Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.394
Peer-review started: June 2, 2015
First decision: August 31, 2015
Revised: October 14, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 7, 2016
Processing time: 216 Days and 14 Hours
Obesity and its related metabolic disorders are serious health problems worldwide, and lead to various health-related complications, including cancer. Among human cancers, hepatocellular carcinoma (HCC) is one of the most common malignancies affected by obesity. Therefore, obesity and its related disorders might be a key target for the prevention of HCC. Recently, new research indicates that the molecular abnormalities associated with obesity, including insulin resistance/hyperinsulinemia, chronic inflammation, adipokine imbalance, and oxidative stress, are possible molecular mechanisms underlying the pathogenesis of obesity-related hepatocarcinogenesis. Green tea catechins and branched-chain amino acids, both of which are classified as nutraceutical agents, have been reported to prevent obesity-related HCC development by improving metabolic abnormalities. The administration of acyclic retinoid, a pharmaceutical agent, reduced the incidence of HCC in obese and diabetic mice, and was also associated with improvements in insulin resistance and chronic inflammation. In this article, we review the detailed molecular mechanisms that link obesity to the development of HCC in obese individuals. We also summarize recent evidence from experimental and clinical studies using either nutraceutical or pharmaceutical agents, and suggest that nutraceutical and pharmaceutical approaches targeting metabolic abnormalities might be a promising strategy to prevent the development of obesity-related HCC.
Core tip: Obesity and its related metabolic disorders increase the risk of hepatocellular carcinoma (HCC). In particular, the molecular abnormalities represented by insulin resistance/hyperinsulinemia, chronic inflammation, adipokine imbalance, and oxidative stress play a central role in the development of obesity-related HCC. Administration of green tea catechins, branched-chain amino acids, and acyclic retinoid has improved these metabolic abnormalities, and resulted in the inhibition of HCC development in obese and diabetic mice models. In this review, we highlight the possibility that nutraceutical and pharmaceutical approaches targeting metabolic abnormalities are a promising strategy to prevent the development of obesity-related HCC.
- Citation: Sakai H, Shirakami Y, Shimizu M. Chemoprevention of obesity-related liver carcinogenesis by using pharmaceutical and nutraceutical agents. World J Gastroenterol 2016; 22(1): 394-406
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/394.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.394
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm and the third leading cause of cancer-related deaths in the world. It accounts for more than 90% of primary liver cancers, and its incidence is increasing[1,2]. It is well known that HCC primarily develops from chronic liver inflammation and subsequent cirrhosis induced by persistent infection with the hepatitis B or hepatitis C viruses[3]. However, more recently, the incidence of HCC attributed to nonalcoholic fatty liver disease (NAFLD), which is related to obesity and systemic insulin resistance[4], has been rapidly increasing, especially in developed countries[5].
Obesity is now recognized as one of the most serious health problems worldwide, and its prevalence has dramatically increased in the last few decades[6]. It often causes a number of medical disorders, including type-2 diabetes mellitus, hypertension, and hyperlipidemia, which are collectively known as “metabolic syndrome”. In addition, recent publications indicate that obesity and its related metabolic abnormalities, especially diabetes mellitus, are important risk factors for the development of many types of human malignancies, including HCC[7-16]. Moreover, obesity-associated neoplasms are likely to be more aggressive, and have an increased risk of recurrence, thereby resulting in higher mortality[17,18]. Indeed, in a prospective study conducted in a large cohort of American adults, Calle et al[19] reported that men with a body mass index (BMI) greater than 35 kg/m2 had significantly higher mortality rates due to HCC when compared to men with a normal BMI.
Accumulating evidence from epidemiological and experimental studies indicates that several pathophysiological mechanisms link obesity to liver carcinogenesis, including insulin resistance and adipocytokine imbalance, alterations in the insulin-like growth factor-1 (IGF-1)/IGF-1 receptor (IGF-1R) axis, a state of chronic inflammation, and the induction of oxidative stress[9,10,15,20,21]. Meanwhile, several experimental studies have revealed that the improvement of chronic inflammation through the inhibition of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, plays an important role in the suppression of obesity-related colorectal cancer and HCC[22-25]. These facts suggest that pathophysiological disorders due to obesity and its related metabolic alterations could be critical targets for the chemoprevention of obesity-related liver carcinogenesis.
In this review, we summarize the multiple pathogenic mechanisms by which obesity and its related metabolic disorders induce the development of HCC, with a special focus on the emergence of insulin resistance and subsequent inflammatory cascades. We also discuss the possibility of nutraceutical or pharmaceutical approaches for targeting obesity-induced pathophysiological disorders for the prevention of obesity-related liver carcinogenesis.
NAFLD has been referred to as the hepatic manifestation of obesity and metabolic syndrome and has become one of the most common liver diseases in developed countries[26]. NAFLD is mostly limited to liver steatosis, but approximately 20% of all cases present as nonalcoholic steatohepatitis (NASH) featuring hepatocyte injury, chronic liver inflammation, and various degrees of fibrosis with an elevated risk of developing liver cirrhosis and HCC[27-30]. Obesity is the main risk factor for NAFLD and up to 90% of obese people have some degree of liver steatosis[31]; however, NAFLD can be observed even in non-obese individuals with insulin resistance and hyperinsulinemia[4,32]. In contrast, obesity with only abdominal adiposity and without insulin resistance does not appear to play a role in liver steatosis[33]. These results indicate that obesity-induced metabolic abnormalities, especially insulin resistance, might be a crucial factor in the development of NAFLD.
Most NAFLD-related HCCs are believed to develop in the background of cirrhotic liver, similar to other etiologies such as chronic hepatitis virus infection[3]. Accumulating evidence indicates that NAFLD-induced cirrhosis increases the risk of HCC development in the absence of other risk factors[34-37]. Primary liver carcinomas, including HCC, often occur in patients with NASH, especially in those with advanced fibrosis and cirrhosis[38,39]. However, evidence is also accumulating indicating that NAFLD is strongly associated with the development of non-cirrhotic HCC[40-43].
A recent study from Yasui et al[44] revealed that approximately half of the 87 patients with HCC and biopsy-proven NASH had no established cirrhosis. In addition, a population-based study reported that 2863 cases of HCC (16% of the total number of HCC cases) were due to histologically confirmed NAFLD without other etiologies[45]. Notably, 1031 cases (36%) of NAFLD-related HCC were found in non-cirrhotic livers, and 18% of these cases developed in simple fatty liver without steatohepatitis[45]. Thus, liver cirrhosis is not necessarily linked to the occurrence of obesity-related HCC.
As described above, NAFLD-related HCCs are likely to occur even in individuals with either NASH or simple fatty liver, in the absence of advanced liver fibrosis[34,40-44,46]. In these cases, the presence of metabolic syndrome, especially type 2 diabetes mellitus and obesity, plays a positive role in the development of HCC[47]. Notably, the features of HCC arising in these individuals are different from those arising in patients with chronic viral hepatitis, in terms of tumor size, the degree of tumor differentiation, and the extent of liver fibrosis[43]. Thus, given these differences between NAFLD-related and hepatitis virus-induced HCC, it is anticipated that specific pathophysiological mechanisms may present in the background of NAFLD-related hepatocarcinogenesis.
Although the pathways linking obesity to hepatocarcinogenesis remain poorly defined, accumulating evidence has led to the identification of potential pathophysiological mechanisms, including insulin resistance and the subsequent inflammatory cascades. Liver-specific and systemic insulin resistance are major consequences of obesity[20,48] and lead to fat accumulation in the hepatocyte by lipolysis and hyperinsulinemia, resulting in the development of liver steatosis, including NAFLD[32,47]. In addition, insulin resistance and hyperinsulinemia increase the biological activity of IGF-1, an important endocrine and paracrine regulator of tissue growth and metabolism[49-51].
The binding of insulin and IGF-1 to their respective cell surface receptors on tumors activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is responsible for cellular processes like growth, proliferation, and survival[52,53] Indeed, alterations in the IGF-1/IGF-1R axis have been shown to contribute to the pathogenesis of HCC[52-55]. Moreover, insulin resistance also leads to an increased expression of pro-inflammatory cytokines, including TNF-α and IL-6, through the creation of an oxidative stress environment in the tissues[56]. The dysregulation of these cytokines is associated with the development of steatosis, chronic liver inflammation, and liver tumor formation[9,10,15,20,25,57]. Furthermore, DNA damage due to increased oxidative stress activates the PI3K/Akt pathway[58,59], suggesting that both of these may promote liver tumorigenesis in obese individuals. Thus, insulin resistance and its related inflammatory cascades are thought to be key factors involved in the development of obesity-associated HCC.
Excess fat accumulation in obesity results from a chronic increase in nutrient intake and a decrease in physical exercise, which leads to expansion of adipose tissue and recruitment of various immune cells, such as macrophages[60-62]. Hypertrophic adipocytes and infiltrated macrophages secrete free fatty acids and various pro-inflammatory cytokines, including TNF-α and IL-6[9,10,15,20], and contribute to the development of insulin resistance and low-grade, chronic inflammation[63,64]. Moreover, excess production of storage lipids causes an imbalance of serum adipokine levels, which is related to obesity-associated carcinogenesis[65,66].
The serum levels of adiponectin, an anti-inflammatory and tumor growth-limiting cytokine, are reduced in obese individuals[67] and are negatively correlated with obesity[68,69]. On the other hand, high-circulating levels of leptin, a major adipokine with pro-inflammatory and pro-fibrogenic effects, are observed in patients with obesity and NAFLD[70]. Notably, leptin has a growth-promoting effect through the activation of Janus kinase (JAK), a signal transducer and activator of transcription-3 (Stat3), PI3K/Akt, and extracellular signal-regulated kinase (Erk) signaling pathways[71]. In addition, leptin can induce the expression of TNF-α and IL-6[72,73], and result in tumor growth and progression as described above. Leptin also induces oxidative stress and inflammation in endothelial cells[74]. Indeed, in HCC patients, higher levels of serum leptin increase the risk of HCC recurrence after curative treatment[75]. Moreover, the positive association between leptin levels and the development of HCC has been elucidated by recent in vitro studies[71,76-79]. Taken together, these facts suggest that obesity-related metabolic abnormalities work simultaneously with, and complementary to, one another, and that they increase the risk of cancer, including HCC, in obese individuals (Figure 1).
Recently published research has highlighted the relevance of genetic risk factors in the predisposition toward hepatocarcinogenesis in patients with NAFLD[80]. In particular, the I148M variant of patatin-like phospholipase domain-containing protein 3 (PNPLA3) is a risk factor for HCC development in obese and NAFLD patients[81,82]. Indeed, one recent cohort study involving 3473 obese individuals observed a high incidence of HCC development in the subjects with the I148M risk allele[83]. Interestingly, this risk allele is associated with HCC development independently of its effect on the progression of liver fibrosis and cirrhosis[82,84,85]. Given that NAFLD-related HCC is likely to occur in individuals without advanced liver fibrosis, it is believed that genetic risk factors, such as the I148M variant, also play an important role in the development of NAFLD-related HCC (Figure 1).
The relationship between the intestinal microbiome and metabolic regulation is attracting an increasing amount of attention. Indeed, several experimental studies have demonstrated that intestinal dysbiosis is associated with the development of metabolic disorders, including obesity, insulin resistance, and NAFLD[86-92]. Interestingly, obesity-induced alteration of gut microbiota promotes liver carcinogenesis through the activation of hepatic stellate cells (HSCs). Dietary and genetic obesity induced an alteration of gut microbiota, resulting in increased levels of deoxycholic acid (DCA)[93]. The enterohepatic circulation of DCA induced senescence-associated secretory phenotype (SASP) in the activated HSCs, leading to hepatocarcinogenesis via the secretion of various tumor-promoting factors in the liver[93]. Notably, the inhibition of DCA production or the reduction of gut bacteria prevented the development of HCC in obese mice[93]. Thus, these results indicate that the SASP in the activated HSCs due to obesity-induced gut microbial metabolites plays a key role in the development of obesity-related HCC (Figure 1).
Although several agents have been evaluated in clinical trials, there are currently no well-established therapies for NAFLD[94]. However, several recent clinical studies have elucidated the beneficial effects of weight reduction in the improvement of NAFLD[95-100]. Notably, weight reduction based on dietary and lifestyle modifications improved the histological features of NAFLD in overweight subjects[101]. Interestingly, this beneficial effect was associated with an improvement in biological parameters (aspartate aminotransferase/alanine aminotransferase/γ-glutamyltransferase), metabolic ones (body mass index/fasting glucose/insulin resistance) or in the imbalance of adipocytokines[101]. Besides, recent studies examined the association between the magnitude of weight reduction and changes in histological features of liver steatosis, and reported that a weight loss of over 7% is essential to yield histological outcomes[96,97,100,102]. Moreover, Vilar-Gomez et al[103] reported that weight reduction of over 10% through lifestyle modification significantly reduced NASH-related histological features, including fibrosis and portal inflammation. Thus, weight reduction based on lifestyle modification can be effective in the management of patients with NASH, and is currently recommended as a first line therapeutic intervention for this disease[94,103,104].
As stated earlier, obesity and its related metabolic abnormalities, including insulin resistance and chronic inflammation in the liver, play an important role in the development of HCC. This indicates that metabolic abnormalities induced by obesity may be a valuable target in the prevention of liver carcinogenesis in obese individuals. Indeed, genetic ablation of TNF-α and IL-6 signaling could reduce the incidence of obesity-promoted hepatocarcinogenesis through the reduction of liver steatosis and steatohepatitis[25]. In support of this idea, administration of adiponectin resulted in the reduction of leptin-induced liver tumorigenesis in nude mice[105]. Thus, targeting obesity-related metabolic abnormalities is a promising strategy for the prevention of HCC.
An improvement of metabolic abnormalities through nutraceutical or pharmaceutical intervention might be an effective strategy to inhibit obesity-related liver carcinogenesis, as has already been reported experimentally for colon carcinogenesis[106]. In order to verify this hypothesis, we experimentally investigated the chemopreventive effects of nutritional agents, including green tea catechins (GTCs) and branched-chain amino acids (BCAA) in obese and diabetic C57BL/KsJ-db/db (db/db) mice, and supplemented our findings by summarizing the relevant results of recent publications.
The db/db mice have a functional defect in the long-form leptin receptor, leading to hyperleptinemia and obesity due to overeating. Because of the obesity, hyperinsulinemia, and hyperleptinemia, these mice are regarded as a suitable animal model that mimics metabolic syndrome in humans[107]. In addition, the mice are susceptible to chemical carcinogens and develop N-diethylnitrosamine (DEN)-induced liver tumorigenesis through the activation of IGF/IGF-1R and the induction of chronic inflammation in the liver[54,55,108]. Thus, the db/db mice are thought to be a suitable mouse model of obesity-related hepatocarcinogenesis[54].
Recently, the beneficial effects of GTCs on the improvement of obesity have been reported[109]. A mechanistic review reported that the anti-obesity effects of GTCs results from underlying mechanisms that promote energy expenditure, fatty acid oxidation, and a reduction in nutrient absorption[110]. In addition, GTCs improved hyperglycemia, insulin resistance, and hyperleptinemia, and result in an improvement in liver steatosis and liver dysfunction in rodent diabetic models[111-113]. Treatment with GTCs decreases serum levels of insulin, TNF-α and IL-6 in insulin-resistant rats[114]. Thus, GTCs possess the ability to improve obesity and its related metabolic abnormalities.
In addition to the improvement of obesity, other studies have reported on the anti-cancer and cancer-preventative effects of GTCs[115-118]. A number of studies have demonstrated that GTCs inhibit the proliferation of, and induce apoptosis in, cancer cells by modulating the activation of several receptor tyrosine kinases (RTKs) and their downstream signaling pathways, such as Ras/Erk and PI3K/Akt[115-117,119,120]. Moreover, the down-regulation of IGF/IGF-1R and the activation of IGF-binding protein-3 (IGFBP-3), which negatively controls IGF/IGF-1R signaling, are responsible for the growth inhibition of colorectal and HCC cells[121,122]. Given that IGF/IGF-1R signaling plays an important role in the development of obesity-related HCC as stated above, GTCs are a promising candidate for the chemoprevention of this malignancy.
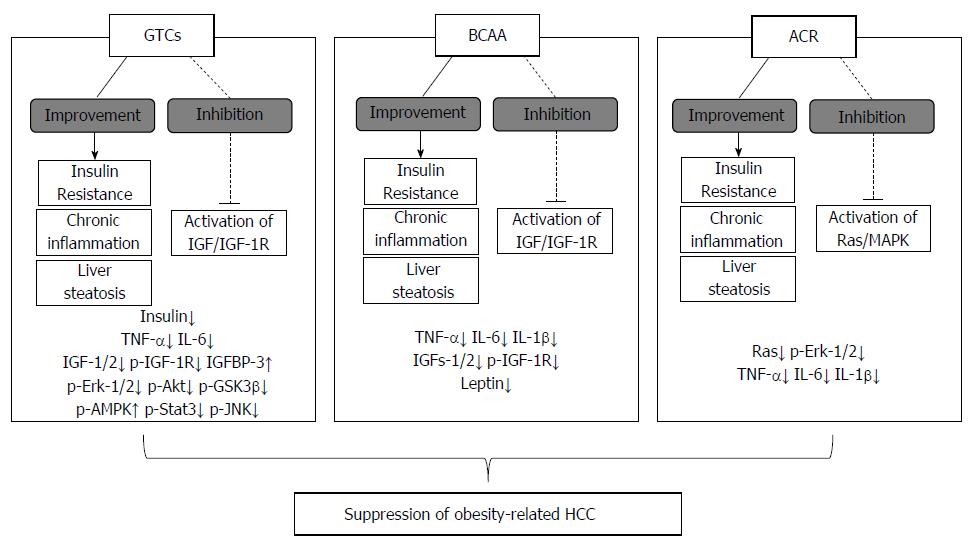
Indeed, our recent publication reported that (-)-epigallocatechin gallate (EGCG), a major biologically active component of green tea catechins, significantly inhibited the development of liver foci and adenoma in DEN-treated db/db mice[55]. Moreover, EGCG decreased the serum levels of insulin, IGF-1, IGF-2, and inhibited the phosphorylation of IGF-1R, Erk, Akt, and GSK-3β in the liver[55]. Furthermore, mRNA expression of TNF-α, IL-6, IL-1β, and IL-18 in the liver was reduced by EGCG treatment[55]. Thus, EGCG prevents obesity-related HCC development by modulating IGF/IGF-1R signaling, and by improving both insulin resistance and chronic liver inflammation. These data also indicate that GTCs, especially EGCG, may be useful for the chemoprevention of obesity-related HCC development (Figure 2).
To date there are no clinical studies that evaluate the chemopreventive effects of GTCs on obesity-related hepatocarcinogenesis in humans. However, our pilot study showed that oral supplementation of GTCs (1.5 g/d) for 1 year significantly reduced the incidence of metachronous colorectal adenomas after polypectomy[123]. In addition, a randomized, double-blinded, placebo-controlled study reported that oral administration of GTCs for 1 year prevented the progression of high-grade prostate intraepithelial neoplasia to prostate cancer[124]. Considering its chemopreventive effects in several human malignancies, an interventional approach using GTCs might also be effective in the prevention of obesity-related hepatocarcinogenesis. However, further clinical studies are needed in this field to verify our hypothesis.
As the liver plays an important role in the regulation of metabolism, patients with chronic liver disease are often malnourished and have metabolic abnormalities, such as hypoalbuminemia and insulin resistance[125-128]. The decreased serum levels of BCAA (valine, leucine, and isoleucine) and hypoalbuminemia worsen the outcomes of cirrhotic patients; however, supplementation with BCAA improves protein-energy malnutrition and hypoalbuminemia, resulting in an improvement in the quality of life and in the prognosis of cirrhotic patients[125,127,129,130]. In addition, the beneficial effects of BCAA on the regulation of glucose metabolism have been demonstrated in recent clinical and experimental studies[131-134]. Thus, BCAA possesses the ability to improve not only malnutrition induced by chronic liver diseases, but also glucose intolerance, such as insulin resistance.
Notably, a long-term survival study reported that the continuous administration of BCAA was associated with a reduced incidence of HCC in obese cirrhotic patients[12]. This result also suggests the hypothesis that BCAA might have an anti-cancer effect on the development of HCC in obese cirrhotic patients. Therefore, in order to verify our hypothesis and clarify the detailed mechanisms of BCAA in the prevention of obesity-related HCC development, we conducted an experimental study by using a DEN-induced HCC model in obese and diabetic db/db mice[54]. In this study, BCAA supplementation significantly inhibited the incidence of liver neoplasms, including hepatic adenoma and HCC, through the inhibition of IGF-1, IGF-2, and IGF-1R protein expression in the liver[54]. The reduced incidence of liver neoplasms in this model was associated with improvements in insulin resistance, hyperleptinemia, and liver steatosis[54]. Moreover, our recent publication demonstrated that the administration of BCAA significantly suppressed the spontaneous development of liver neoplasms in db/db mice by inhibiting the expression of TNF-α, IL-6, and IL-1β mRNA in the liver[135]. Furthermore, BCAA inhibited the infiltration of macrophages into white adipose tissues, resulting in a reduction of TNF-α and IL-6 mRNA production from the tissue[135]. Thus, BCAA inhibited the development of liver tumorigenesis in obese rodents by regulating various obesity-induced metabolic abnormalities, especially insulin resistance and chronic inflammation, suggesting that BCAA is a promising agent for the chemoprevention of liver carcinogenesis in obese patients (Figure 2).
Retinoids, derivatives of vitamin A, exert their biological functions by regulating the transcription of target genes through two distinct nuclear receptors - retinoic acid (RA) receptors (RARs) and retinoid X receptors (RXRs), both of which consist of three subtypes (α, β, and γ) characterized by a modular domain structure[136,137]. Among these receptors, RXRα is the most abundant RXR subtype in the adult liver[138]. Once RXRα is activated by its specific ligands, including RA or 9-cis-RA (9cRA), RXRα forms homodimers with itself or heterodimers with other RARs and then interacts with their respective DNA response elements, resulting in the regulation of proliferation, differentiation, and apoptosis of liver cells. Thus, RXRα plays a crucial role in maintaining the homeostasis of liver cells.
Reduced expression of RXRα has been associated with carcinogen-induced rat hepatocarcinogenesis[139]. The impact of impaired receptor function of RXRα in the development of HCC is demonstrated through experimental studies. In HCC, RXRα is highly phosphorylated by an activated Ras-Erk 1/2 pathway, and accumulates in HCC by preventing its normal degradation through the ubiquitin-proteasome pathway[140]. The accumulated RXRα abrogates the function of the remaining intact RXRα in a dominant-negative manner, thereby inhibiting the formation of heterodimers with the partner molecules, including a tumor suppressor gene, RARβ[141-144]. In addition, phosphorylated RXRα is refractory to its potent ligand, 9cRA, and evades 9cRA-induced apoptosis[145]. Thus, the impaired receptor function of RXRα due to phospho-modification also plays a critical role in the development of HCC, suggesting that phosphorylated-RXRα may in the future be a key target for HCC chemoprevention and treatment.
Acyclic retinoid (ACR), which is equivalent to NIK-333 or peretinoin (Kowa Pharmaceutical Co., Tokyo, Japan), is a synthetic retinoid developed for HCC chemoprevention[139]. Recently, the chemopreventive effects of ACR were reported in our clinical studies. A randomized, controlled clinical trial examined the chemopreventive effects of ACR on secondary HCC in patients who underwent curative treatment for initial HCC[146-148]. In this study, oral administration of ACR (n = 44 patients; dose = 600 mg/d) for 12 mo significantly reduced the incidence of post-therapeutic recurrence or new HCC development compared to the placebo group (n = 45 patients) (median follow-up time = 38 mo; P = 0.04)[146,147]. Moreover, the preventative effects of ACR lasted for up to 3 years following the completion of ACR administration[148]. In addition, a subgroup analysis of a large-scale, randomized, placebo-controlled study (n = 401 patients) also showed that ACR (n = 100 patients; dose = 600 mg/d) reduced the risk of HCC recurrence or death by approximately 40% compared to placebo (n = 106 patients), especially in patients with Child-Pugh A and small tumors (size < 20 mm) (P = 0.0347)[149].
The possible molecular mechanisms by which ACR prevents the recurrence and the development of secondary HCC have been elucidated in experimental studies using HCC cell lines. We found that ACR restores the impaired receptor functions of RXRα by inhibiting RXRα phosphorylation. Namely, ACR inhibits the activated Ras-Erk 1/2 pathway independent of RXRα, and consequently prevents phospho-modification of RXRα, thereby restoring the function of RXRα in HCC cells[150]. Furthermore, we found that ACR inhibits not only the Ras-Erk 1/2 pathways but also several types of growth factors and their corresponding RTKs in several malignancies, including HCC[151-156]. Moreover, ACR itself functions as a ligand for RXRα and regulates the expression of its downstream genes such as p21, RARβ, and Cyclin D1, thereby preventing HCC development through inhibition of cell proliferation or induction of differentiation and apoptosis[145,153,157,158]. Thus, ACR may prevent the development of HCC via the pleiotropic responses of ACR target molecules, including phosphorylated RXRα.
Interestingly, our experimental study also elucidated the chemopreventive effects of ACR on the development of obesity-related HCC using the DEN-induced HCC model of obesity and diabetic db/db mice[108]. In this study, ACR significantly reduced the incidence of obesity-related HCC by inhibiting Ras activation and the phosphorylation of Erk-1/2 and RXRα, thereby restoring RXRα function in the liver[108]. Notably, the administration of ACR improved obesity-related metabolic abnormalities, such as insulin resistance and liver steatosis[108]. Moreover, ACR treatment decreased the levels of serum TNFα, as well as the expression of TNFα, IL-6, and IL-1β mRNA in the liver, resulting in an improvement of chronic inflammation[108]. As stated above, insulin resistance and chronic inflammation are significant risk factors for the development of obesity-related HCC. Therefore, the use of ACR in obese and cirrhotic patients with diabetes might be an effective strategy in preventing obesity-related HCC (Figure 2).
In this review, we highlighted nutraceutical and pharmaceutical approaches to targeting metabolic abnormalities as promising strategies to prevent the development of HCC in obese individuals. The molecular abnormalities represented by (1) insulin resistance/hyperinsulinemia; (2) chronic inflammation; (3) adipokine imbalance; and (4) oxidative stress, are regarded as the likely molecular mechanisms linking obesity to cancer development, including HCC. As stated above, the nutraceutical agents GTCs and BCAA prevented the development of obesity-related HCC by inhibiting those abnormalities in obese and diabetic mice. The safety of both GTCs and BCAA has been shown in recent clinical studies[123-125,127,129,130], suggesting that an intervention using GTCs and BCAA might be a practical approach for the chemoprevention of obesity-related HCC.
In addition, the pharmaceutical agent ACR has chemopreventive effects for recurrence or secondary HCC after curative treatment[146-149], and the safety of the agent was demonstrated in our recent clinical studies[146-149,159]. Moreover, ACR significantly reduced the incidence of obesity-related HCC in obese and diabetic mice, and this effect was associated with an improvement in metabolic abnormalities, including insulin resistance and chronic inflammation[108]. While the detailed mechanism by which ACR improves metabolic homeostasis remains unclear, the positive effects of ACR observed in the experimental study[108] may encourage the use of the agent for the prevention of HCC in obese individuals.
In conclusion, recent evidence highlights that nutraceutical and pharmaceutical approaches that target metabolic abnormalities are a promising strategy for preventing the development of obesity-related HCC. GTCs, BCAA, and ACR might be candidates for this strategy. Further clinical studies are needed to investigate if active intervention using one or more of these agents can prevent the development of obesity-related HCC.
P- Reviewer: Mihaila RG, Tai DI, Valenti L S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2140] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 3. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 4. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] |
| 5. | Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 433] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 7. | Chow WH, Gridley G, Fraumeni JF, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 436] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 601] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 9. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4267] [Article Influence: 237.1] [Reference Citation Analysis (2)] |
| 10. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [PubMed] |
| 11. | Imai K, Takai K, Nishigaki Y, Shimizu S, Naiki T, Hayashi H, Uematsu T, Sugihara J, Tomita E, Shimizu M. Insulin resistance raises the risk for recurrence of stage I hepatocellular carcinoma after curative radiofrequency ablation in hepatitis C virus-positive patients: A prospective, case series study. Hepatol Res. 2010;40:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 16. | Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006;113:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000;152:847-854. [PubMed] |
| 19. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 20. | Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2509] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 21. | Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Kubota M, Shimizu M, Sakai H, Yasuda Y, Ohno T, Kochi T, Tsurumi H, Tanaka T, Moriwaki H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem Biophys Res Commun. 2011;410:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T, Moriwaki H. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012;64:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Yasuda Y, Shimizu M, Shirakami Y, Sakai H, Kubota M, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010;101:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1369] [Article Influence: 91.3] [Reference Citation Analysis (1)] |
| 26. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3720] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 27. | Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1708] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 29. | Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 31. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 32. | Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 606] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 33. | Tarantino G, Colicchio P, Conca P, Finelli C, Di Minno MN, Tarantino M, Capone D, Pasanisi F. Young adult obese subjects with and without insulin resistance: what is the role of chronic inflammation and how to weigh it non-invasively? J Inflamm (Lond). 2009;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 35. | Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13:631-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291-293. [PubMed] |
| 38. | Diehl AM. Hepatic complications of obesity. Gastroenterol Clin North Am. 2010;39:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Chagas AL, Kikuchi LO, Oliveira CP, Vezozzo DC, Mello ES, Oliveira AC, Cella LC, Herman P, Bachella T, Caldwell SH. Does hepatocellular carcinoma in non-alcoholic steatohepatitis exist in cirrhotic and non-cirrhotic patients? Braz J Med Biol Res. 2009;42:958-962. [PubMed] |
| 41. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 42. | Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 43. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 44. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 45. | Rahman R, Ibdah J. Nonalcoholic fatty liver disease without cirrhosis is an emergent and independent risk factor of hepatocellular carcinoma: a population based study. Hepatology. 2012;56:241A. |
| 46. | Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (2)] |
| 47. | Rahman R, Hammoud GM, Almashhrawi AA, Ahmed KT, Ibdah JA. Primary hepatocellular carcinoma and metabolic syndrome: An update. World J Gastrointest Oncol. 2013;5:186-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3583] [Cited by in RCA: 3655] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 49. | Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972-980. [PubMed] |
| 51. | Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 52. | Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 53. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1567] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 54. | Iwasa J, Shimizu M, Shiraki M, Shirakami Y, Sakai H, Terakura Y, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Shimizu M, Sakai H, Shirakami Y, Yasuda Y, Kubota M, Terakura D, Baba A, Ohno T, Hara Y, Tanaka T. Preventive effects of (-)-epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db Mice. Cancer Prev Res (Phila). 2011;4:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067-2072. [PubMed] |
| 57. | Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 58. | Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37-56. [PubMed] |
| 60. | Haslam DW, James WP. Obesity. Lancet. 2005;366:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3211] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 61. | Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1388] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 62. | Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 709] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 63. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3626] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 64. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 2506] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 65. | Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858-s866. [PubMed] |
| 66. | Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4210] [Cited by in RCA: 4200] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 67. | Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 475] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 68. | Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol. 2005;5:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-1599. [PubMed] |
| 70. | Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497-2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 72. | Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Molina A, Vendrell J, Gutiérrez C, Simón I, Masdevall C, Soler J, Gómez JM. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Matarese G, La Cava A, Sanna V, Lord GM, Lechler RI, Fontana S, Zappacosta S. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23:182-187. [PubMed] |
| 75. | Watanabe N, Takai K, Imai K, Shimizu M, Naiki T, Nagaki M, Moriwaki H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J Clin Biochem Nutr. 2011;49:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Ramani K, Yang H, Xia M, Ara AI, Mato JM, Lu SC. Leptin’s mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2beta. Hepatology. 2008;47:521-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin-leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29:1596-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20:12945-12955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C & gt; G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 82. | Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, Valenti L. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969-6978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 170] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 83. | Burza MA, Pirazzi C, Maglio C, Sjöholm K, Mancina RM, Svensson PA, Jacobson P, Adiels M, Baroni MG, Borén J. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Corradini SG, Burza MA, Molinaro A, Romeo S. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology. 2011;53:1776; author reply 1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Trepo E, Guyot E, Ganne-Carrie N, Degre D, Gustot T, Franchimont D, Sutton A, Nahon P, Moreno C. PNPLA3 (rs738409 C& gt; G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. 2012;55:1307-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4417] [Article Influence: 210.3] [Reference Citation Analysis (4)] |
| 87. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 88. | Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948-4959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 89. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 90. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 556] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 91. | Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8:e62885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 92. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 752] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 93. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1652] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 94. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 95. | Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 526] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 96. | Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L, Yasells Garcia A, Del Rosario Abreu Vazquez M. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;30:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 97. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 98. | Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 99. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 100. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 101. | Copaci I, Lupescu I, Caceaune E, Chiriac G, Ismail G. Noninvasive Markers of Improvement of Liver Steatosis Achieved by Weight Reduction in Patients with Nonalcoholic Fatty Liver Disease. Rom J Intern Med. 2015;53:54-62. [PubMed] |
| 102. | Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 103. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-378.e5; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1629] [Article Influence: 162.9] [Reference Citation Analysis (1)] |
| 104. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 105. | Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762-1773, 1773.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Shimizu M, Shirakami Y, Sakai H, Adachi S, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila). 2008;1:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 107. | Scheen AJ, Luyckx FH. Obesity and liver disease. Best Pract Res Clin Endocrinol Metab. 2002;16:703-716. [PubMed] |
| 108. | Shimizu M, Sakai H, Shirakami Y, Iwasa J, Yasuda Y, Kubota M, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- +(db)/+Lepr(db) mice. Cancer Prev Res (Phila). 2011;4:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 110. | Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 111. | Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677-1683. [PubMed] |
| 112. | Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 113. | Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br J Nutr. 2009;102:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol Nutr Food Res. 2010;54 Suppl 1:S14-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 115. | Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. 2011;55:832-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 116. | Shimizu M, Shirakami Y, Moriwaki H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int J Mol Sci. 2008;9:1034-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Shimizu M, Weinstein IB. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat Res. 2005;591:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 118. | Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 830] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 119. | Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493-6501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 120. | Adachi S, Nagao T, To S, Joe AK, Shimizu M, Matsushima-Nishiwaki R, Kozawa O, Moriwaki H, Maxfield FR, Weinstein IB. (-)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis. 2008;29:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, Weinstein IB, Moriwaki H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 123. | Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 124. | Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 528] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 125. | Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 126. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [PubMed] |
| 127. | Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405-409. [PubMed] |
| 128. | Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616-627. [PubMed] |
| 129. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [PubMed] |
| 130. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [PubMed] |
| 131. | Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T, Nakamuta M, Enjoji M, Kotoh K, Takayanagi R. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem. 2011;112:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 132. | Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105-112. [PubMed] |
| 133. | She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 134. | Urata Y, Okita K, Korenaga K, Uchida K, Yamasaki T, Sakaida I. The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol Res. 2007;37:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Terakura D, Shimizu M, Iwasa J, Baba A, Kochi T, Ohno T, Kubota M, Shirakami Y, Shiraki M, Takai K. Preventive effects of branched-chain amino acid supplementation on the spontaneous development of hepatic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Carcinogenesis. 2012;33:2499-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940-954. [PubMed] |
| 137. | Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 138. | Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329-344. [PubMed] |
| 139. | Muto Y, Moriwaki H. Antitumor activity of vitamin A and its derivatives. J Natl Cancer Inst. 1984;73:1389-1393. [PubMed] |
| 140. | Adachi S, Okuno M, Matsushima-Nishiwaki R, Takano Y, Kojima S, Friedman SL, Moriwaki H, Okano Y. Phosphorylation of retinoid X receptor suppresses its ubiquitination in human hepatocellular carcinoma. Hepatology. 2002;35:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 585] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 142. | Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S-482S. [PubMed] |
| 143. | Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41-55. [PubMed] |
| 144. | Yoshimura K, Muto Y, Shimizu M, Matsushima-Nishiwaki R, Okuno M, Takano Y, Tsurumi H, Kojima S, Okano Y, Moriwaki H. Phosphorylated retinoid X receptor alpha loses its heterodimeric activity with retinoic acid receptor beta. Cancer Sci. 2007;98:1868-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Yasuda I, Shiratori Y, Adachi S, Obora A, Takemura M, Okuno M, Shidoji Y, Seishima M, Muto Y, Moriwaki H. Acyclic retinoid induces partial differentiation, down-regulates telomerase reverse transcriptase mRNA expression and telomerase activity, and induces apoptosis in human hepatoma-derived cell lines. J Hepatol. 2002;36:660-671. [PubMed] |
| 146. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 477] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 147. | Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340:1046-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 148. | Takai K, Okuno M, Yasuda I, Matsushima-Nishiwaki R, Uematsu T, Tsurumi H, Shiratori Y, Muto Y, Moriwaki H. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Updated analysis of the long-term follow-up data. Intervirology. 2005;48:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Okita K, Izumi N, Matsui O, Tanaka K, Kaneko S, Moriwaki H, Ikeda K, Osaki Y, Numata K, Nakachi K. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 150. | Matsushima-Nishiwaki R, Okuno M, Takano Y, Kojima S, Friedman SL, Moriwaki H. Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid. Carcinogenesis. 2003;24:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Kagawa M, Sano T, Ishibashi N, Hashimoto M, Okuno M, Moriwaki H, Suzuki R, Kohno H, Tanaka T. An acyclic retinoid, NIK-333, inhibits N-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of TGF-alpha expression and cell proliferation. Carcinogenesis. 2004;25:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Komi Y, Sogabe Y, Ishibashi N, Sato Y, Moriwaki H, Shimokado K, Kojima S. Acyclic retinoid inhibits angiogenesis by suppressing the MAPK pathway. Lab Invest. 2010;90:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 153. | Nakamura N, Shidoji Y, Yamada Y, Hatakeyama H, Moriwaki H, Muto Y. Induction of apoptosis by acyclic retinoid in the human hepatoma-derived cell line, HuH-7. Biochem Biophys Res Commun. 1995;207:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 154. | Sano T, Kagawa M, Okuno M, Ishibashi N, Hashimoto M, Yamamoto M, Suzuki R, Kohno H, Matsushima-Nishiwaki R, Takano Y. Prevention of rat hepatocarcinogenesis by acyclic retinoid is accompanied by reduction in emergence of both TGF-alpha-expressing oval-like cells and activated hepatic stellate cells. Nutr Cancer. 2005;51:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 155. | Shao RX, Otsuka M, Kato N, Taniguchi H, Hoshida Y, Moriyama M, Kawabe T, Omata M. Acyclic retinoid inhibits human hepatoma cell growth by suppressing fibroblast growth factor-mediated signaling pathways. Gastroenterology. 2005;128:86-95. [PubMed] |
| 156. | Shimizu M, Suzui M, Deguchi A, Lim JT, Weinstein IB. Effects of acyclic retinoid on growth, cell cycle control, epidermal growth factor receptor signaling, and gene expression in human squamous cell carcinoma cells. Clin Cancer Res. 2004;10:1130-1140. [PubMed] |
| 157. | Araki H, Shidoji Y, Yamada Y, Moriwaki H, Muto Y. Retinoid agonist activities of synthetic geranyl geranoic acid derivatives. Biochem Biophys Res Commun. 1995;209:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 158. | Suzui M, Masuda M, Lim JT, Albanese C, Pestell RG, Weinstein IB. Growth inhibition of human hepatoma cells by acyclic retinoid is associated with induction of p21(CIP1) and inhibition of expression of cyclin D1. Cancer Res. 2002;62:3997-4006. [PubMed] |
| 159. | Okusaka T, Ueno H, Ikeda M, Morizane C. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol Res. 2011;41:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |