INTRODUCTION
Ischemia and reperfusion (IR) lead to damage in aerobically metabolizing tissues or organs[1]. It is an important injury mechanism in many clinical settings, e.g., liver surgical resection and liver transplantation[2,3]. Hepatocyte death due to IR insults results in the release reactive oxygen species (ROS) that initiate a sterile immune response. This activates Kupffer cells first and then recruits neutrophils to infiltrate[4]; hence, liver IR injury has a biphasic pattern: the acute phase, which involves activation of Kupffer cells after 4-6 h of reperfusion, and the subacute phase, which results from neutrophil infiltration at 18-24 h[5-7].
Autophagy, including macroautophagy, microautophagy and chaperone-mediated autophagy, is a process that degrades macromolecules and organelles via lysosomes[8]. It is very important for cell differentiation, survival during nutrient depletion and maintenance of homeostasis[9]. When hepatocytes suffer from nutrient starvation and anoxia during IR insults, autophagy can ameliorate liver damage[8,10].
All-trans retinoic acid (ATRA) has been shown to protect the liver from IR injury. The protective mechanism of ATRA relies on inhibiting ROS generation and the nuclear factor-kappa B (NF-κB) pathway[11,12]. In addition, ATRA has been shown to mediate autophagy in acute promyelocytic leukemia cells[13]. Similarly, ATRA promotes autophagy by redistributing the cation-independent mannose-6-phosphate/IGFII receptor[14]. ATRA increases the transcription of Forkhead box O (Foxo) 3a, which is a strong inducer of Foxo1[15,16]. The Foxo3a/p-Akt/Foxo1 signaling pathway also augments autophagy[17]. To date, there has been little research into how ATRA alleviates liver IR injury through autophagy. This report is the first to demonstrate the effects of the retinoic acid receptor α (RARα)/Foxo3a/p-Akt/Foxo1 pathway on liver IR injury.
MATERIALS AND METHODS
Animals
Male wild-type C57BL/6 mice (8-12-wk-old) were obtained from Vital River Experimental Animal Co., P.R. China. Mice were housed in special pathogen-free conditions with a 12-h light-dark cycle and free access to standard laboratory diet and water, and they received humane care in accordance with the guidelines of the Chinese Association of Laboratory Animal Care. The standards for animal use and care were approved by the Institutional Animal Care Committee of Nanjing Medical University (Protocol Number: NJMU08-092).
Operative procedure
Before the experiment, the mice received ATRA (Sigma-Aldrich, Shanghai, China) by gavage at 15 mg/kg per day for two weeks. We induced 70% hepatic ischemia by occluding the hepatic artery, portal vein and bile duct to the cephalad hepatic lobes for 90 min. During the ischemic process, mice were anesthetized using isoflurane, and the environmental temperature was maintained at 25 °C. After liver reperfusion for 6 h or 20 h, the mice were sacrificed, and then blood and liver tissue samples were harvested for analysis.
Hepatocellular function assay
Serum alanine aminotransferase (sALT) and serum aspartate aminotransferase (sAST) levels were measured to assess hepatocyte injury using an automated chemical analyzer (Olympus Automated Chemistry Analyzer AU5400, Tokyo, Japan).
Histopathological study
Liver samples were fixed with 10% neutral formaldehyde and embedded in paraffin. Paraffin sections at 5 μm were stained with hematoxylin and eosin and then blindly analyzed and scored from 0 to 4, as described by Suzuki et al[18].
Western blot analysis
Proteins were extracted from liver samples and cell lysates, and the concentration was detected using a BCA Protein Assay Kit (Thermo Fisher Scientific, Shanghai, China). The nuclear proteins were extracted to detect the level of Foxo1. The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transblotted onto polyvinylidene fluoride membranes (Millipore, United States). These membranes were blocked in non-fat dry milk (5% wt/vol) with Tris-buffered saline containing 0.1% Tween 20 (TBS-T) at 4 °C overnight and then incubated with primary antibodies against RARα (Santa Cruz Biotechnology, Santa Cruz, CA, United States), Bcl-2, Beclin-1, LC3B, β-actin (Cell Signaling Technology, Danvers, MA, United States), cleaved caspase 3, p-Akt, Foxo1, Foxo3a, and p62 (Abcam, Shanghai, China). Following three washes with TBS-T, the membranes were incubated with a peroxidase-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA, United States) for 1 h at room temperature. Bands were quantified by densitometry using the Quantity One software for image analysis.
Quantitative real-time reverse transcriptase-polymerase chain reaction
RNA was isolated using TRIzol reagent (Invitrogen, Shanghai, China). cDNA was synthesized according to the manufacturer’s instructions using the PrimeScriptTM RT Reagent Kit with gDNA Eraser (Takara, Japan). Polymerase chain reaction (PCR) was performed using SYBR Premix Ex TaqTM (Takara, Japan). The primer sets were as follows: β-actin: forward, 5’-CTA CAA TGA GCT GCG TGT GG-3’ and reverse, 5’-AAG GAA GGC TGG AAG AGT GC-3’; IL-6: forward, 5’-GAC TTC CAT CCA GTT GCC TTC T-3’ and reverse, 5’-TTT CTC ATT TCC ACG ATT TCC CA-3’; IL-1β: forward, 5’-GTG TTT TCC TCC TTG CCT CTG AT-3’ and reverse, 5’-GCT GCC TAA TGT CCC CTT GAA T-3’; tumor necrosis factor (TNF)-α: forward, 5’-CTC TGT GAA GGG AAT GGG TGT-3’ and reverse, 5’-TCT TGT GTT TCT GAG TAG TTG TTG A-3’.
Cell culture and treatment
FL83B mouse hepatocytes were cultured in Ham’s F-12K medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Before being co-cultured with 200 μmol/L H2O2 for 6 h, cells were preincubated with ATRA (10 μmol/L) or LE540 (10 μmol/L, Wako Pure Chemical Industries Ltd., Osaka, Japan) for 24 h.
Annexin V and propidium iodide labeling
The cell samples were stained using an Annexin V (AV)-fluorescein isothiocyanate Apoptosis Detection Kit (Sigma-Aldrich, St Louis, United States) following the manufacturer’s instructions. For confocal microscopy, both probes were activated by a 488 nm diode laser, and the fluorescence emission was detected at 510 and 560 nm for AV and propidium iodide (PI), respectively. The fluorescence intensity was obtained for 20000 events, and the data were analyzed using Cell-Quest™ (Becton-Dickinson, San Jose). The data are presented as the percentage of cells in four different population phenotypes-unstained, stained only with AV, stained only with PI or stained with both markers-relative to the total number of cells analyzed.
Statistical analysis
Data are presented as mean ± SD from at least three independent experiments. SPSS software (Chicago, IL, United States) was used to calculate the statistical significance by performing one-way analysis of variance. All the P values were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
ATRA pretreatment ameliorates liver injury after IR
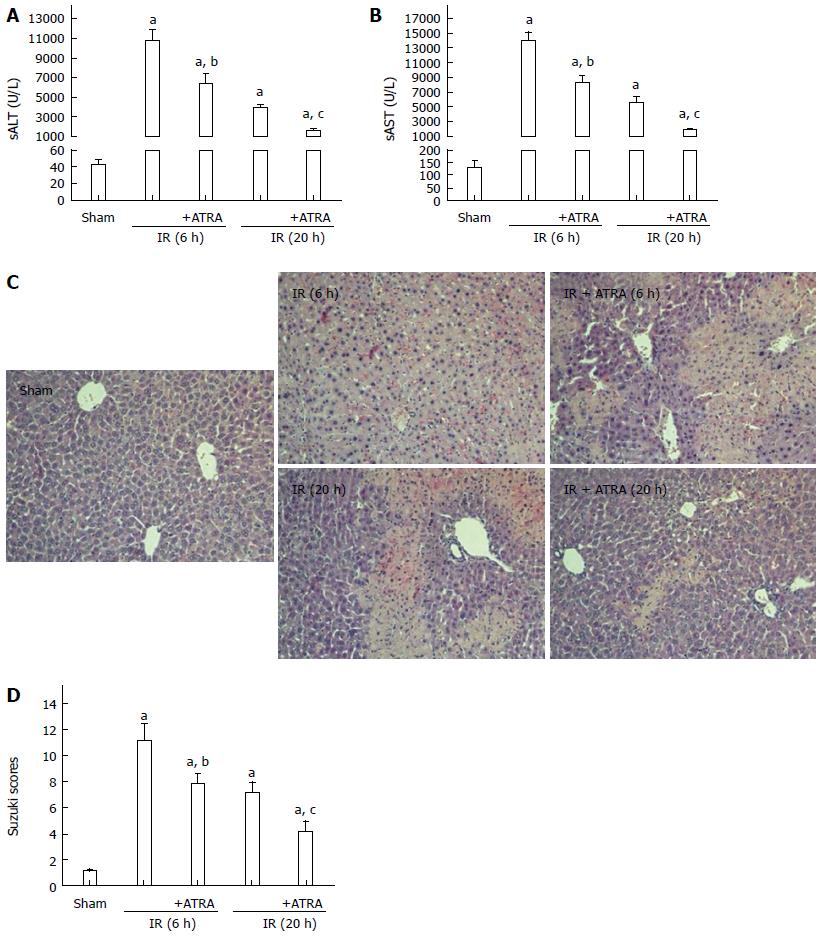
We used an established model of hepatic damage in mice, inducing 90 min of ischemia and then sacrificing the mice at 6 h or 20 h after reperfusion. Before the operation, ATRA was administered by gavage once a day for two weeks. The levels of sALT and sAST, the area of necrosis on liver histopathology and the samples’ Suzuki scores were obviously decreased in the ATRA group compared with the IR control group (Figure 1).
Figure 1 All-trans retinoic acid pretreatment ameliorates liver ischemia and reperfusion injury.
Hepatic ischemia was induced in mice for 90 min, and then the mice were sacrificed at 6 h or 20 h after reperfusion. A, B: Hepatocellular function as assessed by sALT and sAST levels; C: Liver samples were stained with hematoxylin and eosin (magnification × 200); D: Liver histological grade by the Suzuki criteria. Mean ± SD, n = 3-5/group, aP < 0.05 vs the sham group; bP < 0.05 vs the IR 6 h group; cP < 0.05 vs the IR 20 h group. ATRA: All-trans retinoic acid; IR: Ischemia and reperfusion; sALT: Serum alanine aminotransferase; sAST: Serum aspartate aminotransferase.
ATRA pretreatment reduces apoptosis and inflammation in liver IR injury
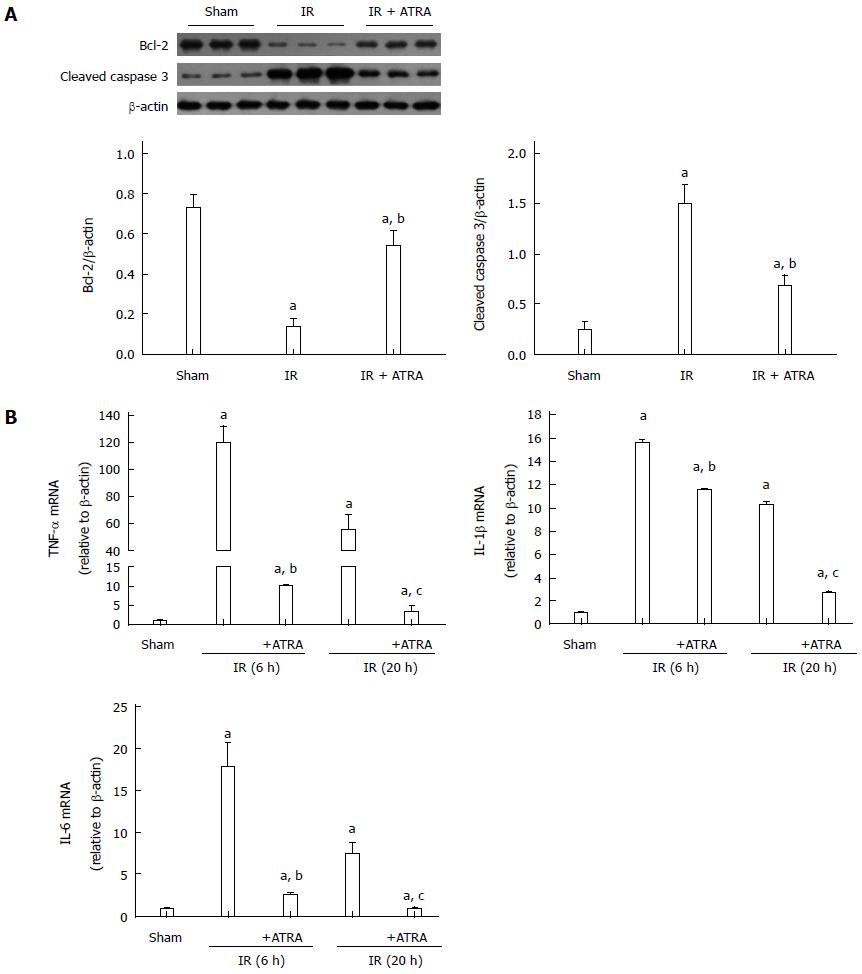
When IR injury occurs, hepatocellular apoptosis is induced, and this condition results in liver injury[19]. According to liver function and histological score, liver damage was more serious at 6 h after reperfusion. So, we just chose the liver samples at 6 h after reperfusion to analyze by Western blot. ATRA enhanced the expression of the anti-apoptotic protein Bcl-2 while inhibited the expression of the pro-apoptotic protein cleaved caspase 3 in liver IR injury (Figure 2A). This mechanism explains the downregulation of hepatocellular apoptosis. Besides, the role of IR injury in inflammation was weakened, because the mRNA levels of TNF-α, interleukin (IL)-1β and IL-6 in the ATRA group were less than those in the IR group (Figure 2B).
Figure 2 All-trans retinoic acid pretreatment reduces apoptosis and inflammation in liver ischemia and reperfusion injury.
A: Apoptosis-related proteins Bcl-2 and cleaved caspase 3 expression was detected by Western blot to demonstrate relative quantities in liver tissues; B: The levels of TNF-α, IL-1β and IL-6 mRNAs were analyzed. Mean ± SD, n = 3-5/group, aP < 0.05 vs the sham group; bP < 0.05 vs the IR 6h group; cP < 0.05 vs the IR 20h group. TNF: Tumor necrosis factor; IL: Interleukin; IR: Ischemia and reperfusion; ATRA: All-trans retinoic acid.
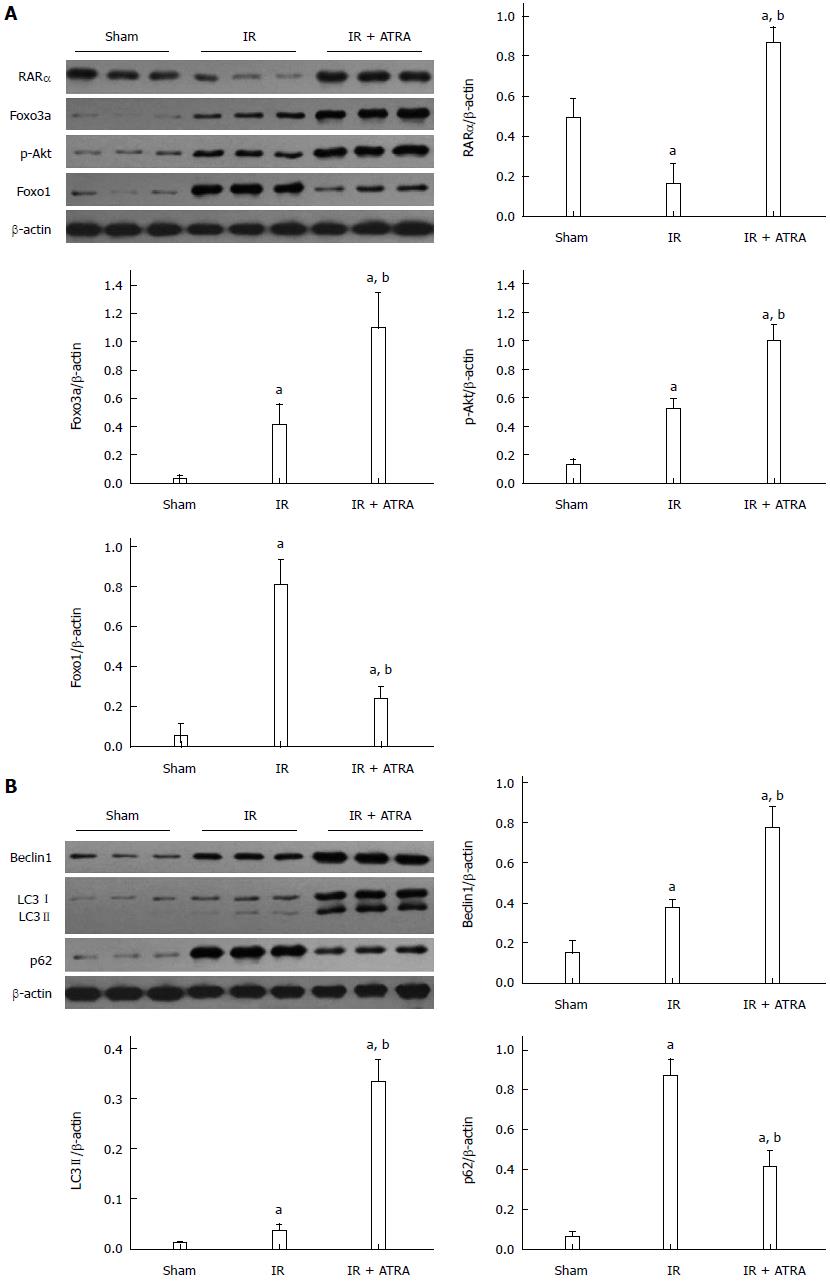
Autophagy is promoted by ATRA
RARα was activated by ATRA. Similarly, the Foxo3a/p-Akt/Foxo1 pathway was induced by RARα activation (Figure 3A). Foxo3a/p-Akt/Foxo1 has been shown to promote autophagy[17]. The proteins Beclin1, LC3 II and p62 are involved in the process of autophagy. As a substrate, p62 is degraded by autolysosome and inversely correlates with the autophagic activity[20-22]. ATRA pretreatment enhanced the levels of Beclin1 and LC3 II but decreased the level of p62 (Figure 3B). These results suggest that ATRA promotes autophagy in liver IR injury.
Figure 3 Autophagy is promoted by all-trans retinoic acid.
A: Western blot assessment of RARα, Foxo3a, p-Akt, Foxo1, and β-actin expression levels; B: Western blot analysis of Beclin1, LC3I/II, p62 and β-actin expression levels. Mean ± SD, n = 3-5/group, aP < 0.05 vs the sham group; bP < 0.05 vs the IR 6 h group. IR: Ischemia and reperfusion; ATRA: All-trans retinoic acid.
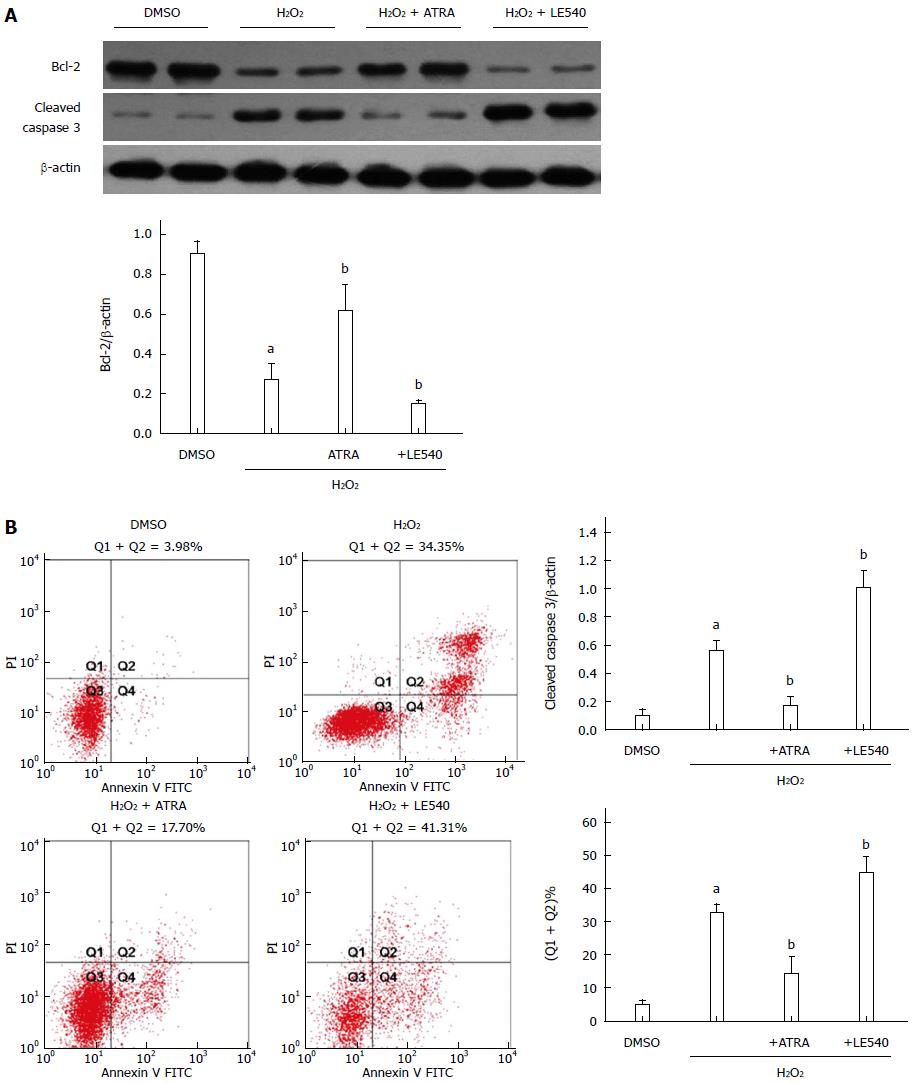
RARα inhibits ROS-induced apoptosis in vitro
To further analyze the effect of RARα, LE540 was used to inhibit RARα activity. Following H2O2 stimulation, ATRA, the RARα agonist, upregulated the expression of Bcl-2, downregulated cleaved caspase 3 expression and ultimately decreased the number of apoptotic cells detected by Annexin V staining (Figure 4). However, LE540, an antagonist of RARα, aggravated the hepatocyte damage in comparison with the H2O2 group.
Figure 4 Retinoic acid receptor α inhibits reactive oxygen species-induced apoptosis in vitro.
LE540, an inhibitor of RARα, was cocultured with FL83B cells for 24 h, and then the cells were harvested 6 h after H2O2 treatment. A: Expression levels of Bcl-2, cleaved caspase 3 and β-actin were measured by Western blot; B: The number of apoptotic cells was assessed by AV and PI labeling. Mean ± SD, n = 3-5/group, aP < 0.05 vs the DMSO group; bP < 0.05 vs the H2O2 group. RARα: Retinoic acid receptor α; AV: Annexin V; PI: Propidium iodide; FITC: Fluorescein isothiocyanate; ATRA: All-trans retinoic acid.
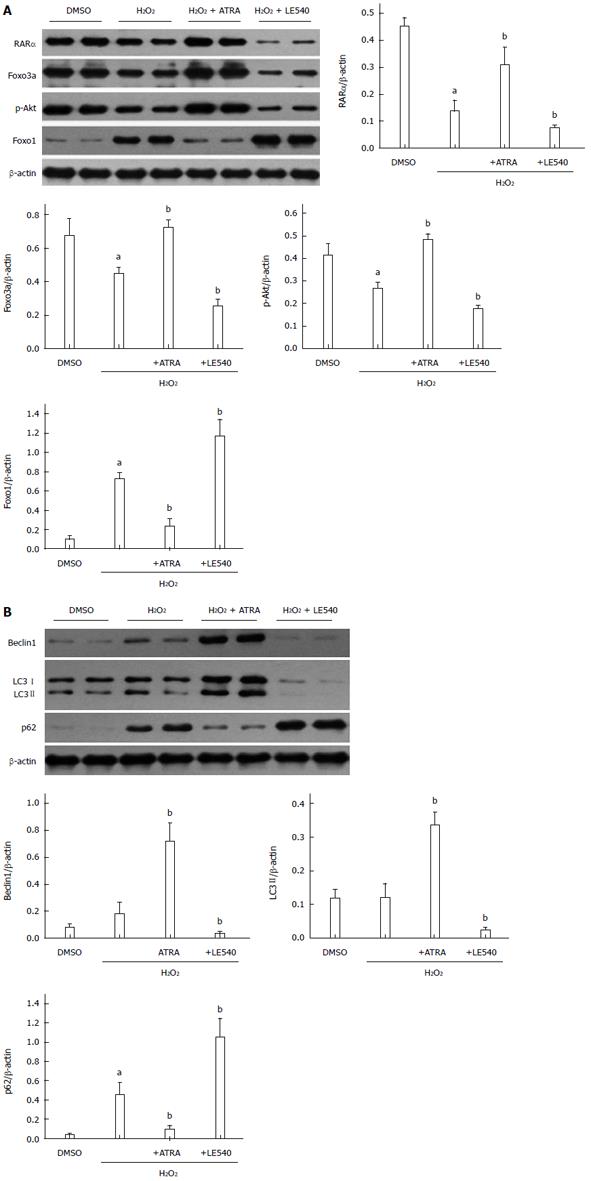
RARα mediates autophagy through the Foxo3a/p-Akt/Foxo1 pathway
As mentioned above, RARα activates the Foxo3a/p-Akt/Foxo1 pathway to mediate autophagy. Although Foxo3a and Foxo1 are both members of the Foxo family, they have opposing roles in autophagy. To enhance the level of autophagy, Foxo3a needs phosphorylated Akt to decrease Foxo1 accumulation in the nucleus[17]. In our H2O2-induced damage model, RARα promoted the transcription of Foxo3a, which resulted in an autophagy-related chain reaction including increased Beclin1 and LC3 II levels and decreased p62 levels (Figure 5B). This finding was confirmed in another manner by LE540. Because the level of Foxo3a expression was consistent with RARα activation, the levels of RARα, Foxo3a, p-Akt and Foxo1 were reversed in the LE540 group (Figure 5A). Ultimately, the degree of ROS-induced cell damage was strengthened by LE540 induced inhibition of autophagy.
Figure 5 Retinoic acid receptor α mediates autophagy through the Foxo3a/p-Akt/Foxo1 pathway.
A: Western blot assessment of RARα, Foxo3a, p-Akt, Foxo1, and β-actin expression levels. B: Western blot analysis of Beclin1, LC3I/II, p62 and β-actin expression levels. Mean ± SD, n = 3-5/group, aP < 0.05 vs the DMSO group; bP < 0.05 vs the H2O2 group. RARα: Retinoic acid receptor α; ATRA: All-trans retinoic acid.
DISCUSSION
ATRA is the activated form of vitamin A, and its biosynthesis process is controlled by cytosolic alcohol dehydrogenases, retinol dehydrogenases and retinaldehyde dehydrogenases[23-25]. ATRA can also attenuate liver IR injury through suppressing NF-κB p65 expression and phosphorylating p38 mitogen-activated protein kinase and Akt to increase the expression of manganese superoxide dismutase[11,12]. Moreover, it can regulate the expression of many genes to mediate its biological effects by binding with RAR[26-28]. In this study, ATRA increased the transcription of Foxo3a, which had a protective role in hepatic tissue after IR injury; in turn, this effect could be blocked by the RARα inhibitor LE540 in vivo.
Autophagy, which has an important role in cellular development, differentiation and survival, is necessary to maintain homeostasis and provide energy by degrading cytosolic components in vivo[8,29]. During IR insults, autophagy can degrade abnormal or dysfunctional mitochondria to decrease ROS generation. In addition, the formation of autophagic vesicles and autophagic flux is decreased in IR injury[29]. The process of autophagy is divided into three sections including the formation of autophagosomes and autolysosomes, and degration[30-32]. Beclin1 and LC3II are involved in the initiation of autophagosomse[33-35], but they cannot absolutely reflect the function of autophagy. Because autophagy is a degration system. Despite the enhancement of Beclin1 and LC3II in the IR group, it does not mean that autophagy was increasing. In addition, p62, which is the substrate of autophagy and used to evaluate the degree of autophagy[36-38], was increasing in the IR group. Hence, the autophagy was inhibited and this was associated with the exacerbation of apoptotic cells and inflammation response in the IR group. We also found the similar results in the H2O2 treatment group. However, these findings were reversed by ATRA. Thus, ATRA can reduce apoptosis and inflammation by autophagy partially.
The Foxo family, which includes Foxo1, Foxo3a, Foxo4 and Foxo6 in mammals, can control many cellular processes[39,40]. Except for Foxo6, the Foxo proteins are widely expressed in most tissues. Foxo1 and Foxo3a have been shown to be important for normal and abnormal liver function[41]. Foxo3a is a strong inducer of the transcription factor Foxo1 because it positively regulates the promoter of Foxo1[15]. Foxo1 plays a different role, depending on locating in the cytoplasm or nucleus. This process is controlled by Akt kinase, which phosphorylates Foxo1 at Thr24, Ser256 and Ser319, and makes phosphorylated Foxo1 translocate from the nucleus to cytoplasm. Moreover, cytoplasmic Foxo1 promotes autophagy[42-44]. We also found that the increased level of Akt phosphorylation diminished nuclear Foxo1 accumulation. Foxo3a is involved in mediating autophagy through the Akt/Foxo1 pathway[17]. This finding is consistent with our results.
In addition, ATRA can influence the expression of Foxo3a[16,45,46]. In the ATRA group, the expression of Foxo3a was increased. This effect is related to RARα activity, which was completely inhibited by LE540 treatment. Additionally, ATRA can regulate phosphoinositide 3-kinase (PI3K) activity and the expression of PI3K catalytic subunit p110β[47]. ATRA can also redistribute the cation-independent mannose-6-phosphate/IGFII receptor to mediate autophagy[14]. Whether this effect is dependent on Foxo3a requires further investigation.
In conclusion, our study is the first to demonstrate that ATRA enhances autophagy to protect the liver from IR injury. This effect depends on RARα activation of the Foxo3a/p-Akt/Foxo1 pathway. This mechanism provides a new strategy for treating liver IR injury.
COMMENTS
Background
Ischemia and reperfusion (IR) injury is an important clinical factor in the process of hepatic surgery. It can affect the prognosis of the patient. Liver IR injury, in nature, is a sterile immune response. All-trans retinoic acid (ATRA) can mitigate liver IR injury, but the underlying mechanism is not clear.
Research frontiers
ATRA can activate retinoic acid receptor (RAR) α to induce autophagy, and Foxo3a is the target of ATRA-induced apoptosis and granulocytic polarization in the treatment of acute promyelocytic leukemia (APL). In addition, the expression of Foxo3a is increased by ATRA treatment. Foxo3a also activates the Akt/Foxo1 pathway to regulate autophagy. However, this report is the first to discuss the relationship between ATRA and autophagy in liver IR injury.
Innovations and breakthroughs
ATRA pretreatment induces autophagy to decrease hepatocyte apoptosis in liver IR injury. This protective role depends on the Foxo3a/p-Akt/Foxo1 pathway through the activation of RARα.
Application
This study confirmed that ATRA pretreatment is an effective therapeutic treatment in liver IR injury. Moreover, ATRA has already been applied to treat APL in the clinic. Hence, ATRA is a potential drug for patients undergoing hepatic operations.
Peer-review
This study demonstrated the mechanism of ATRA-mediated autophagy in liver IR injury. The results are adequate to support the conclusions, and the design is reasonable.