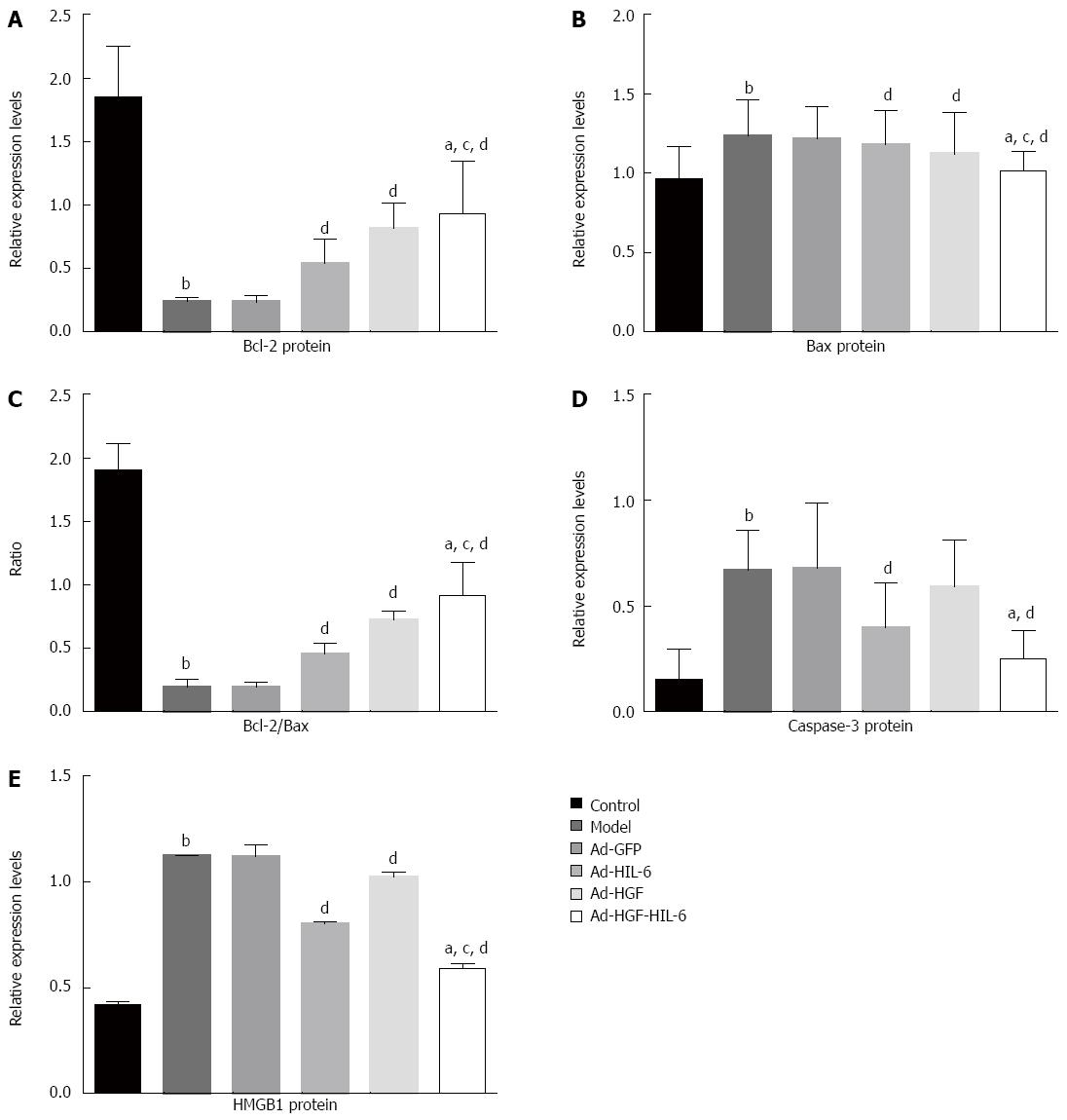
As shown in Figure 5A and B and Figure 6A-D, Bax and caspase-3 proteins in the model group (1.23 ± 0.23; 0.67 ± 0.19) were dramatically enhanced compared to the control group (0.97 ± 0.20, 0.15 ± 0.14; P < 0.01), however, there was no statistically significance between the model group and Ad-GFP group (1.21 ± 0.20; 0.68 ± 0.31, P > 0.05). The Ad-HIL-6 (1.18 ± 0.21; 0.40 ± 0.21) and Ad-HGF-HIL-6 (1.01 ± 0.12; 0.25 ± 0.14) treatments evidently decreased the levels of Bax and caspase-3 proteins compared to Ad-GFP (P < 0.05), and the effect of Ad-HGF-HIL-6 treatment was more obvious (P < 0.05). The levels of Bax protein in the Ad-HGF group were lower than those of the Ad-GFP group (1.12 ± 0.27, P < 0.01) but significantly higher than those of the Ad-HGF-HIL-6 group (P < 0.05). However, Ad-HGF treatment failed to decrease the expression of caspase-3 protein levels (0.59 ± 0.22) compared to Ad-GFP (P > 0.05). Bcl-2 protein of the model group (0.24 ± 0.03) was remarkably lower than that of the control group (1.85 ± 0.40, P < 0.01). The Ad-HIL-6 (0.54 ± 0.19), Ad-HGF (0.81 ± 0.20) and Ad-HIL-6-HGF (0.93 ± 0.41) treatments dramatically enhanced Bcl-2 protein expression compared to Ad-GFP (P < 0.01), and the effect of Ad-HGF-HIL-6 was more obvious (P < 0.05). The trends of Bcl-2/Bax (protein levels; ratio) in each group were consistent with those of Bcl-2. In addition, Bcl-2 protein and Bcl-2/Bax displayed no statistical discrepancy between the model and Ad-GFP groups (P > 0.05).
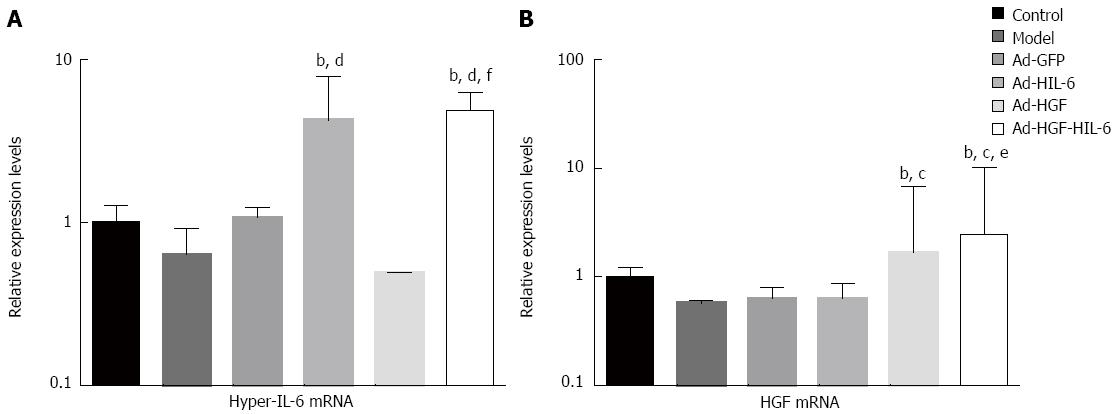
As shown in Figure 7, real-time quantitative PCR revealed that the expression of HIL-6 or HGF mRNA in the liver samples in the Ad-HIL-6 or Ad-HGF group had no significant difference compared to the Ad-HGF-HIL-6 group (HIL-6: 4.27 ± 3.69 vs 4.92 ± 1.34, HGF: 1.68 ± 5.13 vs 2.56 ± 7.79; P > 0.05) and had a very small intersection of false-positive expression in the control (HIL-6: 1.02 ± 0.27, HGF: 1.01 ± 0.21; P < 0.01), model (HIL-6: 0.64 ± 0.28, HGF: 0.57 ± 0.04; P < 0.01), Ad-GFP (HIL-6: 1.08 ± 0.15, HGF: 0.64 ± 0.16; P < 0.01), HIL-6 (HGF: 0.63 ± 0.25; P < 0.01) and HGF (HIL-6: 0.49 ± 0.01; P < 0.01) groups.
As shown in Figure 5C and Figure 6E, the Western blot assay exhibited that the HMGB1 protein expression in liver samples was dramatically enhanced in the model group (1.12 ± 0.00) compared to the control group (0.41 ± 0.01, P < 0.01); however, it was remarkably reduced in the Ad-HGF (1.02 ± 0.02), Ad-HIL-6 (0.80 ± 0.01) and Ad-HGF-HIL-6 (0.59 ± 0.02) groups compared to the Ad-GFP group (P < 0.01), and the change was more considerable in the Ad-HGF-HIL-6 group (P < 0.01). Meanwhile, the HMGB1 protein expression had no statistical significance between the model and Ad-GFP groups (1.12 ± 0.06, P > 0.05).
Various strategies have been attempted in vivo to search for effective ways to treat ACLF[17-21]. In our study, an ACLF rat model was used to perform a comparative study on the efficacy of Ad-HGF, Ad-HIL-6 or Ad-HGF-HIL-6 in ACLF. We demonstrated that Ad-HGF-HIL-6 significantly improved pathological damage compared to Ad-HGF and Ad-HIL-6 in ACLF rats. Meanwhile, greater effects in correcting coagulation and liver function were observed in ACLF rats with Ad-HGF-HIL-6 treatment. Ki67 is an important index for evaluating the regeneration of hepatocytes. At the 24 h and 48 h detection points of our study, Ad-HGF-HIL-6 treatment significantly enhanced the frequency of Ki67+ hepatocytes compared to Ad-HGF or Ad-HIL-6, indicating a better ability to promote hepatocyte regeneration.
The apoptosis of hepatocytes plays a significant part in the development of multifarious liver diseases[22,23]. By reducing the hepatocyte injury that proceeds apoptosis, HGF may prevent or diminish the initiation of the apoptotic cascade[24]. In our study, we also detected that Ad-HGF-HIL-6 remarkably enhanced Bcl-2 (anti-apoptotic) protein expression and apparently reduced Bax (pro-apoptotic) protein expression in hepatocytes of ACLF rats compared to Ad-HGF or Ad-HIL-6. Although both Ad-HGF-HIL-6 and Ad-HIL-6 could conspicuously decrease the activities of caspase-3, the former had a more significant effect. In addition, apoptotic characteristics were viewed by the TUNEL assay of liver tissues. These results displayed that Ad-HGF-HIL-6 could protect the liver of rats against ACLF more effectively by reducing apoptosis.
HMGB1 positively reacts upon various inflammatory diseases[20,25-27]. A protective effect against ACLF is detected by blockading HMGB1 at 24 h since the ACLF model was established[21]. A previous study proved that the high level of HMGB1 plays a considerable part in the pathogenic mechanism of liver failure[28]. Our study found that Ad-HGF-HIL-6 could significantly decrease the serum and tissue HMGB1 concentrations in ACLF compared to Ad-HGF or Ad-HIL-6, suggesting reduced inflammatory response and necrotic hepatocytes. These results further indicate that the mechanism of Ad-HGF-HIL-6 in protecting against ACLF is likely to be related to HMGB1 signalling, which is worthy of future research.
In the progress of ACLF, systemic inflammatory response is tightly related to its prognosis[29]. Anti-TNF treatment protects against experimental hepatic damage due to bacteria endotoxin[30], demonstrating that TNF-α plays a pivotal part in the development of liver failure. In the current research, serum TNF-α in the Ad-HGF, Ad-HIL-6 and Ad-HGF-HIL-6 groups significantly decreased compared with the Ad-GFP group; however, there was no statistical discrepancy between the Ad-HGF-HIL-6 and Ad-HGF treatments at 24 h and 48 h, but these levels were lower than those of the Ad-HIL-6 treatment. TNF-α can mediate apoptosis by interacting with TNF-R1, giving rise to the activation of caspase cascades[21], as confirmed by the similar alteration trend between TNF-α and the apoptotic indicator caspase-3 in this experiment. Similarly, Ad-HGF-HIL-6 inhibited the release of IFN-γ and endotoxin more effectively than did Ad-HGF or Ad-HIL-6, also suggesting the alleviation of the liver inflammatory response.
Our study confirmed the functional synergistic effect of HIL-6 and HGF by combining them into an adenovirus vector. Real-time PCR results suggest that the constructions of recombinant adenovirus were successful, as the expression of the two genes in the liver tissues of ACLF rats was excellent and did not produce mutual interference. Moreover, the enhancement of treatment did not increase immunogenicity or toxicity.
In summary, our results suggest that Ad-HGF-HIL-6 may confer a more powerful protective effect against ACLF than do Ad-HGF or Ad-HIL-6 in rats and can restrain the release of various inflammatory cytokines, reduce the apoptosis of hepatocytes, and protect rats against experimental ACLF even 48 h since ACLF model was established, although the precise molecular events and cell signalling pathways warrant further studies. Our findings indicate that Ad-HGF-HIL-6 is likely to be a feasible and neoteric protective agent for severe liver injury.
Acute-on-chronic liver failure (ACLF) is a life-threatening medical emergency, which is usually associated with a precipitating event and results in high mortality. Therefore, it is urgent to develop new therapeutic reagents for ACLF. Gene therapy by delivering a target gene to the patients via viral and non-viral vectors appears to be a promising approach for the treatment of ACLF. Previous studies have shown that hyper-interleukin-6 (HIL-6), comprising interleukin-6 (IL-6) and the soluble IL-6 receptor, can reduce the necrosis of remnant liver cells during the process of liver failure, and hepatocyte growth factor (HGF) can stimulate the DNA synthesis of hepatocytes, suggesting a possible protective effect of these two factors on liver injury.
IL-6 is the crucial transcriptional factor during the process of early-stage hepatic regeneration response. Hyper-IL-6 is an artificial fusion cytokine comprising IL-6 linked by an artificial linker with a soluble variant of gp80 (sIL-6R). HIL-6 is a stable protein displaying biological activity in vitro or in vivo 10-1000-fold higher than that of IL-6/sIL-6R soluble complex. HGF initiates liver regeneration after liver excision or chemical injuries. The coadministration of IL-6 and HGF most effectively increased both the wet weight of the unoccluded lobes and the hepatocellular DNA synthesis of the animals that underwent PBL of the left lateral and median branches, suggesting a possible synergistic effect of these two factors. In the current study, the authors evaluated the protective efficacy of recombinant adenovirus containing HIL-6 and HGF (Ad-HGF-HIL-6) compared to that of recombinant adenovirus HIL-6 (Ad-HIL-6) or HGF (Ad-HGF) in an ACLF rat model.
ACLF is a condition with acute liver function decompensation secondary to chronic liver diseases. Infectious etiologies constitute the majority of acute insults in the East. In this study, the authors established a rat model of ACLF by immune system-induced liver cirrhosis induced with HSA, and later with D-galactosamine and lipopolysaccharide. Since this practical animal model can well simulate the pathophysiological processes of ACLF, the experimental results of treatment with Ad-HGF-HIL-6 are more persuasive. On the other hand, they sought to deliver two genes by a recombinant adenovirus vector into rats simultaneously but avoiding significant side effects. These findings demonstrated that the protective efficacy of Ad-HGF-HIL-6 is more potent than that of Ad-HGF or Ad-HIL-6 in ACLF rats, with no significant side effects.
These findings indicate that Ad-HGF-HIL-6 is likely to be a potential and novel protective agent for severe liver inflammatory injury.
ACLF refers to an acute deterioration of known or unknown chronic liver disease. It is primarily caused by bleeding and infections, which could result in a series of pathophysiological process, including systemic hemodynamic changes, systemic inflammatory response syndrome, hepatorenal syndrome, and hepatic encephalopathy. IL-6 is a pleiotropic cytokine mediating acute-phase responses, cell regeneration, and transition from innate to acquired immunity. In the classical pathway, IL-6 binds to a membrane bound IL-6R and the complex associates with gp130, for intracellular signalling. Interactions of the IL-6/sIL-6R complex with gp130 provides IL-6 sensitivity to many cell types that do not express IL-6R, thereby expanding biological effects. HIL-6 is a chimera of recombinant human IL-6 bound to IL-6Rα by a short peptide chain. Compared to IL-6/IL-6Rα, HIL-6 has a 10-1000-fold higher receptor binding affinity while the half-life is similar to IL-6. HIL-6 has enhanced and longer activation of mitogen-activated protein kinase pathways. HGF initiates liver regeneration after liver excision or chemical injuries. The coadministration of IL-6 and HGF most effectively increased both the wet weight of the unoccluded lobes and the hepatocellular DNA synthesis of the animals that underwent portal branch ligation of the left lateral and median branches, suggesting a possible synergistic effect of these two factors. In the current study, we evaluated the therapeutic efficacy of recombinant adenovirus containing HIL-6 and HGF compared to that of recombinant adenovirus HIL-6 or HGF in an ACLF rat model.
This is a very well designed research and the literature relating to the principle for using this recombinant adenovirus in the ACLF model in rats to explicate its potential effects has been exhaustively explored. The study is well presented in terms of its aims and purposes. There are confident data to indicate that Ad-HGF-HIL-6 may be explored as a potential therapeutic agent for severe liver inflammatory injury.
P- Reviewer: Grizzi F, Wei D S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Limbach KJ, Richie TL. Viral vectors in malaria vaccine development. Parasite Immunol. 2009;31:501-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18:546-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Kato H, Shimomura T, Murai R, Gonda K, Ishii K, Yoshida Y, Kanbe T, Hashiguchi K, Sakabe T, Takubo K. Regulation of hepatic oval cell proliferation by adenoviral mediated hepatocyte growth factor gene transfer and signal transduction inhibitors. Hepatogastroenterology. 2007;54:821-825. [PubMed] |
| 4. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1205] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 5. | Peters M, Blinn G, Solem F, Fischer M, Meyer zum Büschenfelde KH, Rose-John S. In vivo and in vitro activities of the gp130-stimulating designer cytokine Hyper-IL-6. J Immunol. 1998;161:3575-3581. [PubMed] |
| 6. | Makino H, Shimizu H, Ito H, Kimura F, Ambiru S, Togawa A, Ohtsuka M, Yoshidome H, Kato A, Yoshitomi H. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol. 2006;12:2053-2059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Hamada T, Eguchi S, Takatsuki M, Yamanouchi K, Sugiyama N, Kawashita Y, Okudaira S, Tajima Y, Ishii T, Kanematsu T. Low-dose recombinant human hepatocyte growth factor enhances effect of hepatocyte transplantation in rats treated with retrorsine. Hepatogastroenterology. 2009;56:1466-1470. [PubMed] |
| 8. | Franco-Gou R, Roselló-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, Bartrons R, Peralta C. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Kaido T, Oe H, Imamura M. Interleukin-6 augments hepatocyte growth factor-induced liver regeneration; involvement of STAT3 activation. Hepatogastroenterology. 2004;51:1667-1670. [PubMed] |
| 10. | Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. Adv Pharmacol. 1997;40:137-206. [PubMed] |
| 12. | Balasubramaniyan V, Wright G, Sharma V, Davies NA, Sharifi Y, Habtesion A, Mookerjee RP, Jalan R. Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G145-G152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Liu XH, Chen Y, Wang TL, Lu J, Zhang LJ, Song CZ, Zhang J, Duan ZP. [Establishment of a D-galactosamine/lipopolysaccharide induced acute-on-chronic liver failure model in rats]. Zhonghua Gan Zang Bing Za Zhi. 2007;15:771-775. [PubMed] |
| 14. | Ni HM, Chen X, Ding WX, Schuchmann M, Yin XM. Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice. Am J Pathol. 2008;173:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Takahashi K, Imaeda H, Fujimoto T, Ban H, Bamba S, Tsujikawa T, Sasaki M, Fujiyama Y, Andoh A. Regulation of eotaxin-3/CC chemokine ligand 26 expression by T helper type 2 cytokines in human colonic myofibroblasts. Clin Exp Immunol. 2013;173:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133956] [Article Influence: 5581.5] [Reference Citation Analysis (1)] |
| 17. | Hu C, Shen S, Zhang A, Ren B, Lin F. The liver protective effect of methylprednisolone on a new experimental acute-on-chronic liver failure model in rats. Dig Liver Dis. 2014;46:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Wang H, Bao S, Tabassam F, Cai W, Xiang X, Zhao G, Wu H, Gao T, Li H. Amelioration of liver injury by continuously targeted intervention against TNFRp55 in rats with acute-on-chronic liver failure. PLoS One. 2013;8:e68757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yang F, Li X, Wang LK, Wang LW, Han XQ, Zhang H, Gong ZJ. Inhibitions of NF-κB and TNF-α result in differential effects in rats with acute on chronic liver failure induced by d-Gal and LPS. Inflammation. 2014;37:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Wang LW, Wang LK, Chen H, Fan C, Li X, He CM, Gong ZJ. Ethyl pyruvate protects against experimental acute-on-chronic liver failure in rats. World J Gastroenterol. 2012;18:5709-5718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Li X, Wang LK, Wang LW, Han XQ, Yang F, Gong ZJ. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm Res. 2013;62:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Tsamandas AC, Thomopoulos K, Zolota V, Kourelis T, Karatzas T, Ravazoula P, Tepetes K, Petsas T, Karavias D, Karatza C. Potential role of bcl-2 and bax mRNA and protein expression in chronic hepatitis type B and C: a clinicopathologic study. Mod Pathol. 2003;16:1273-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Zhang M, He W, Liu F, Zou P, Xiao J, Zhong ZD, Hu ZB. Inhibition of mouse hepatocyte apoptosis via anti-Fas ribozyme. World J Gastroenterol. 2004;10:2567-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Thatch KA, Schwartz MZ, Yoo EY, Mendelson KG, Duke DS. Modulation of the inflammatory response and apoptosis using epidermal growth factor and hepatocyte growth factor in a liver injury model: a potential approach to the management and treatment of cholestatic liver disease. J Pediatr Surg. 2008;43:2169-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 845] [Cited by in RCA: 939] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 26. | Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M, Yamada S, Hashimoto S, Fukata S, Abraham E. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Yang R, Miki K, Oksala N, Nakao A, Lindgren L, Killeen ME, Mennander A, Fink MP, Tenhunen J. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am J Physiol Regul Integr Comp Physiol. 2009;297:R362-R369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2010;9:499-507. [PubMed] |
| 29. | Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |