Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.72
Peer-review started: May 8, 2015
First decision: August 25, 2015
Revised: September 24, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 7, 2016
Processing time: 245 Days and 20.5 Hours
Liver cirrhosis is the common endpoint of many hepatic diseases and represents a relevant risk for liver failure and hepatocellular carcinoma. The progress of liver fibrosis and cirrhosis is accompanied by deteriorating liver function. This review summarizes the regulatory and functional changes in phase I and phase II metabolic enzymes as well as transport proteins and provides an overview regarding lipid and glucose metabolism in cirrhotic patients. Interestingly, phase I enzymes are generally downregulated transcriptionally, while phase II enzymes are mostly preserved transcriptionally but are reduced in their function. Transport proteins are regulated in a specific way that resembles the molecular changes observed in obstructive cholestasis. Lipid and glucose metabolism are characterized by insulin resistance and catabolism, leading to the disturbance of energy expenditure and wasting. Possible non-invasive tests, especially breath tests, for components of liver metabolism are discussed. The heterogeneity and complexity of changes in hepatic metabolism complicate the assessment of liver function in individual patients. Additionally, studies in humans are rare, and species differences preclude the transferability of data from rodents to humans. In clinical practice, some established global scores or criteria form the basis for the functional evaluation of patients with liver cirrhosis, but difficult treatment decisions such as selection for transplantation or resection require further research regarding the application of existing non-invasive tests and the development of more specific tests.
Core tip: Liver cirrhosis is a common endpoint for many hepatic diseases and is accompanied by the extensive gene regulation of cytokines and enzymes for hepatic metabolism. The resulting organ deficiency complicates treatment decisions, especially regarding transplantation and the resection of hepatocellular carcinoma. This review summarizes the regulatory events involving the metabolism in the cirrhotic liver and puts these events into the context of the non-invasive testing of liver function. This combination can help to better estimate the liver function of individual patients.
- Citation: Dietrich CG, Götze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol 2016; 22(1): 72-88
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/72.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.72
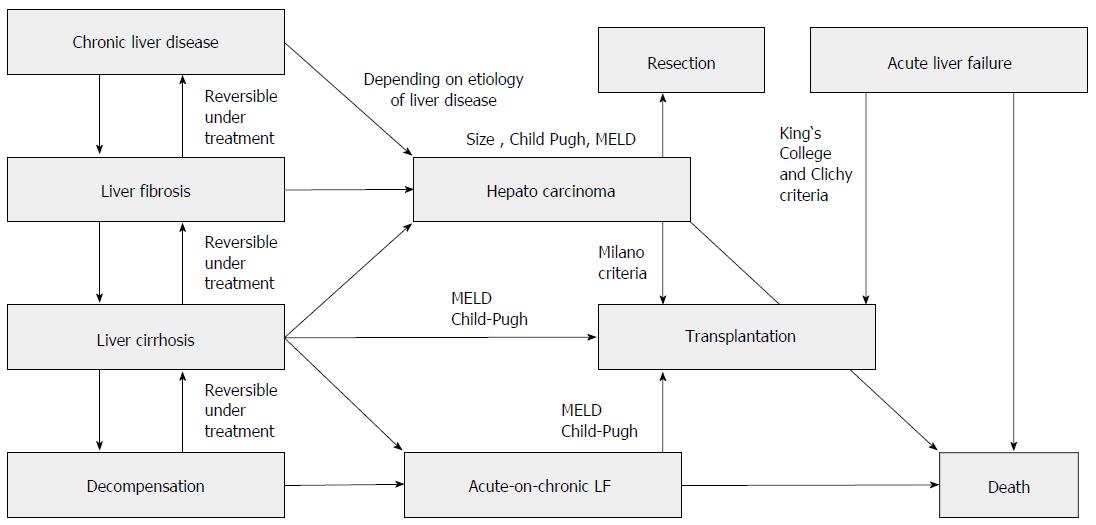
Liver cirrhosis is the common final pathway of inflammatory liver diseases of different origins. In general, it takes many years or even decades to develop the full picture of liver cirrhosis that is associated with the complete destruction of liver architecture (represented by the liver lobes) through bridging fibrosis. Liver cirrhosis per se is the main risk factor for hepatocellular carcinoma and leads, if the underlying disease is not treated adequately, to chronic liver failure. Other pathways to liver failure include acute and acute-on-chronic liver failure (Figure 1). Liver failure of various origins necessitates the transplantation of 5500 livers per year in Europe[1].
Chronic hepatitis B and C and alcoholic and non-alcoholic steatohepatitis are quantitatively the most important causes of cirrhosis, the first mainly in sub-Saharan Africa and most parts of Asia and the latter three in more developed countries in Europe and North America[2]. Other less frequent causes of liver cirrhosis comprise autoimmune diseases such as autoimmune hepatitis, primary biliary cirrhosis or primary sclerosing cholangitis and hereditary entities such as Wilson disease, hemochromatosis and alpha-1-antitrypsin-deficiency. Vascular diseases such as Budd-Chiari syndrome or Osler disease rarely cause liver cirrhosis, while right-heart failure is likely a more frequent cause than is commonly assumed[3]. Finally, drug-induced liver injury[4], recurrent biliary obstruction and rare metabolic disorders such as porphyria can lead to liver cirrhosis and decompensation[5].
The development of cirrhosis is a continuous process from inflammation to fibrosis and ultimately cirrhosis and is complicated by decompensation, liver failure and/or hepatocellular carcinoma (Figure 1). It is accompanied by molecular changes in the hepatocytes and other liver cells modulating the inflammatory and fibrosing process itself that also influence the metabolism of endo- and xenobiotics as well as the synthesis of liver-derived proteins[6]. These changes are the result of the up- or down-regulation of the respective genes in the liver cell or changes in translational mechanisms in the cell. Preliminary data from microarray analysis imply that there are distinct molecular differences between the different etiologies of cirrhosis[7,8]. Research over the past 20 years has been focused on examining molecular mechanisms in the liver for the development of methods to block or retard the development of cirrhosis but has also focused on molecular changes in hepatic metabolism to identify additional risks elicited by insufficient liver function. The limited function of the liver in cirrhosis has significant importance and limits therapeutic options in not only chronic liver disease but also in 70% of all hepatocellular carcinomas (intermediate and advanced stages). The molecular changes in cirrhosis can alter the transport and metabolism of drugs and carcinogens as well as endogenous metabolic intermediates and therefore lead to a higher risk of side effects, drug interactions and genotoxic effects as well as to changes in glucose or lipid metabolism. Many previous studies have used animal models (see below), with unclear transferability to humans. Human studies are scarce and mostly include small sample sizes. This review summarizes the existing knowledge and puts the results into the clinically relevant framework of non-invasive metabolic tests and their application in the clinical routine.
Experimental liver cirrhosis has been induced by bile duct ligation, toxic compounds such as carbon tetrachloride and the generation of fatty liver for almost a century[9-11]. Over the years, various animal models have been developed in rodents that closely reflect relevant human disease entities and their unique differences[12,13]. The pattern of hepatic fibrosis varies with the model used.
Carbon tetrachloride (CCl4) is one of the oldest and most widely used toxins for the experimental induction of liver fibrosis in laboratory animals but does not currently resemble a clinically relevant human disease[10,14]. Alternatively, thioacetamide can be used as a supplement to the drinking water to induce severe toxic bridging fibrosis[12,13]. Diethylnitrosamine induces toxic fibrosis that mechanistically resembles CCl4-induced fibrosis. As rodents develop malignancies under treatment with this carcinogen, it is best for those studying fibrosis in the context of hepatocellular carcinoma (HCC)[13].
Several models have been developed to represent biliary fibrosis and cirrhosis. These include surgical common bile duct ligation, which leads to cirrhosis with signs of portal hypertension and ascites[13]. Genetic alterations of the biliary transporters involved in bile formation are reflected by mice with targeted disruption of the Mdr2 (Abcb4) gene, encoding a canalicular phospholipid flippase (Mdr2-/- mice) that spontaneously develops sclerosing cholangitis with macroscopic and microscopic features of human primary sclerosing cholangitis (PSC) and serves as a model for biliary liver cirrhosis[15,16]. Immunological models include a range from simple heterologous serum application to sophisticated knockout models of autoimmune liver disease[17]. Beyond autoimmune hepatitis models, different spontaneous autoimmune biliary disease mouse models, including the interleukin (IL)-2Rα-/- mouse model, have been reported[18].
Non-alcoholic fatty liver disease (NAFLD)-associated cirrhosis currently represents the most frequent liver disease in developed countries. Various rodent models for fatty liver-derived fibrosis are available either nutritionally or based on a genetic modification[19-22]. Among the most widely used models for different stages of the metabolic syndrome including NAFLD are ob/ob and db/bd mice with genetic alterations in the leptin/leptin receptor pathway and feeding models with high fat (or high fat high fructose). In contrast, the methionine-choline-deficient diet model does not resemble typical human NAFLD in the context of the metabolic syndrome but rather full-blown non-alcoholic steatohepatitis (NASH) with peripheral cachexia[20]. Animal models of alcohol-induced liver disease include a range of different exposure modalities from acute binge ethanol feeding to chronic ethanol feeding (Lieber-DeCarli model)[23].
Genetically humanized mouse models for hepatitis C virus infection are just emerging and have their focus on the immunological pathogenesis rather than on the induction of advanced fibrosis[24]. The same is true for hepatitis B models, which provide clues for understanding host-virus immunologic interactions rather than serve as a disease-specific fibrosis model[25].
As human studies providing data on molecular changes in hepatic metabolism and transport in cirrhosis are very scarce, data from animal models of cirrhosis have been incorporated into this work as far as these models adequately reflect human disease counterparts.
The human Cytochrome P450s are more than 50 oxygenases present mainly in the liver and the intestine, divided into several families with different substrate specificities. CYP P450 families 1-3 are most important for the metabolism of xenobiotics, including drugs and carcinogens, while the other families catalyze the metabolism of endogenous substrates. A comprehensive review of the structure and function of the CYPs can be found elsewhere[26]. It is very important to note that the CYP450s not only catalyze the detoxification of drugs or carcinogens, but can also promote their carcinogenicity by activating such compounds via hydroxylation[27]. Changes in the gene regulation or functional activity of the CYP450s therefore do not have a uniform effect on xenobiotics.
Additionally to this central role of hepatic CYPs in the handling of xenobiotics, the rate-limiting step of bile acid synthesis is mediated by the liver-specific CYP 7A1. Thus, changes in CYP gene regulation also have an impact on bile acid synthesis and lipid metabolism.
CYP isoforms are intensely regulated by nuclear receptors aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane X receptor (PXR) and peroxisome proliferator-activated receptors (PPAR) in coordination with phase II enzymes and transporters[28]. Not unexpectedly, inter-individual variations in gene expression are much higher in human samples than in animal livers[29]. Additionally, end-stage livers often exhibit even higher variation than normal livers[30]. Depending on the study design, conflicting data exist regarding the regulation of CYP isoforms in cirrhosis.
In an animal study from 30 years ago, rats with biliary cirrhosis (35 d of bile-duct ligation) showed a reduction in the total protein mass of cytochrome P450 by 45%[31]. In the same study however, toxic post-necrotic cirrhosis in rats (induced by CCl4) did not lead to a change in the CYP450 protein mass in the diseased livers. This result is in line with recent data regarding non-ascitic animals with cirrhosis, while ascitic cirrhotic rats (assuming a later cirrhosis stage for those animals) had a significant decrease in the total CYP450 protein mass in this newer study[32]. Certainly the differential regulation of specific isoforms of CYP450 contributes to unchanged total protein mass in cirrhosis. CYP450 11B2, for example, was upregulated in rats with fibrotic liver due to CCl4[33].
In (uninduced) rats, CYP 1A1 is virtually absent, while CYP 1A2 is abundant[32]. Basically, the CYP 1A2 protein mass was decreased to approximately half in cirrhotic rats without ascites; this reduction was intensified to less than 20% in rats with ascites, indicating a dependency on the cirrhosis stage[32]. The mRNA levels correlated well with the protein amount in this study. Interestingly, treatment with benzo[a]pyrene led to the normalization of CYP 1A2 mRNA and protein mass in cirrhotic rats without ascites, while in rats with ascites, a significant decrease remained (albeit lower than in uninduced animals). The same results were obtained for CYP 1A1 in these animals, and all these changes in regulation correlated well with AhR mRNA as well as protein expression[32]. Practically, this means that the ability to have microsomal oxidation (accompanied by possible molecular activation) of carcinogens is preserved in compensated liver cirrhosis (in rats).
Human studies began 20 years ago, when an analysis of 50 end-stage livers showed the differential regulation of CYP isoforms, with the clear basal downregulation of CYP 1A2 as one of the most important activators of xenobiotic carcinogens. In contrast, the CYP 3A protein mass (as the most important isoform for drug metabolism) was only slightly decreased, reaching significance only in non-cholestatic cirrhosis due to hepatocellular diseases, and CYP 2E1 was significantly reduced only in cholestatic cirrhosis[34]. This result implies an interesting isoform-specific regulation in different subforms of cirrhosis. The mechanisms of regulation were diverse and were not restricted to classic translational regulation[35]. These results question an interpretation of gene regulation that rests solely upon mRNA analysis. Very recently, a clear correlation of midazolam clearance (as a surrogate for CYP 3A activity) with the Child Pugh and model of end stage liver disease (MELD) score was shown in 24 patients with end-stage liver disease without any differentiation between cholestatic and non-cholestatic types[36]. Likewise, CYP 1A2, 2C19, 3A4 and 2E1 mRNA were negatively correlated with increasing liver stiffness in additional studies analyzing patients with alcoholic fibrosis[37] or viral hepatitis[38,39]. Combined, these data support the hypothesis that the expression and function of many CYP isoenzymes declines with cirrhosis progression.
In Chinese patients with cirrhosis based on hepatitis B, CYP 1A2 activity measured via phenacetin metabolism was decreased by 91%[40]. This result was accompanied by unchanged sulfotransferase (SULT) 1A1 activity in 159 cirrhotic patients, while a subgroup of 46 patients exhibited normal CYP 1A2 activity and elevated SULT 1A1 activity. Interestingly, in a 2 year follow up, three patients from this latter group, but none from the first group, developed HCC, indicating a higher carcinogenic risk with preserved CYP 1A2 activity[40]. These results may offer a chance for identifying a subgroup of cirrhotic patients prone to HCC but were obtained in a simple metabolism analysis of phenacetin without any determination of the molecular expression levels. Even if these results fit with the theoretical considerations regarding the importance of CYP 1A2 in the activation of carcinogens, they should be confirmed in further studies.
In another Chinese study with human end-stage liver disease samples, almost all CYP isoforms (and especially 1A2, 2E1, 2C19, 3A4 and 4A11) were down-regulated to various degrees[30]. This result was accompanied by the down-regulation of the nuclear receptor PPAR, while CAR, PXR and AhR expression was preserved. The data regarding metabolic enzymes are in line with another study already cited above showing the significant down-regulation of CYP 1A2, 2C19 and 2E1 in advanced fibrosis and cirrhosis in patients with viral hepatitis[39]. However, in this study, CAR, PXR and AhR expression were reduced as well, significantly in contrast to the study above. In both studies, the analysis was restricted to mRNA data, while protein expression and functional data were lacking[30,39].
The down-regulation of CYP 2E1 mRNA in cirrhosis, including alcoholic cirrhosis[30], is interesting as this isoform is up-regulated in acute and chronic alcohol consumption and is implicated in the genesis of alcoholic liver damage due to its ability to produce ROS[41,42]. Obviously, cirrhosis as the end stage of alcoholic disease evens this up-regulation into a more dominant general regulatory event associated with severe cell damage.
CYP 7A1 as the rate-limiting enzyme of bile acid synthesis was shown to be up-regulated in primary biliary cholangitis (PBC) patients, but when determined in end-stage patients with PBC (defined as Child Pugh class C), the investigators observed the significant mRNA down-regulation of this enzyme[43]. This mechanism of late down-regulation may be associated with protective regulation events in liver cirrhosis (avoidance of intracellular bile acid accumulation in hepatocytes) and is confirmed by an additional study[44]. Interestingly, in this latter study, CYP 8B1 as well as CYP 27 mRNAs were preserved, and CYP 3A4 mRNA was only mildly reduced. PBC patients with a certain CYP 7A1 polymorphism leading to higher protein expression of this isoform in hepatocytes were at risk for a rapid PBC progression in a study of more than 300 Japanese patients[45].
In vitro studies in human hepatocytes showed that PXR not only induces CYP 3A4 in normal cells but also mediates the IL-6-induced repression of this CYP isoform[46]. These results may confer an explanation of CYP 3A4 down-regulation in an IL-6 productive state, such as chronic inflammation and liver cirrhosis, but it needs confirmation in in vivo studies. A recent human study implies that microRNA-155, a known regulator of liver inflammation, may contribute to lower CYP 3A4 activity in liver cirrhosis[47], but the data presented in this study are merely descriptive and lack a clear mechanistic explanation.
The effect of liver fibrosis or cirrhosis on CYP450 expression and function outside the liver remains controversial. In a human study with 23 patients with various degrees of cirrhosis, duodenal CYP 3A expression and total midazolam hydroxylation were both reduced to less than 50% of normal control patients[48]. In a pharmacokinetic animal study of ofloxacin, the authors found increased CYP enzyme activity in cirrhotic rats (CCl4, ethanol and high fat) as a reason for prolonged and reduced bioavailability of the test substance[49]. It must be clarified in additional studies whether these conflicting data are species-specific or relate to the different study designs.
In summary, most CYP isoforms are reduced in expression and activity in advanced fibrosis and cirrhosis. This especially holds true for the important isoenzymes 1A2, 2E1 and 3A4 (for exogenous compounds) but also for CYP 7A1 (for bile acids). One important problem in many studies is that only mRNA data are available for the respective animal model or the human liver disease. Additionally, even protein expression data do not necessarily reflect the enzyme activity in vivo in advanced liver disease (see also below).
Phase II in metabolism of xenobiotics is conferred by several groups of enzymes, the most important of which are uridine diphosphate (UDP)-glucuronosyltransferases (2 families with more than 20 isoforms[50]) and sulfotransferases (13 isoforms in 4 groups[51]). The ultimate goal of phase II metabolism is the solubilization of metabolites in water and thereby the potential for excretion in urine and bile. Nevertheless, sulfatation by sulfotransferases can also potentiate the genotoxic effect of a certain carcinogen. In general however, these metabolites are no longer toxic or carcinogenic, and therefore phase II -metabolism is the final step of detoxification (before transport into urine or bile). Not surprisingly, both groups of enzymes are regulated coordinately by the nuclear receptors AhR, CAR, PXR and PPAR[28,52] together with phase I and transporters, and this principle also holds for human liver disease[39]. In the latter study, coordination between nuclear receptors and metabolic enzymes was even stronger in severe liver disease (METAVIR score 3-4 in patients with mainly HCV) than in mild liver disease, indicating increasing cross-talk between transcription factors[39]. Human SULT 2A1, an important sulfotransferase for endogenous compounds including bile acids, has been shown to be regulated by the retinoid-related orphan receptors RORα and β[53]. For UDP-glucuronosyltransferase (UGT) 1A7, a clear association of low-activity genotypes with cirrhosis, functional hepatic impairment and HCC was shown[54], indicating the importance of detoxification by this isoenzyme in the pathophysiology of chronic liver disease.
Increasing amounts of deposited cholesterol in the livers of rats fed with high cholesterol diet, with final development of fibrotic steatohepatitis, led to a progressive down-regulation of the mRNA expression of SULT 2A1 and UGT 1A1 as well as UGT activity, most likely due to the parallel down-regulation of PXR and CAR[55]. In a rat model of toxic fibrosis/cirrhosis (treatment with thioacetamide), major UGT isoforms were up-regulated, but the enzyme activity was unchanged[56]. In CCl4-cirrhotic rat livers, however, UGT protein expression was completely preserved while enzyme activity was not measured[57]. In biliary cirrhotic rats, we also found the protein content of UGT 1A isoforms unchanged, but the enzyme activity of both UGT and SULT isoforms was clearly reduced[58]. As Mrp2 expression (the main transporter of glucuronidated and sulfated metabolites) is extensively down-regulated in biliary cirrhosis, these results can be interpreted as end-product inhibition of UGT 1A activity via lack of efflux[58].
Zollner et al[44] investigated the mRNA expression of a few UGT and SULT isoforms (UGT 2B4 and 2B7 and SULT 2A1) in 11 PBC patients and found them unaltered. This result is only partially in line with an earlier human study showing the mRNA expression levels of several UGT isoforms (1A4, 2B4, 2B7) to be significantly down-regulated in inflammation but not in fibrotic livers[59]. Later, the same group confirmed unaltered UGT isoenzyme mRNA in advanced fibrosis or cirrhosis in viral hepatitis[39]. In a recent human study, the authors analyzed UGT and SULT expression along with NAFLD from steatosis via NASH to cirrhosis[60]. In this comprehensive study, the mRNA expression levels of numerous UGT and SULT isoforms were almost invariably preserved in cirrhosis. Protein expression of the tested UGT isoforms (1A1, 1A6, 1A9 and 2B10) was also very similar in normal, steatotic and cirrhotic tissue, with a significant down-regulation in cirrhosis only for 1A6. SULT isoenzymes protein expression levels (analyzed for 1A1, 1C4 and 2A1) were significantly up-regulated for SULT 1C4 (important for endocrine metabolism) and down-regulated for 2A1 in cirrhotic NASH. The significance of these specific regulatory events on enzyme activity or hepatic metabolism must remain open as in this study, UGT activity was unchanged in all NASH patients, and SULT activity was significantly reduced in fatty and cirrhotic NASH patients[60]. In another study analyzing the SULT isoenzymes 1A1, 2A1, 1E1 and 1A3 in fatty liver disease (both NASH and ASH), the authors showed a clear reduction of SULT 1A1, 1A3 and 1E1 activity and protein expression correlating to the extent of fatty liver disease[61]. This down-regulation was pronounced in alcoholic cirrhotic patients, where additionally SULT 2A1 activity was reduced. In general, this study found a clear reduction in the protein expression and function of SULT isoenzymes with the progression of liver disease[61]. In contrast, SULT 1A1 activity was unchanged in cirrhosis in a study analyzing phenacetin metabolism, while cirrhotic patients with elevated SULT 1A1 activity (along with preserved CYP 1A2 activity) were at higher risk for HCC (see above[40]). These partially conflicting data may indicate on the one hand high interindividual differences and on the other hand the decoupling of molecular expression and activity of isoenzymes during advancing fibrosis. Additionally, a decrease in SULT expression or activity can be modified by sulfatase activity, which is known to be reduced in cirrhosis as well[62].
In general, UGT and SULT expression seem to be largely unchanged in advanced fibrosis and cirrhosis. However, with regard to the enzyme activity of these two important phase II enzymes, we are facing a serious methodological problem. Most studies (especially in humans) have used in vitro methods with defined substrates to determine the activity of the respective isoenzymes[60,61]. In these isolated test situations, the pure enzymatic activity of each specific isoenzyme can be determined accurately and is preserved for most isoenzymes. In contrast, data from rat in vivo experiments indicate that in the cirrhotic liver, where the transport of some phase II metabolites is impaired (see below), the phenomenon of end product inhibition can occur[58]. As phase II metabolism is crucially linked to transporter-mediated export into bile, these results in a reduced in vivo activity of phase II isoenzymes despite preserved in vitro activity. The validity of in vitro data is therefore in question, which in turn indicates the increasing importance of in vivo test systems for hepatic metabolism in health and disease.
Functional changes in enterohepatic transport systems have been described in experimental liver disease and specific human disease entities[63,64].
Basolateral import transporters of the liver are down-regulated in inflammatory and cholestatic conditions[63]. In human cholestatic liver disease, decreased Na-taurocholate co-transporting polypeptide (NTCP) (SLC 10A1), mRNA and protein levels have been observed in PBC patients with stage III and IV disease[65,66] as well as biliary atresia[67]. Reduced organic anion-transporting polypeptides (OATP1B1) and OATP1B3 mRNA and protein expression have also been described in the later stages of PBC[65,66]. In line with these findings, OATP1B1 down-regulation can be observed in other cholestatic conditions such as PSC[68]. Remarkably, the expression of NTCP is only reduced in PBC stage IV (cirrhosis), whereas OATP1B1 is diminished at an earlier stage III[65,68]. The down-regulation of NTCP (SLC 10A1) and OATP1B1 may not only contribute to impaired hepatic bile salt uptake in the advanced stages of cholestatic liver disease but could also represent a defense mechanism that is partially limiting the accumulation of potentially toxic bile salts[65]. As another line of defense, the compensatory upregulation of basolateral escape transporters such as the multidrug resistance-associated proteins MRP3 (ABCC3) and MRP4 (ABCC4) is already induced at a precirrhotic PBC stage, while canalicular ATP-dependent export pumps remain stably expressed in the cirrhotic stage[44,65,66,69].
Canalicular transport systems of the liver are less tightly regulated in inflammatory and cholestatic conditions. For Multidrug resistance-associated protein 2 (MRP2, ABCC2), decreased immunostaining has been described in a subset of PBC patients with stage IV disease and progressive cholestasis[70]. Similarly, decreased MRP2 (ABCC2) mRNA levels have also been observed in PSC patients and patients with poorly drained obstructive cholestasis[68,71]. In the latter study, the mRNA levels of MRP2 (ABCC2) and bile salt export pump (BSEP, ABCB11) were decreased in poorly drained compared to well-drained patients, who were at the levels of control subjects. Immunostaining of MRP2 (ABCC2) and BSEP (ABCB11) at the canalicular membrane domain were fuzzy to varying degrees in the specimens obtained from poorly drained cholestatic liver but linear and intense in the liver of well-drained patients and control subjects, correlating with impaired bilirubin conjugate and bile acid secretion[71].
The down-regulation of hepatic transport systems has also been observed in patients with non-cholestatic chronic inflammation of the liver such as hepatitis C infection. Together with the expression levels of nuclear receptors as the transactivators, the mRNA levels of various transporter genes, including NTCP (SLC10A1), OATP1B1, BSEP (ABCB11) and MRP2 (ABCC2), are decreased depending on the stage of fibrosis, with an approximately 50 % decrease between F3 and F1 patients[72]. In another study investigating viral hepatitis C patients, inflammatory cytokines such as tumor necrosis factor (TNF)α have been found to be increased with fibrosis stage F3, while transporters including OATP1B1 were decreased[38]. Additional cell culture experiments have also demonstrated a functional contribution of interleukin (IL)-1 and -6, which was most prominent for NTCP (SLC 10A1).
The functional consequences of decreased transporter expression during the progression of fibrogenesis have been studied in rats with experimental biliary cirrhosis[58]. A significant down-regulation of canalicular multidrug-resistance transporters, including Mrp2 (Abcc2) and Bcrp (Abcg2), has been detected, while the biliary excretion of a radiolabelled food-derived carcinogen into the bile was significantly decreased. Of note, the mRNA and protein expression of MRP2 (ABCC2) was only moderately decreased in human livers with alcoholic cirrhosis, whereas BCRP (ABCG2) was increased[73]. Therefore, the potential contribution of decreased carcinogen defense transporters to the increased hepatic and extrahepatic incidence of cancers in cirrhosis patients remains to be evaluated in more detail. Additional findings for hepatic uptake systems have been obtained in rat liver perfusion experiments. Here, a linear relationship was found to exist between the histopathologic fibrosis index and the hepatic extraction ratio of 3H-taurocholate[74].
In summary, changes in transporter expression in cirrhosis fit into the “cholestatic paradigm”[75] of transporter regulation. Cirrhosis also represents a cholestatic state with the intracellular accumulation of bile acids in hepatocytes. Consequently, import transporters (at the basolateral membrane) are downregulated, and export transporters (especially basolaterally) are simultaneously upregulated. At the canalicular membrane, the regulation events are less uniform and also depend upon the stage and pathogenesis of fibrosis and cirrhosis.
In clinical practice, decreased hepatic transport and metabolic function may be critical for decision making in critically ill patients or those undergoing hepatic intervention or surgery. Methods to assess hepatic function quantify the abundance and functional integrity of the basolateral uptake and canalicular export systems described above. The indocyanine green (ICG) disappearance rate reflects a direct non-invasive measure of the actual functional state of these hepatic transport systems at the time of assessment[76-78]. Albumin-bound water-soluble ICG, which is not metabolized by hepatocytes[79], is selectively taken up by the basolateral uptake systems NTCP (SLC10A1) and OATP1B3 and is later excreted unchanged into the bile by the canalicular MRP2 (ABCC2) transporter[80]. At the basolateral membrane, OATP1B1 and OATP2B1 are both inhibited by ICG[80]. ICG clearance thus reflects the overall hepatic uptake, and excretory function and can be used to assess liver function in patients with chronic liver failure and as a prognostic factor in critically ill patients[76]. However, a delayed residual ICG excretion indicates an additional transcellular pathway, which can be blocked by colchicines[81]. This might be an explanation for ICG plasma disappearance that occurs in humans during the anhepatic phase of orthotopic liver transplantation, possibly hampering the validity of the test[82].
Although less frequently used in clinical practice today, hepatobiliary radiotracers such as 99mTc-mebrofenin and 99mTc-N-pyridoxyl-5-methyltryptophan (99mTc-PMT) share a transporter spectrum that is partially overlapping with ICG, which involves OATP1B1 and OATP1B3 for basolateral uptake[80,83,84]. 99mTc-mebrofenin and 99mTc-PMT excretion into bile canaliculi is facilitated by the canalicular ATP-dependent export pumps MDR1 (ABCB1) and MRP2 (ABCC2)[83,84], which contribute to the visualization of biliary structures in clinical scintigraphy.
Changes in transporter expression in chronic liver disease associated with fibrosis or even cirrhosis also have implications for MRI-based imaging. MRI contrast agents are taken up into and excreted out of hepatocytes by the same transporters of the OATP and MRP family. Experimental cirrhosis in rats is associated with the decreased entry of Gd-BOPTA into hepatocytes in a radioactivity distribution compartment model[85] in agreement with the reduced expression of Oatp transporters in experimental cirrhosis[86]. Although the entry of contrast agent into hepatocytes was lower in cirrhotic than in normal livers, the accumulation of Gd-BOPTA was higher in cirrhotic livers because biliary excretion was totally abolished[85], again correlating with decreased Mrp2 (Abcc2) expression in previous studies. Additionally, the Gd-EOB-DTPA uptake in hepatocytes is strongly affected by liver function[87]. Gd-EOB-DTPA-enhanced MRI and the assessment of relative enhancement during the hepatobiliary phase may serve as a useful image-based test in liver imaging for determining regional and global liver function[88].
Interaction and changes in hepatic lipid and glucose metabolism are important for the etiology and progression of non-alcoholic steatohepatitis[89]. These changes are not confined to the liver, and they contribute to the development of liver cirrhosis rather than being the result of cirrhosis development. Insulin resistance, increased synthesis and the release of free fatty acids and changes in the production of leptin, adiponectin and interleukins 1 and 6 are the central players in NAFLD and NASH-dependent liver fibrosis[90,91], and these changes are connected to excessive fat accumulation in obese NASH patients[92].
However, studies investigating changes in lipid and glucose metabolism in liver cirrhosis are rare. Insulin resistance is also an important hallmark in liver cirrhosis, but here it is a catabolic disease associated with muscle wasting, anorexia and weight loss. Twenty years ago, a receptor/postreceptor dysfunction was already postulated as the explanation for the glucose metabolism disturbances and malnutrition found in cirrhotic patients, based on altered membrane lipid composition, hyperinsulinemia and a lack of liver-derived humoral factors[93]. Protein synthesis in general is disturbed in liver cirrhosis[94], intensifying ER stress and organ failure[95], but the changes in glucose and lipid metabolism are more complex and are individually very diverse depending on the general metabolic status. This concept is also illustrated by the results of a microarray analysis of fibrosis progression in hepatitis c patients. In this study, the amino acid metabolism enzymes were more severely and uniformly down-regulated than were the glucose and lipid metabolism enzymes[6].
Patients with cirrhosis were shown to have a lower energy intake and a higher resting energy expenditure, higher fasting leptin and higher insulin resistance than controls[96]. In a follow-up study with 42 cirrhotic patients, the same group found increased postprandial glucose, insulin and glucagon-like peptide 1 responses and reduced postprandial ghrelin. Interestingly, in this latter study, these metabolic changes were related to delayed gastric emptying and prolonged small bowel transit[97], and a high proportion of these patients suffered from gastrointestinal symptoms. Additionally, cirrhotic patients showed increased rates of gluconeogenesis but lower net hepatic glycogenolysis[98]. These effects explain the higher insulin resistance and the diminished reaction to hypoglycemia in cirrhotic patients. Hyperinsulinemia in cirrhotic patients has also been linked to an increased pancreatic beta-cell sensitivity to glucose[99], again an extrahepatic metabolic effect of liver cirrhosis. In the explanation of higher circulating plasma levels of enzymes (e.g., insulin or glucagon) or proteins in general, the decreasing hepatic extraction capacity as represented by, for example, the asialoglycoprotein-receptor certainly plays a role[100] but must be quantified individually[99]. However, again, effects such as this may form a basis for another non-invasive test of a part of liver function, in this case the extraction of circulating proteins from the portal blood[101-104].
Fibroblast growth factor (FGF) 15/19 acts as a FXR-activated negative feedback regulator that signals from the intestine to the liver to repress bile acid synthesis and has recently been recognized to regulate energy homeostasis and lipid metabolism (Jahn and Geier, Mechanisms of enterohepatic Fibroblast Growth Factor (FGF) 15/19 signaling in health and disease, manuscript submitted). Intestinal FGF19 has emerged as a novel endocrine regulator of hepatic bile salt and lipid metabolism, and an impaired hepatic response to FGF19 may contribute to the dysregulation of lipid homeostasis in NAFLD patients[105]. Although no data on FGF19 expression and signaling exist in human liver cirrhosis, recent data from mouse experiments indicate that activated ileal FGF15 may contribute to HCC development in the context of chronic liver injury and fibrosis[106].
Many associations between cytokine regulation and metabolism have been identified. The level of the adipokine resistin was increased in 57 cirrhotic patients and correlated negatively with hepatic glucose production and positively with circulating free fatty acids and TNF-α levels, implicating an effect in glucose and lipid metabolism. However, resistin was not associated with the degree of insulin resistance after transplantation; the resistin levels remained unchanged, while the insulin resistance was significantly improved[107]. Adiponectin has also been shown to be elevated in liver cirrhosis without any etectable correlation with the parameters of lipid or glucose metabolism or proinflammatory cytokines[108]. So far, the overall influence of these cytokine regulatory events on lipid and glucose metabolism is unclear, and a causal relationship has not continuously been shown. However, it is obvious that altered cytokine profiles in liver cirrhosis contribute to systemic alterations in lipid and glucose metabolism that concern many extrahepatic sites, such as the pancreas, the gut and the muscle tissue. Still, the exact mechanisms of altered lipid and glucose metabolism during liver cirrhosis deserve further research.
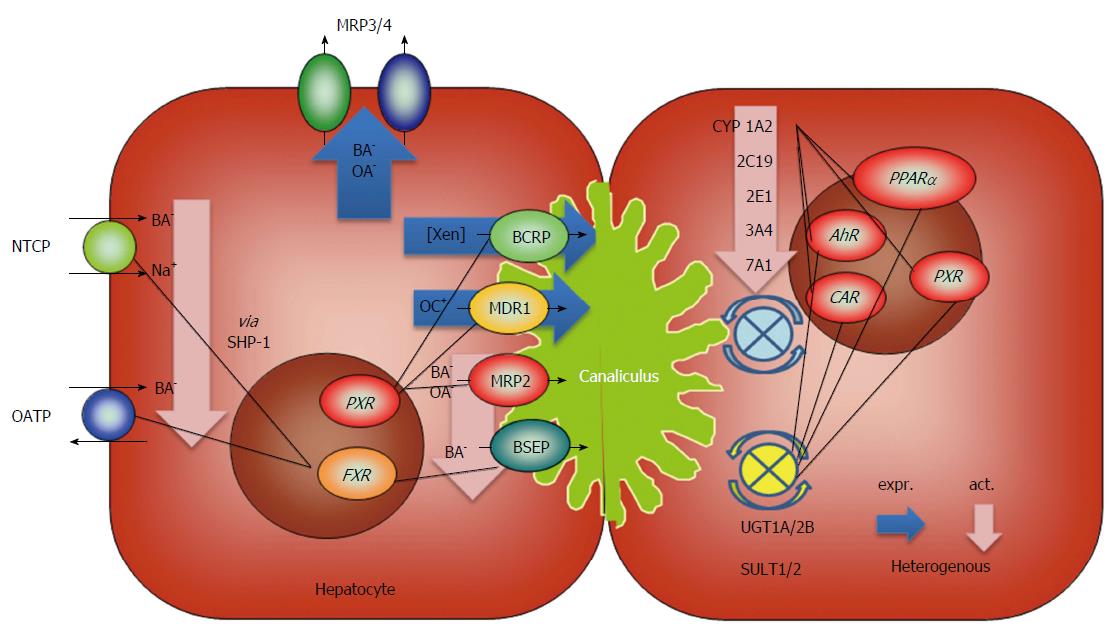
In addition to the general changes in cirrhotic livers, disease-specific metabolic events must be considered in these patients. As such, hepatitis B virus (HBV) infection alters bile acid and cholesterol metabolism as a consequence of impaired NTCP-mediated bile acid uptake into hepatocytes[109]. Using human liver-chimeric uPA/SCID mice (SCID - severe combined immunodeficiency) and human liver biopsies from HBV patients, Oehler et al[110] showed that nuclear FXR localization and SHP expression are decreased with chronic HBV infection, leading to relevant expression changes in genes involved in bile acid synthesis as well as cholesterol uptake and synthesis. The metabolic consequences for patients with chronic HBV infection and particularly end-stage liver disease remain to be determined. The regulatory events of liver cirrhosis described in this chapter are summarized in Figure 2.
Several functions of hepatic metabolism can be monitored non-invasively using specific breath test analysis. The determination of pCO2 in breath following the ingestion of a meal was the first application of a breath analysis in hepato-gastroenterology in the early twentieth century[111-113]. Because of their minimally invasive nature and feasibility, breath tests were attractive for their clinical use but had no clinical applications for almost 40 years. Later on, a large variety of breath tests were introduced as “no-touch” functional diagnostic tests and are currently performed to investigate gastrointestinal motility and liver disorders[114]. Typical stable isotope breath tests for liver function are listed in Table 1.
| Investigated function | 13C-labelled compound |
| Bile acid malabsorption | Glycocholic acid |
| Hepatic microsomal function (CYP P450) | Aminopyrine, methacetin, phenacetin caffeine, diazepam, erythromycin |
| Hepatic cytosolic function (galactokinase) | Galactose, phenylalanine |
| Hepatic mitochondrial function (α-keto acid dehydrogenase complex) | Methionine, octanoate, α-ketoisocaproic acid |
Liver function tests using unlabeled and labeled (13C, 14C, D, 15N) compounds as marker substances play an important role for the management of patients with chronic liver disease. Chronic liver diseases comprise pathomorphological features such as necrosis, inflammation, fibrosis, changes of intra- and extrahepatic blood flow and impaired function. These changes result in the typical clinical manifestations of complications governing overall patient outcome, whereas the degree of functional impairment has been described as a stronger predictor for the clinical outcome than are histological changes in patients with chronic hepatitis C infection[115]. Indices for the prediction of survival are essential tools for assessing prognosis, establishing priority for liver transplantation and identifying those at high risk for developing complications due to disease progression or following interventions[116-118]. Determining the hepatic reserve is essential for assessing the prognosis, predicting decompensation and organ allocation[119,120]. Decision-making in the treatment of patients with chronic liver disease focuses on the early identification of these patients[118,120]. For the non-invasive evaluation of “quantitative liver function” exogenous/xenobiotic or natural liver, specific test substances have been introduced to specify partial function, such as hepatic blood flow (HBF) by ICG clearance or cholate clearance, hepatic plasma flow by sorbitol elimination capacity, cytosolic liver function by (13C) galactose elimination capacity, mitochondrial function by alpha-ketoisocaproic acid or 13C-methionine breath test (MeBT), hepatic cytochrome P450 function by 13C aminopyrine (CYP 2C19, 2D6) (ABT), caffeine test or 13C-methacetin (CYP 1A2) (MBT) breath test or lidocaine/monoethylglycinexylidite (CYP 3A4) (MEGX) test[121-134]. The dual cholate test is a novel oral (D4-cholate) and intravenous (13C-cholate) simultaneous function test that quantifies clearance from the systemic circulation, portal circulation and portal systemic shunting[134].
Hepatic clearance (in mL of plasma/min/kg of substance cleared) can be flow or functional liver cell mass-dependent (comprising extraction and metabolic efficiency of hepatocytes) or both and is given by[134,135]:
clearance = [c (arterial) - c (hepatic venous)]/c (arterial) × hepatic blood flow
with [c (arterial) - c (hepatic venous)]/c (arterial) defined as first pass hepatic extraction E, calculated from the concentrations of the substance measured in the arterial and hepatic venous blood[136]. For substances with high extraction, which is facilitated by high affinity transport systems, e.g., for orally administered bile acids[137], the membrane sodium-dependent bile acid transporter and organic solute transporter (OST-α/OST-β in enterocytes, the Na-dependent taurocholate cotransporter (NTCP, SLC10A1) and the Na-independent superfamily of organic anion transporting polypeptides (OATP) at the basolateral membrane of hepatocytes, elimination half-lives are in the range of minutes, and the clearance is close to HBF. If flow-dependent substances are administered, such as ICG[138] or lidocaine[139], then the hepatic extraction of ICG in normal controls measured by hepatic venous catheter is 0.7-0.9. In patients with liver diseases, it is reduced, with values < 0.3.
Test substances with E < 0.25, such as the CYP-metabolized xenobiotics aminopyrine or diazepam, are only extracted to a small amount during liver passage, i.e., the hepatic disposition of the substance is determined only by the metabolic capacity of the liver and not by HBF. Interestingly, for most substrates frequently used for the assessment of hepatic biotransformation function (methacetin, aminopyrine), the transport mechanisms are not well described. For example, erythromycin applied as a 13C-labeled substrate in a breath test for hepatic CYP 3A4 activity inhibits OATP1B1 and OATP1B3[140], is transported by OATP1B1 and is a substrate for MRP2 (see also ICG transport above[135]). Again, transport function alters erythromycin metabolism, showing a close relationship with hepatocyte metabolism and transport in humans as well[141]. This interrelationship must be implemented in the interpretation of the 13C erythromycin breath test. The biotransformation of drugs is reduced in patients with severe liver diseases, whereas the microsomal monooxygenase system is the most affected. The well-known reduction of total cytochrome P450 protein in patients with liver cirrhosis[142,143] could be characterized in detail by George and co-workers for specific CYP subfamilies in patients with cholestatic (primary biliary cirrhosis, primary sclerosing cholangitis, biliary atresia, idiopathic cholestasis) and non-cholestatic liver cirrhosis (auto-immune hepatitis, alcoholic cirrhosis, chronic viral hepatitis) in different Child Pugh stages (see above[34]). Furthermore, the CYP1A2 protein amount and catalytic activity is significantly and homogenously reduced in both cholestatic and non-cholestatic liver cirrhosis compared to a control group, which serves as a pathobiochemical basis of the frequently used 13C-methacetin breath test[34,144-147]. It is important to note that the activity of the NADPH-cytochrome P450 reductase is not decreased in patients with liver cirrhosis, and thus the reduction of specific CYP isoforms in hepatocytes for the selective change of the mixed functional monooxygenase activity is causative[34].
In summary, transport systems are an important component of well-established flow and liver cell mass-dependent liver function tests. The involvement of multiple processes, including substance uptake in the intestine and the hepatocyte, metabolism, and excretion from the hepatocyte indicate that the (patho)-physiological interpretation of a liver function test is multilayered and might influence the clinical value of a single test[135].
Liver cirrhosis is a serious disease with far-reaching changes in gene expression and function. The impairment of hepatic function has consequences for the treatment and prognosis of patients. These consequences mainly comprise the resection of hepatocellular carcinoma (or other tumors including metastasis) and an indication for liver transplantation. Additionally, decisions about medical treatment must take impaired liver function into account (dose adjustment), and the altered metabolism of xenobiotics including carcinogens can have an impact on toxic or carcinogenic effects in the body.
Metabolism in the liver represents a coordinated sequence of enzymatic steps: (1) extraction of compounds from the portal blood is followed by uptake into the hepatocyte (phase 0, basolateral import); (2) oxygenation/activation of compounds (phase I, CYP 450); (3) glucuronidation or sulfation, and rarely acetylation, methylation or conjugation to glutathione (phase II , UGT, SULT and others); and (4) secretion via the basolateral or canalicular membrane to the caval blood or the bile (phase III, basolateral or canalicular export transporters).
The close connection between these steps is also embodied by the coordinated regulation of these metabolic steps by central nuclear receptors, which is even stronger in diseased livers[28,39]. The data summarized in this review show the down-regulation of important CYP 450 isoforms[34,35] and basolateral import transporters[65,66]. Phase II enzymes (UGT, SULT) are mostly preserved in their expression[39,44,60], but data from animal in vivo experiments point to a reduction of enzymatic function[58]. Lipid or glucose metabolism is individually altered as a result of cytokine regulation, differential enzyme expression and basic metabolic status but is in general characterized by hyperinsulinemia, insulin resistance and catabolism[96,98]. These data show the complexity of metabolic processes and their regulation in cirrhosis.
Experimental data clearly show that every metabolic step can influence the preceding steps of metabolism in their functional capacity or differentiation. In a study in Gunn rats (an animal model for Crigler-Najjar syndrome), the lack of UGT1 isoforms significantly changed the metabolic ratio of phase I metabolism conferred by CYP isoforms[148]. The influence of a single transporter function on hepatic metabolism is best exemplified by MRP2. MRP2 represents a transporter for organic anions[149] but also transports amphipathic compounds in co-transport with glutathione[150,151]. The genetic loss of MRP2 expression forms the basis for the rare Dubin Johnson syndrome[152], but acquired MRP2 deficiency is common in cholestatic diseases, including liver cirrhosis[72,73]. In Mrp2 knockout mice but also in patients with a MRP2 polymorphism, metabolism of erythromycin was altered independently from CYP 3A4 expression and function[141]. In animal studies, the pronounced down-regulation of the canalicular organic anion transporter MRP2 is supposed to be responsible for the pronounced functional inhibition of phase II enzymes[58] despite preserved enzyme expression. The up-regulation of Mrp3 and 4 at the basolateral membrane can obviously only partially compensate for this acquired Mrp2 deficiency. Phase II metabolism is extremely important in the detoxification of endogenous and exogenous toxicants, including carcinogens. At least in rats, the accumulation of metabolized but also activated carcinogens in the liver and in other organs as a consequence of genetic or acquired Mrp2 deficiency was shown and may have consequences for the toxic and carcinogenic effects of xenobiotics[58,153]. However, species differences can complicate the interpretation of these results. Hepatic BCRP, a transporter with overlapping substrate specificity, can compensate for MRP2 deficiency in humans, where it is preserved or even upregulated[73], while in rats, it is down-regulated during liver cirrhosis[58]. As a further complication, extrahepatic expression of these transporters also contributes to metabolism, tissue accumulation and the excretion of toxic compounds. In colonic adenomas, BCRP is more down-regulated in humans than in mice, where the accumulation of a food-derived carcinogen has been shown to promote carcinogen accumulation[154]. The overall effect of impaired liver function the metabolism of toxins and carcinogens is not sufficiently defined and needs further studies, especially epidemiological data[155].
Dose adjustments in patients with liver cirrhosis are also difficult and mostly based on rough calculation of hepatic function with Child Pugh criteria[156]. Many of the tests mentioned above, using either blood or breath samples, exploit pharmaceutical compounds such as midazolam or erythromycin, but no test can be recommended for dosing decisions in cirrhotic patients[114,156].
The summarized data show that in humans, enzymatic functions are difficult to test in vivo, and therefore ex vivo methods (e.g., microsomal assays) have been used. In these assays, however, the enzymatic function is tested in an isolated manner, leaving out the necessary connection between phases 0 and III. For almost all steps in hepatic metabolism, there is a test that can be applied, but the full picture is hidden behind many complex regulatory events. No single test is able to reliably estimate liver function simply because liver function is extremely complex and encompasses many diverse functions. Breath tests have advantages in daily patient care as they are non-invasive, readily available and can be applied in vivo in the intact metabolic sequence[114,115,134]. Nevertheless, even these tests are dependent on test substances, and therefore the used test substance and its rate-limiting step determines the value of the test[114]. From a logical point of view, it certainly is useful to combine breath tests with different test substances and different rate-limiting metabolic steps to examine different aspects of liver function in a test panel.
In every-day practice, established global easy-to-measure scores such as MELD or Child Pugh will be used for a first estimation of liver function (Table 2 and Figure 1). The continuing discussion about the efficacy and validity of these scores already shows their limitations[157-160]. We know too little about functional tests in liver cirrhosis, and all available tests only represent parts of the individual’s liver function. As a consequence, even applying multiple tests on different aspects of liver function cannot avoid the misjudgment of individual patients[161,162]. If difficult decisions in treatment must be made (e.g., partial liver resection or liver transplantation), the application of two or more complementary breath tests as outlined above may help in an appropriate classification of liver function to live up to the expectations of clinicians and patients in legitimate treatment decisions.
| Name | Parameters | Used for |
| MELD[163] | INR, creatinine, bilirubin | CLF, LTX, resection |
| Child Pugh[164] | Prothrombin time, bilirubin, ascites, encephal., albumin | CLF, resection |
| King’s College[165] | Paracetamol-ALF: pH, INR, creatinine, encephal | ALF, LTX |
| Non-paracetamol-ALF: INR, age, bilirubin, timing of jaundice, etiology | ||
| Clichy[165] | Age, factor V, encephalopathy | ALF, LTX |
| Milano[166] | Size and number of tumor nodules | HCC, LTX |
P- Reviewer: Guo XZ S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 909] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 2. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 3. | Ford RM, Book W, Spivey JR. Liver disease related to the heart. Transplant Rev (Orlando). 2015;29:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Dietrich CG, Götz M, Fischbach W, Al-Taie O. Severe hepatitis and subacute liver failure with “fast track” cirrhosis in an elderly lady. Z Gastroenterol. 2010;48:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Wiegand J, Berg T. The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013;110:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Takahara Y, Takahashi M, Zhang QW, Wagatsuma H, Mori M, Tamori A, Shiomi S, Nishiguchi S. Serial changes in expression of functionally clustered genes in progression of liver fibrosis in hepatitis C patients. World J Gastroenterol. 2008;14:2010-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Mas VR, Fassnacht R, Archer KJ, Maluf D. Molecular mechanisms involved in the interaction effects of alcohol and hepatitis C virus in liver cirrhosis. Mol Med. 2010;16:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lederer SL, Walters KA, Proll S, Paeper B, Robinzon S, Boix L, Fausto N, Bruix J, Katze MG. Distinct cellular responses differentiating alcohol- and hepatitis C virus-induced liver cirrhosis. Virol J. 2006;3:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Rous P, Larimore LD. The biliary factor in liver lesions. J Exp Med. 1920;32:249-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Cameron GR, Karunaratne WAE. Carbon tetrachloride cirrhosis in relation to liver regeneration. J Pathol Bacteriol. 1936;42:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 294] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Chaikoff IL, Gillman T. Cirrhosis and other hepatic lesions produced in dogs by thyroidectomy and by combined hypophysectomy and thyroidectomy. J Exp Med. 1948;88:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Fickert P, Pollheimer MJ, Beuers U, Lackner C, Hirschfield G, Housset C, Keitel V, Schramm C, Marschall HU, Karlsen TH. Characterization of animal models for primary sclerosing cholangitis (PSC). J Hepatol. 2014;60:1290-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Trauner M, Fickert P, Baghdasaryan A, Claudel T, Halilbasic E, Moustafa T, Wagner M, Zollner G. New insights into autoimmune cholangitis through animal models. Dig Dis. 2010;28:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Jaeckel E, Hardtke-Wolenski M, Fischer K. The benefit of animal models for autoimmune hepatitis. Best Pract Res Clin Gastroenterol. 2011;25:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Chuang YH, Ridgway WM, Ueno Y, Gershwin ME. Animal models of primary biliary cirrhosis. Clin Liver Dis. 2008;12:333-47; ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 579] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 20. | Varela-Rey M, Embade N, Ariz U, Lu SC, Mato JM, Martínez-Chantar ML. Non-alcoholic steatohepatitis and animal models: understanding the human disease. Int J Biochem Cell Biol. 2009;41:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300-2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 379] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (2)] |
| 22. | Nagarajan P, Mahesh Kumar MJ, Venkatesan R, Majundar SS, Juyal RC. Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819-G823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Inuzuka T, Takahashi K, Chiba T, Marusawa H. Mouse models of hepatitis B virus infection comprising host-virus immunologic interactions. Pathogens. 2014;3:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Hasler JA. Pharmacogenetics of cytochromes P450. Mol Aspects Med. 1999;20:12-24, 25-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Walsh AA, Szklarz GD, Scott EE. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J Biol Chem. 2013;288:12932-12943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos. 2012;40:1366-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Hata S, Miki Y, Fujishima F, Sato R, Okaue A, Abe K, Ishida K, Akahira J, Unno M, Sasano H. Cytochrome 3A and 2E1 in human liver tissue: Individual variations among normal Japanese subjects. Life Sci. 2010;86:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Chen H, Shen ZY, Xu W, Fan TY, Li J, Lu YF, Cheng ML, Liu J. Expression of P450 and nuclear receptors in normal and end-stage Chinese livers. World J Gastroenterol. 2014;20:8681-8690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Babany G, Descatoire V, Corbic M, Gendre S, Degott C, Larrey D, Letteron P, Wandscheer JC, Funck-Brentano C, Pessayre D. Regulation of renal cytochrome P-450. Effects of two-thirds hepatectomy, cholestasis, biliary cirrhosis and post-necrotic cirrhosis on hepatic and renal microsomal enzymes. Biochem Pharmacol. 1985;34:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Floreani M, De Martin S, Gabbia D, Barbierato M, Nassi A, Mescoli C, Orlando R, Bova S, Angeli P, Gola E. Severe liver cirrhosis markedly reduces AhR-mediated induction of cytochrome P450 in rats by decreasing the transcription of target genes. PLoS One. 2013;8:e61983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Li X, Yang X, Wu P, Meng Y, Li S, Lai W. Gene-CYP11B2 expression in rat liver in hepatic fibrogenesis induced by CCl4. Chin Med J (Engl). 2001;114:64-68. [PubMed] |
| 34. | George J, Murray M, Byth K, Farrell GC. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology. 1995;21:120-128. [PubMed] |
| 35. | George J, Liddle C, Murray M, Byth K, Farrell GC. Pre-translational regulation of cytochrome P450 genes is responsible for disease-specific changes of individual P450 enzymes among patients with cirrhosis. Biochem Pharmacol. 1995;49:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Albarmawi A, Czock D, Gauss A, Ehehalt R, Lorenzo Bermejo J, Burhenne J, Ganten TM, Sauer P, Haefeli WE. CYP3A activity in severe liver cirrhosis correlates with Child-Pugh and model for end-stage liver disease (MELD) scores. Br J Clin Pharmacol. 2014;77:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Theile D, Haefeli WE, Seitz HK, Millonig G, Weiss J, Mueller S. Association of liver stiffness with hepatic expression of pharmacokinetically important genes in alcoholic liver disease. Alcohol Clin Exp Res. 2013;37 Suppl 1:E17-E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Nakai K, Tanaka H, Hanada K, Ogata H, Suzuki F, Kumada H, Miyajima A, Ishida S, Sunouchi M, Habano W. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab Dispos. 2008;36:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Congiu M, Mashford ML, Slavin JL, Desmond PV. Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol. 2009;24:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Wang XR, Qu ZQ, Li XD, Liu HL, He P, Fang BX, Xiao J, Huang W, Wu MC. Activity of sulfotransferase 1A1 is dramatically upregulated in patients with hepatocellular carcinoma secondary to chronic hepatitis B virus infection. Cancer Sci. 2010;101:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Kessova I, Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003;3:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 43. | Takeyama Y, Kanegae K, Inomata S, Takata K, Tanaka T, Ueda S, Yokoyama K, Morihara D, Nishizawa S, Anan A. Sustained upregulation of sodium taurocholate cotransporting polypeptide and bile salt export pump and downregulation of cholesterol 7α-hydroxylase in the liver of patients with end-stage primary biliary cirrhosis. Med Mol Morphol. 2010;43:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Zollner G, Wagner M, Fickert P, Silbert D, Gumhold J, Zatloukal K, Denk H, Trauner M. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Inamine T, Higa S, Noguchi F, Kondo S, Omagari K, Yatsuhashi H, Tsukamoto K, Nakamura M. Association of genes involved in bile acid synthesis with the progression of primary biliary cirrhosis in Japanese patients. J Gastroenterol. 2013;48:1160-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Yang J, Hao C, Yang D, Shi D, Song X, Luan X, Hu G, Yan B. Pregnane X receptor is required for interleukin-6-mediated down-regulation of cytochrome P450 3A4 in human hepatocytes. Toxicol Lett. 2010;197:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Vuppalanchi R, Liang T, Goswami CP, Nalamasu R, Li L, Jones D, Wei R, Liu W, Sarasani V, Janga SC. Relationship between differential hepatic microRNA expression and decreased hepatic cytochrome P450 3A activity in cirrhosis. PLoS One. 2013;8:e74471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | McConn DJ, Lin YS, Mathisen TL, Blough DK, Xu Y, Hashizume T, Taylor SL, Thummel KE, Shuhart MC. Reduced duodenal cytochrome P450 3A protein expression and catalytic activity in patients with cirrhosis. Clin Pharmacol Ther. 2009;85:387-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Wang H, Liao ZX, Chen M, Hu XL. Effects of hepatic fibrosis on ofloxacin pharmacokinetics in rats. Pharmacol Res. 2006;53:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1107] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 51. | Lindsay J, Wang LL, Li Y, Zhou SF. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr Drug Metab. 2008;9:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 53. | Ou Z, Shi X, Gilroy RK, Kirisci L, Romkes M, Lynch C, Wang H, Xu M, Jiang M, Ren S. Regulation of the human hydroxysteroid sulfotransferase (SULT2A1) by RORα and RORγ and its potential relevance to human liver diseases. Mol Endocrinol. 2013;27:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Tang KS, Lee CM, Teng HC, Huang MJ, Huang CS. UDP-glucuronosyltransferase 1A7 polymorphisms are associated with liver cirrhosis. Biochem Biophys Res Commun. 2008;366:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Jia X, Naito H, Yetti H, Tamada H, Kitamori K, Hayashi Y, Wang D, Yanagiba Y, Wang J, Ikeda K. Dysregulated bile acid synthesis, metabolism and excretion in a high fat-cholesterol diet-induced fibrotic steatohepatitis in rats. Dig Dis Sci. 2013;58:2212-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Hao H, Zhang L, Jiang S, Sun S, Gong P, Xie Y, Zhou X, Wang G. Thioacetamide intoxication triggers transcriptional up-regulation but enzyme inactivation of UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Debinski HS, Mackenzie PI, Lee CS, Mashford ML, Danks JA, Tephly TR, Green M, Desmond PV. UDP glucuronosyltransferase in the cirrhotic rat liver. J Gastroenterol Hepatol. 1996;11:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Dietrich CG, Geier A, Wasmuth HE, Matern S, Gartung C, de Waart DR, Elferink RP. Influence of biliary cirrhosis on the detoxification and elimination of a food derived carcinogen. Gut. 2004;53:1850-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Congiu M, Mashford ML, Slavin JL, Desmond PV. UDP glucuronosyltransferase mRNA levels in human liver disease. Drug Metab Dispos. 2002;30:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington NJ. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2013;41:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 61. | Yalcin EB, More V, Neira KL, Lu ZJ, Cherrington NJ, Slitt AL, King RS. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab Dispos. 2013;41:1642-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773:283-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 64. | Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 65. | Zollner G, Fickert P, Zenz R, Fuchsbichler A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 66. | Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Shneider BL, Fox VL, Schwarz KB, Watson CL, Ananthanarayanan M, Thevananther S, Christie DM, Hardikar W, Setchell KD, Mieli-Vergani G. Hepatic basolateral sodium-dependent-bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology. 1997;25:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Takeyama Y, Uehara Y, Inomata S, Morihara D, Nishizawa S, Ueda S, Matsumoto T, Tanaka T, Anan A, Nishimura H. Alternative transporter pathways in patients with untreated early-stage and late-stage primary biliary cirrhosis. Liver Int. 2009;29:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Kullak-Ublick GA, Baretton GB, Oswald M, Renner EL, Paumgartner G, Beuers U. Expression of the hepatocyte canalicular multidrug resistance protein (MRP2) in primary biliary cirrhosis. Hepatol Res. 2002;23:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Shoda J, Kano M, Oda K, Kamiya J, Nimura Y, Suzuki H, Sugiyama Y, Miyazaki H, Todoroki T, Stengelin S. The expression levels of plasma membrane transporters in the cholestatic liver of patients undergoing biliary drainage and their association with the impairment of biliary secretory function. Am J Gastroenterol. 2001;96:3368-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Hanada K, Nakai K, Tanaka H, Suzuki F, Kumada H, Ohno Y, Ozawa S, Ogata H. Effect of nuclear receptor downregulation on hepatic expression of cytochrome P450 and transporters in chronic hepatitis C in association with fibrosis development. Drug Metab Pharmacokinet. 2012;27:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | More VR, Cheng Q, Donepudi AC, Buckley DB, Lu ZJ, Cherrington NJ, Slitt AL. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab Dispos. 2013;41:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Hung DY, Chang P, Cheung K, Winterford C, Roberts MS. Quantitative evaluation of altered hepatic spaces and membrane transport in fibrotic rat liver. Hepatology. 2002;36:1180-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Dietrich CG, Geier A. Effect of drug transporter pharmacogenetics on cholestasis. Expert Opin Drug Metab Toxicol. 2014;10:1533-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc. 2006;38:801-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;13:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 78. | Kortgen A, Recknagel P, Bauer M. How to assess liver function? Curr Opin Crit Care. 2010;16:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958;99:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | de Graaf W, Häusler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, Kullak-Ublick GA, Hesselmann R, van Gulik TM, Stieger B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 81. | Mori M, Oyamada M, Sakauchi F, Ogawa K. Effects of colchicine on the hepatocellular transport of indocyanine green in the rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Bruegger L, Studer P, Schmid SW, Pestel G, Reichen J, Seiler C, Candinas D, Inderbitzin D. Indocyanine green plasma disappearance rate during the anhepatic phase of orthotopic liver transplantation. J Gastrointest Surg. 2008;12:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Neyt S, Huisman MT, Vanhove C, De Man H, Vliegen M, Moerman L, Dumolyn C, Mannens G, De Vos F. In vivo visualization and quantification of (Disturbed) Oatp-mediated hepatic uptake and Mrp2-mediated biliary excretion of 99mTc-mebrofenin in mice. J Nucl Med. 2013;54:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Kobayashi M, Nakanishi T, Nishi K, Higaki Y, Okudaira H, Ono M, Tsujiuchi T, Mizutani A, Nishii R, Tamai I. Transport mechanisms of hepatic uptake and bile excretion in clinical hepatobiliary scintigraphy with 99mTc-N-pyridoxyl-5-methyltryptophan. Nucl Med Biol. 2014;41:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Planchamp C, Gex-Fabry M, Becker CD, Pastor CM. Model-based analysis of Gd-BOPTA-induced MR signal intensity changes in cirrhotic rat livers. Invest Radiol. 2007;42:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Planchamp C, Pastor CM, Balant L, Becker CD, Terrier F, Gex-Fabry M. Quantification of Gd-BOPTA uptake and biliary excretion from dynamic magnetic resonance imaging in rat livers: model validation with 153Gd-BOPTA. Invest Radiol. 2005;40:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Dahlqvist Leinhard O, Dahlström N, Kihlberg J, Sandström P, Brismar TB, Smedby O, Lundberg P. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012;22:642-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 88. | Verloh N, Haimerl M, Zeman F, Schlabeck M, Barreiros A, Loss M, Schreyer AG, Stroszczynski C, Fellner C, Wiggermann P. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol. 2014;24:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 89. | Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 707] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 90. | Anty R, Lemoine M. Liver fibrogenesis and metabolic factors. Clin Res Hepatol Gastroenterol. 2011;35 Suppl 1:S10-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol. 2007;47:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 92. | Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G697-G702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 93. | Nolte W, Hartmann H, Ramadori G. Glucose metabolism and liver cirrhosis. Exp Clin Endocrinol Diabetes. 1995;103:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Tessari P. Protein metabolism in liver cirrhosis: from albumin to muscle myofibrils. Curr Opin Clin Nutr Metab Care. 2003;6:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Paridaens A, Laukens D, Vandewynckel YP, Coulon S, Van Vlierberghe H, Geerts A, Colle I. Endoplasmic reticulum stress and angiogenesis: is there an interaction between them? Liver Int. 2014;34:e10-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Kalaitzakis E, Bosaeus I, Ohman L, Björnsson E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: correlations with energy intake and resting energy expenditure. Am J Clin Nutr. 2007;85:808-815. [PubMed] |
| 97. | Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Björnsson E. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Petersen KF, Krssak M, Navarro V, Chandramouli V, Hundal R, Schumann WC, Landau BR, Shulman GI. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am J Physiol. 1999;276:E529-E535. [PubMed] |
| 99. | Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinaemia in Child’s disease grade B liver cirrhosis investigated in free living conditions. Gut. 2002;51:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 100. | Schytte S, Hansen M, Møller S, Junker P, Henriksen JH, Hillingsø J, Teisner B. Hepatic and renal extraction of circulating type I procollagen aminopropeptide in patients with normal liver function and in patients with alcoholic cirrhosis. Scand J Clin Lab Invest. 1999;59:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Benyair R, Kondratyev M, Veselkin E, Tolchinsky S, Shenkman M, Lurie Y, Lederkremer GZ. Constant serum levels of secreted asialoglycoprotein receptor sH2a and decrease with cirrhosis. World J Gastroenterol. 2011;17:5305-5309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Chang WY, Kao HW, Wang HE, Chen JT, Lin WJ, Wang SJ, Chen CL. Synthesis and biological evaluation of technetium-99m labeled galactose derivatives as potential asialoglycoprotein receptor probes in a hepatic fibrosis mouse model. Bioorg Med Chem Lett. 2013;23:6486-6491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Veselkin E, Kondratyev M, Lurie Y, Ron E, Santo M, Reif S, Elashvili I, Bar L, Lederkremer GZ. A secreted form of the asialoglycoprotein receptor, sH2a, as a novel potential noninvasive marker for liver fibrosis. PLoS One. 2011;6:e27210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | Kokudo N, Vera DR, Tada K, Koizumi M, Seki M, Matsubara T, Ohta H, Yamaguchi T, Takahashi T, Nakajima T. Predictors of successful hepatic resection: prognostic usefulness of hepatic asialoglycoprotein receptor analysis. World J Surg. 2002;26:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Schreuder TC, Marsman HA, Lenicek M, van Werven JR, Nederveen AJ, Jansen PL, Schaap FG. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G440-G445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Uriarte I, Latasa MU, Carotti S, Fernandez-Barrena MG, Garcia-Irigoyen O, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, de Mingo A. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int J Cancer. 2015;136:2469-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Bahr MJ, Ockenga J, Böker KH, Manns MP, Tietge UJ. Elevated resistin levels in cirrhosis are associated with the proinflammatory state and altered hepatic glucose metabolism but not with insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E199-E206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Tietge UJ, Selberg O, Kreter A, Bahr MJ, Pirlich M, Burchert W, Müller MJ, Manns MP, Böker KH. Alterations in glucose metabolism associated with liver cirrhosis persist in the clinically stable long-term course after liver transplantation. Liver Transpl. 2004;10:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Geier A. Hepatitis B virus: the “metabolovirus” highjacks cholesterol and bile acid metabolism. Hepatology. 2014;60:1458-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 110. | Oehler N, Volz T, Bhadra OD, Kah J, Allweiss L, Giersch K, Bierwolf J, Riecken K, Pollok JM, Lohse AW. Binding of hepatitis B virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology. 2014;60:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 111. | Dodds EC. Variations in alveolar carbon dioxide pressure in relation to meals. J Physiol. 1921;54:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Dodds EC, Bennett TI. Variations in alveolar carbon dioxide pressure in relation to meals: a further study. J Physiol. 1921;55:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Ghoos Y, Geypens B, Maes B, Hiele M, Rutgeerts P. Breath test in gastric emptying and transit studies: Technical aspects. Progress in Understanding and Management of gastro-intestinal motility disorders. Leuven: Leuven University Press 1993; 169-180. |
| 114. | Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Everson GT, Shiffman ML, Hoefs JC, Morgan TR, Sterling RK, Wagner DA, Lauriski S, Curto TM, Stoddard A, Wright EC. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 116. | Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 117. | Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, Burroughs AK. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. 2006;24:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Lalazar G, Ilan Y. Assessment of liver function in acute or chronic liver disease by the methacetin breath test: a tool for decision making in clinical hepatology. J Breath Res. 2009;3:047001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 119. | O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 120. | Stravitz RT, Reuben A, Mizrahi M, Lalazar G, Brown K, Gordon SC, Ilan Y, Sanyal A. Use of the methacetin breath test to classify the risk of cirrhotic complications and mortality in patients evaluated/listed for liver transplantation. J Hepatol. 2015;63:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Goetze O, Selzner N, Fruehauf H, Fried M, Gerlach T, Mullhaupt B. 13C-methacetin breath test as a quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry. Aliment Pharmacol Ther. 2007;26:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Stremmel W, Wojdat R, Groteguth R, Zoedler M, Ebener T, Niederau C, Becker H, Strohmeyer G. [Liver function tests in a clinical comparison]. Z Gastroenterol. 1992;30:784-790. [PubMed] |
| 123. | Banasch M, Ellrichmann M, Tannapfel A, Schmidt WE, Goetze O. The non-invasive (13)C-methionine breath test detects hepatic mitochondrial dysfunction as a marker of disease activity in non-alcoholic steatohepatitis. Eur J Med Res. 2011;16:258-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 124. | Banasch M, Emminghaus R, Ellrichmann M, Schmidt WE, Goetze O. Longitudinal effects of hepatitis C virus treatment on hepatic mitochondrial dysfunction assessed by C-methionine breath test. Aliment Pharmacol Ther. 2008;28:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Banasch M, Frank J, Serova K, Knyhala K, Kollar S, Potthoff A, Brockmeyer NH, Goetze O. Impact of antiretroviral treatment on (13) C-methionine metabolism as a marker of hepatic mitochondrial function: a longitudinal study. HIV Med. 2011;12:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Banasch M, Goetze O, Hollborn I, Hochdorfer B, Bulut K, Schlottmann R, Hagemann D, Brockmeyer NH, Schmidt WE, Schmitz F. 13C-methionine breath test detects distinct hepatic mitochondrial dysfunction in HIV-infected patients with normal serum lactate. J Acquir Immune Defic Syndr. 2005;40:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Banasch M, Knyhala K, Kollar S, Serova K, Potthoff A, Schlottmann R, Schmidt WE, Brockmeyer NH, Goetze O. Disease- and treatment-related predictors of hepatic mitochondrial dysfunction in chronic HIV infection assessed by non-invasive (13)C-methionine breath test diagnostic. Eur J Med Res. 2008;13:401-408. [PubMed] |
| 128. | Saft C, Zange J, Andrich J, Müller K, Lindenberg K, Landwehrmeyer B, Vorgerd M, Kraus PH, Przuntek H, Schöls L. Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington’s disease. Mov Disord. 2005;20:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 129. | Stüwe SH, Goetze O, Lukas C, Klotz P, Hoffmann R, Banasch M, Orth M, Schmidt WE, Gold R, Saft C. Hepatic mitochondrial dysfunction in manifest and premanifest Huntington disease. Neurology. 2013;80:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 130. | Stüwe SH, Goetze O, Arning L, Banasch M, Schmidt WE, Schöls L, Saft C. Hepatic mitochondrial dysfunction in Friedreich ataxia. BMC Neurol. 2011;11:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Bircher J, Küpfer A, Gikalov I, Preisig R. Aminopyrine demethylation measured by breath analysis in cirrhosis. Clin Pharmacol Ther. 1976;20:484-492. [PubMed] |
| 132. | Mion F, Rousseau M, Scoazec JY, Berger F, Minaire Y. [13C]-Galactose breath test: correlation with liver fibrosis in chronic hepatitis C. Eur J Clin Invest. 1999;29:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118-125. [PubMed] |
| 134. | Helmke S, Colmenero J, Everson GT. Noninvasive assessment of liver function. Curr Opin Gastroenterol. 2015;31:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Stieger B, Heger M, de Graaf W, Paumgartner G, van Gulik T. The emerging role of transport systems in liver function tests. Eur J Pharmacol. 2012;675:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Rowland M, Tozer N. Clinical Pharmacokinetics and Pharamcodynamics. Elimination. Clinical Pharmacokinetics and Pharmacodynamics. 4th ed. Philadelphia: Lippincott Williams & Wilkins 2011; 111-159. |
| 137. | Gilmore IT, Thompson RP. Plasma clearance of oral and intravenous cholic acid in subjects with and without chronic liver disease. Gut. 1980;21:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 138. | Paumgartner G. The handling of indocyanine green by the liver. Schweiz Med Wochenschr. 1975;105:1-30. [PubMed] |
| 139. | Oellerich M, Raude E, Burdelski M, Schulz M, Schmidt FW, Ringe B, Lamesch P, Pichlmayr R, Raith H, Scheruhn M. Monoethylglycinexylidide formation kinetics: a novel approach to assessment of liver function. J Clin Chem Clin Biochem. 1987;25:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 140. | Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, Dörje F, Fromm MF, König J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 141. | Franke RM, Lancaster CS, Peer CJ, Gibson AA, Kosloske AM, Orwick SJ, Mathijssen RH, Figg WD, Baker SD, Sparreboom A. Effect of ABCC2 (MRP2) transport function on erythromycin metabolism. Clin Pharmacol Ther. 2011;89:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 142. | Farrell GC, Cooksley WG, Powell LW. Drug metabolism in liver disease: activity of hepatic microsomal metabolizing enzymes. Clin Pharmacol Ther. 1979;26:483-492. [PubMed] |
| 143. | Boobis AR, Brodie MJ, Kahn GC, Fletcher DR, Saunders JH, Davies DS. Monooxygenase activity of human liver in microsomal fractions of needle biopsy specimens. Br J Clin Pharmacol. 1980;9:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Palmer CN, Coates PJ, Davies SE, Shephard EA, Phillips IR. Localization of cytochrome P-450 gene expression in normal and diseased human liver by in situ hybridization of wax-embedded archival material. Hepatology. 1992;16:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | Lown K, Kolars J, Turgeon K, Merion R, Wrighton SA, Watkins PB. The erythromycin breath test selectively measures P450IIIA in patients with severe liver disease. Clin Pharmacol Ther. 1992;51:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 146. | Guengerich FP, Turvy CG. Comparison of levels of several human microsomal cytochrome P-450 enzymes and epoxide hydrolase in normal and disease states using immunochemical analysis of surgical liver samples. J Pharmacol Exp Ther. 1991;256:1189-1194. [PubMed] |
| 147. | Iqbal S, Vickers C, Elias E. Drug metabolism in end-stage liver disease. In vitro activities of some phase I and phase II enzymes. J Hepatol. 1990;11:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Dietrich CG, Ottenhoff R, de Waart DR, Oude-Elferink RP. Lack of UGT1 isoforms in Gunn rats changes metabolic ratio and facilitates excretion of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo. Toxicol Appl Pharmacol. 2001;170:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 149. | van der Schoor LW, Verkade HJ, Kuipers F, Jonker JW. New insights in the biology of ABC transporters ABCC2 and ABCC3: impact on drug disposition. Expert Opin Drug Metab Toxicol. 2015;11:273-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 150. | Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 151. | Dietrich CG, de Waart DR, Ottenhoff R, Schoots IG, Elferink RP. Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol Pharmacol. 2001;59:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 152. | Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 340] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 153. | Dietrich CG, de Waart DR, Ottenhoff R, Bootsma AH, van Gennip AH, Elferink RP. Mrp2-deficiency in the rat impairs biliary and intestinal excretion and influences metabolism and disposition of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo. Carcinogenesis. 2001;22:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 154. | Dietrich CG, Vehr AK, Martin IV, Gassler N, Rath T, Roeb E, Schmitt J, Trautwein C, Geier A. Downregulation of breast cancer resistance protein in colon adenomas reduces cellular xenobiotic resistance and leads to accumulation of a food-derived carcinogen. Int J Cancer. 2011;129:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 155. | Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506-507:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 156. | Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 157. | Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre-transplant MELD score: a systematic review of the literature. PLoS One. 2013;8:e80661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 158. | Bahra M, Neuhaus P. Liver transplantation in the high MELD era: a fair chance for everyone? Langenbecks Arch Surg. 2011;396:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Biggins SW, Bambha K. MELD-based liver allocation: who is underserved? Semin Liver Dis. 2006;26:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 160. | Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, Burroughs AK. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 161. | Blei AT. Selection for acute liver failure: have we got it right? Liver Transpl. 2005;S30-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 162. | Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 163. | Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1229] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 164. | Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol. 2013;19:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 165. | Williams R. Acute liver failure--practical management. Acta Gastroenterol Belg. 2007;70:210-213. [PubMed] |
| 166. | Bolondi L, Piscaglia F, Camaggi V, Grazi GL, Cavallari A. Review article: liver transplantation for HCC. Treatment options on the waiting list. Aliment Pharmacol Ther. 2003;17 Suppl 2:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |