Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12218
Peer-review started: May 15, 2015
First decision: July 14, 2015
Revised: September 1, 2015
Accepted: October 23, 2015
Article in press: October 26, 2015
Published online: November 21, 2015
Processing time: 187 Days and 18.8 Hours
Lymph node staging is of crucial importance for the therapy stratification and prognosis estimation in colon cancer. Beside the detection of metastases, the number of harvested lymph nodes itself has prognostic relevance in stage II/III cancers. A stage migration effect caused by missed lymph node metastases has been postulated as most likely explanation for that. In order to avoid false negative node staging reporting of at least 12 lymph nodes is recommended. However, this threshold is met only in a minority of cases in daily practice. Due to quality initiatives the situation has improved in the past. This, however, had no influence on staging in several studies. While the numbers of evaluated lymph nodes increased continuously during the last decades the rate of node positive cases remained relatively constant. This fact together with other indications raised doubts that understaging is indeed the correct explanation for the prognostic impact of lymph node harvest. Several authors assume that immune response could play a major role in this context influencing both the lymph node detectability and the tumor’s behavior. Further studies addressing this issue are need. Based on the findings the recommendations concerning minimal lymph node numbers and adjuvant chemotherapy should be reconsidered.
Core tip: The number of evaluated lymph nodes is prognostic in stage II and III colon cancers. Understaging due to inadequate lymph node harvest causing a stage migration effect is a widely accepted explanation for this. However, there is growing evidence that understaging plays only a minor role in this context. It seems much more likely that immune response has influence on the lymph nodes’ detectability and is associated with outcome in colon cancer.
-
Citation: Märkl B. Stage migration
vs immunology: The lymph node count story in colon cancer. World J Gastroenterol 2015; 21(43): 12218-12233 - URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12218.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12218
Lymph node staging is still of crucial importance for the prognosis and the therapy stratification in colon cancer. The occurrence of lymph node metastases is associated with an adverse clinical course with an indication for adjuvant chemotherapy. In contrast, patients with stage I/II colon cancers show a considerable better outcome with a high rate of long-term survivors. Because only a small number of these patients benefit from adjuvant chemotherapy it is restricted to high risk situations like T4-stage or emergency resections[1,2]. In order to ensure high quality in staging colon cancer several national guidelines recommend the histopathological evaluation of at least 12 lymph nodes[3,4]. On the other hand it is well known that this recommendation is achieved only partially sometimes only in a minority of cases[5,6]. Low lymph node yields, however, are associated with an adverse outcome[7]. Cases with low lymph node harvests might be prone to the missing of positive lymph nodes and understaging. In contrast, high numbers of evaluated lymph nodes could prevent from understaging. Actually, high numbers of investigated lymph nodes are associated with favorable outcome in colon cancer. A stage migration effect also called Will Rogers phenomenon introduced by Feinstein et al[8] would take place resulting in improved survival curves both for stage II and III cancers. The elimination of false node negative cases within the collective of stage I/II cases and the shift of relatively early nodal positive cases into the correct stage III category is believed to cause such a phenomenon.
This prognostic impact of high lymph node yields prompted the demand of more intensive lymph node evaluations with up to 30 lymph nodes or even more[9-11]. Because insufficient lymph node harvest has been identified as an adverse prognostic factor adjuvant chemotherapy is recommended for patients with less than 12 identified lymph nodes regardless of the nodal status[1,2].
However, the achievement of the 12 lymph node threshold is not only of prognostic and therapeutic relevance. It has also implications in terms of the quality measurement in surgical oncology. It is widely accepted that the identification of at least 12 lymph nodes is a good surrogate marker for an adequately performed surgical resection[12]. This is still the case today although it could be shown by several investigation that the number of elevated lymph nodes is not only influenced by the surgeon but by many other very different factors including the pathologist, the age of the patient and the molecular alterations of the tumor[13,14]. The attempt to improve the quality of colon cancer therapy is very likely the reason for the increased rate of sufficiently staged cases in the past[9,15]. This development to an improved lymph node staging should influence the outcome statistics not only mathematically but also effectively because of a higher rate of correct stage adapted therapy. This would be a strong argument that understaging of cases with a poor lymph node yield is the reason for its prognostic impact. However, several authors express doubts that the Will Rogers phenomenon is really the correct explanation for this effect[16,17]. An alternative thesis is that immune response plays a major role in this context[16,18,19]. A strong immunologic reaction against the tumor could result in local lymph node hyperplasia with enlargement and enhanced lymph node detectability.
This review discusses the current literature in order to elucidate the biological nature of the prognostic impact of lymph node count in colon cancer.
For that a broad literature research within the MEDLINE Database was performed. The search terms included “lymph node” in combination with “colon” or “colorectal”. Additionally previously published reviews[7,20,21] were screened for relevant references that might have been missed by the initial MEDLINE search. Because this review emphasizes on colon cancer articles that solely deal with rectal cancers were excluded. Articles in English and German language were considered for integration. In order to answer the question of this review articles providing information about the following topics were of particular interest: Factors influencing the lymph node harvest; Prognostic impact of lymph node harvest; Lymph node positivity rates; Upstaging from N0 to N+ after secondary lymph node dissection; Effect of advanced dissection techniques; Effect of improved lymph node recovery over time; Comparison of differently performing hospitals; Indications for the role of immune response.
Analyzing the literature an increasing interest in identifying factors that influence the lymph node harvest is recognizable. It seems that a search is ongoing for the one who is to blame when the 12 lymph nodes rule could not be achieved and for answer of the question whether it is justified to demand this rule in all situations. Forty-four studies investigating such potential factors, published between 2003 and 2014, are included in this review. Before discussing these factors it might be worth to consider how many lymph nodes can be expected within a colonic specimen. Two studies reporting the results of entire submission of mesenteric tissue (ESMT). Brown et al[22] found about 90 lymph nodes per colonic specimen on average while Kim et al[23] detected about 43 lymph nodes in colorectal cancers. Anecdotally, the authors group found 360 lymph nodes in one specimen of a total colectomy using methylene blue assisted lymph node dissection (unpublished case). It is clear that the vast majority of these nodes are very tiny and barely visible. Nevertheless, these reports indicate that the theoretically achievable numbers are by far above the 12 recommended lymph nodes. The main different factor categories are given in Table 1 and discussed below.
| Surgery | Pathology | Patient | Tumor | Other |
| Experience | Experience | Age | Location | Specimen length |
| Volume | Technique | Gender | T-stage | Hospital status |
| BMI | N-stage | Year of operation | ||
| Lymph node size | ||||
| MSI |
The resection of the complete lymphatic basin is an essential part of the oncological adequate surgical therapy of colon cancer. As mentioned before the total number of evaluated lymph nodes is a well-established but also controversial debated marker for the surgery’s quality. Many of the published studies show significant differences between individual surgeons and/or positive associations between, surgeon’s experience/qualification and/or surgical volume and the number of harvested lymph nodes[13,24-30]. A few studies, however, found no influence of surgery related variables[31,32]. Open and laparoscopic technique were shown to be equal in terms of lymph node retrieval in two meta-analyses[33,34].
The independent influence of the pathologist on lymph node retrieval is also reported in several studies[13,25,27,29,30,35,36]. Interestingly, an inverse association between qualification or level of training and number of identified lymph nodes is reported. Kuijpers et al[37] reported better results of pathology assistants compared to pathologists and Bamboat et al[35] showed that residents in their first year of training are more successful in dissecting lymph nodes than there more experienced colleagues. To pathologists these results are probably less surprising as the might be to others. Dissecting lymph nodes of a surgical specimen is certainly one of the most unpopular task in pathology. Diligence and lack of time play a major role in this context. Pathology assistance and young residents may have more time and patience to do a better job.
The usage of special techniques like fat clearance, methylene blue technique or ESMT improves the lymph node yield effectively in comparison to the conventional manual technique[38]. The same two studies that did not find lymph node retrieval influenced by the surgeon also reported a lacking influence of pathology related factors on[31,32]. This, however, is probably more the result of homogenous performance levels of these specialties within these single centers.
Patient related factors are unmodifiable and therefore different from the former discussed points. Patient’s age is mentioned by many authors as an independent predictor for the lymph node count[19,28,39-47]. All these studies reported consistently an inverse association between higher age and lymph node harvest. To our knowledge there are no studies available that investigated the underlying reasons for that. One can speculate that surgical aggressiveness differ between different age groups. On the other hand increasing age could be accompanied by a diminishing immunologic response resulting in smaller lymph nodes.
The role of patient’s gender is somewhat more controversial. Gender was identified only by a minority of studies as a lymph node yield influencing factor. Nevertheless, three of the four of these studies reported an association of female sex with higher lymph node count[19,41,48]. Only Horzic et al[49] found a higher lymph node count in males.
Whether the body mass index (BMI) plays a role or not remains unclear. There are studies that found a positive association between particular low BMI and lymph node harvest[31,50] others report an association between high BMI and poor harvest[13,32]. Explanations again are speculative and point in the same direction as in age. In several other studies, however, no effect was seen[25,51,52].
Tumor associated factors are also unmodifiable. There is strong evidence from 15 studies[14,19,26,28,31,36,39,42-44,53-57] that right location of the tumor is associated with significant higher lymph node counts compared to left sided tumors. This might be related to anatomic differences between the different parts of the colon. A higher rate of microsatellite instability (MSI) positive cancers - which are mainly located in the right colon - could be another explanation at least in certain a part of cases.
Like location, T-stage and/or tumor size were found to be predictive for the lymph node yield in colon cancer very often[13,31,36,41-43,46,49,55,56,58-60]. The immunogenicity of advanced tumors seems to be higher compared to low stages inducing a stronger reaction in lymph nodes. A more aggressive surgical treatment in advanced diseases is also thinkable.
Several authors report a positive correlation between lymph node number and the detection of lymph node metastases[41,44,56,61]. Nevertheless, it seems questionable whether this means that a greater lymph node yield necessarily results in in higher detection of metastases. The opposite linkage - the metastatic involvement induce a stronger lymph node reaction - is at least as plausible similar to advanced T-stages.
Lymph node size was recently reported as being associated with total lymph node count by the authors group, Märkl et al[62] and Sloothaak et al[63]. MSI is also believed to interfere with the lymph node count. However, we found only four articles addressing this issue. Three authors report higher lymph node numbers in MSI positive cancers[14,53,64]. MacQuarrie et al[65] did not find such an association focusing on stage III cancers. Both factors lymph nodes size and MSI might indicate an immunologic association.
Other factors that influence lymph node yields are the specimen length, the status of the hospital and the year of diagnosis/operation. The latter will discussed in detail in one of the following paragraphs. The specimen length is probably multifactorial influenced by the surgeon, the tumor and patient’s individual anatomy. Several studies report this factor as lymph node count influencing[19,26,29,47,55,56,60].
A view studies investigated the impact of hospital status on lymph node count retrieval[60,66-68]. The results indicate that teaching and high volume centers are more successful in identifying a sufficient number of lymph nodes.
We identified 49 studies published between 1998 and 2014 including total 625279 (range: 94-194459) that investigated the prognostic impact of lymph node count in colon and colorectal cancers, respectively. These studies show a very high heterogeneity in many respects. The study endpoint differ as cut offs, included locations and stages, case numbers and study designs do. Two nested cohort studies[69,70] and 10 register studies[45,71-79] were found. The other studies are mainly performed in single centers.
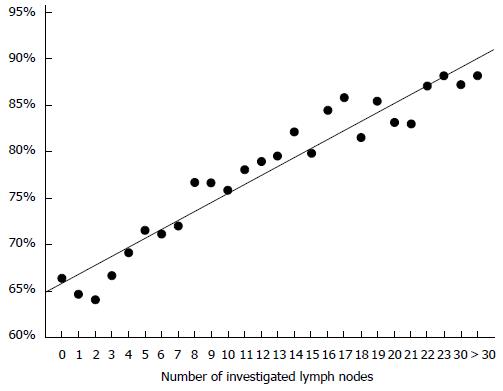
All seven studies that were restricted to stage I/II cancers colon cancers showed survival advantages with considerable risk reductions for the groups with higher lymph node counts (Table 2)[28,76,77,79-82] or increased risk for patients with low lymph node yields. Most authors used defined cut offs for their analyses. Swanson et al[77] however showed a linear increase of the 5 year overall survival rates with increasing numbers of evaluated lymph nodes (Figure 1). The work of Sato and coworkers[82] seems unique because of addressing the issue of adjuvant chemotherapy in patients with poor lymph node yield. The authors reported an improved outcome in the chemotherapy group.
| First author | Year | n | Insuff.-rate | pT3/4 | Prognostic | Endpoints | Cut off | Survival |
| Swanson | 2003 | 35787 | 60% | 100% | Yes | 5yOS | No cut off | linear increase of 5yOS-rate |
| Law | 2003 | 115 | NA | 100% | Yes | 5yOS, 5yDFS | ≥ 7 | 5yOS: < 7LN 69% vs > 6LN 89% |
| Bui | 2006 | 4531 | NA | NA | Yes | OS | 1-3 vs 10-36 | HR = 0.6 (CI: 0.4-1.0), P = 0.03 |
| Bilimoria | 2008 | 142009 | NA | 59% | Yes | 5yOS | ≥ 12 | HR = 0.75 (CI: 0.71-0.8), P < 0.0001 |
| Maggard | 2009 | 11263 | NA | 69% | Yes | 5yOS | 4 (T1) and 10 (T2) | T1: HR = 0.76 (CI: 0.641-0.902), P = 0.002 |
| T2: 0.853 (CI: 0.776-0.937), P = 0.001 | ||||||||
| Stocchi | 2011 | 901 | NA | 100% | Yes | OS, DFS, CsS | ≥ 12 | < 12 LN: HR = 1.93 (1.27-2.94), P = 0.002 |
| Sato | 2011 | 1476 | 56% | 100% | Yes | 5yOS | > 12 | ACT: improved 5yOS for LNs ≤ 12 |
Twelve studies performed between 2002 and 2014 investigated colon cancers with and without lymph node metastases (stage I-III or stage III colon cancers exclusively) (Table 3)[44,45,54,69-71,73,74,78,83-85]. Both nested cohort studies[69,70] which are retrospective analyzes from two large multicenter studies belong to this group. All ten studies that included stage II cases found favorable outcomes of the groups with higher lymph node counts. Again the chosen cut offs differed considerable. In 8 of 12 studies superior survival rates were found in stage III cancers also. One study showed an advantage for cases with low lymph node ratio (number metastatic lymph node divided by total lymph node number). Three groups including Kelder et al[44], Prandi et al[70] and Tsikitis et al[85] however, found no significant association between lymph node harvest and outcome in stage III cancers.
| First author | Year | n | N+ | Insuff rate | pT3/4 | Endpoints | Cut off | Prognostic stage II | Prognostic stage III |
| Prandi1 | 2002 | 3491 | 48% | 501% | n.m. | OS, PFS | 8-12 (RR = 0.46) vs 13-17 (RR = 0.76) vs > 17 (RR = 0.79) | Yes | No |
| Le Voyer2 | 2003 | 3411 | 81% | NA | 89% | CsS | N1: ≥ 12 vs > 10 vs > 40; N2: > 35; N0: ≥ 12 vs≥ 12 vs > 20 and < 35 | Yes | Yes |
| Jestin | 2004 | 3735 | 31% | NA | NA | OS | ≥ 12 | Yes | /3 |
| Johnson | 2006 | 20702 | 100% | NA | 92% | 5yCsS | < 4 neg LN vs > 12 neg LN | / | Yes |
| Kelder | 2009 | 2281 | 32.4% | 79% | 79% | 5yOS | < 6; 6-11; > 11 | Yes | N |
| Tsikitis | 2009 | 329 | 100% | 49% | NA | CsS/DFS | > 12 | / | N |
| Vather | 2009 | 4309 | NA | NA | NA | 5yOS | 4 LN wide steps | Yes | Yes |
| Dillman | 2009 | 574 | NA | NA | NA | OS | ≥ 12 | Yes | No |
| Shanmugam | 2011 | 490 | 46.9% | 24% | NA | 5yCsS/CsS | ≥ 20 | Yes | Yes |
| Chang | 2012 | 9644 | 41% | 27.7% | 80.2% | 5yOS | ≥ 12 | Yes | Yes |
| Gleisner | 2013 | 154208 | 34%4 | NA | 69.4% | OS | Linear risk reduction up to 25 LN in N- and up 10 LN in N+ | Yes | Yes |
| Khan | 2014 | 194459 | NA | 41% | NA | CsS | ≥ 12 LN | Yes | Yes |
Twelve publication between 2002 and 2013 were restricted to stage I/II cases but included both colon and rectal cancers (Table 4)[47,72,86-95]. Despite the very different cut off points and endpoints, all except one paper reported favorable outcomes for the groups with better lymph node harvests. Nir et al[91] were not able to show such an effect. This, however, might be the result of a relatively small sample number with only 117 cases. The authors reported at least a non-significant trend (P = 0.15) towards better disease free survival in the group of ≥ 12 lymph nodes.
| First author | Year | n | Insuff rate | pT3/4 | Prognostic | Endpoint | Cut off | Survival |
| Cserni | 2002 | 8574 | NA | 100% | Yes | OS | No cut off | Continuously improved survival |
| Cianchi | 2002 | 140 | min 40%1 | n.m. | Yes | 5yOS | ≥ 9 | 54.9% vs 79.9%, P < 0.001 |
| Wong | 2002 | 345 | NA | NA | ≥ 68 | DFS | 22.6 vs 11.32 | 40% vs 90%1, P < 0.001 |
| Berberoglu | 2004 | 301 | 53.5%1 | 69% | Yes | 5yOS | ≤ 10 | RR = 2.8 (CI: 1.6-5.2), P = 0.0008 |
| Yoshimatsu | 2005 | 94 | 35% | 100% | Yes | 5yOS | ≥ 9 | 66.7% vs 86.7% |
| Tsai | 2007 | 180 | NA | 70% | Yes | OS | ≥ 18 | 5yOS: 70 vs 98%1, P = 0.015 |
| Norwood | 2009 | 2449 | NA | NA | Yes | OS | < 12 | about 15% difference1, P = 0.001 |
| Ishizuka | 2010 | 205 | min 36%1 | 100% | Yes | CsS | ≤ 9 vs > 9 | 44.5 mo vs 66 mo, P = 0.0042 |
| Nir | 2010 | 117 | 28% | 100% | No | 5yOS, 5yDFS | ≥ 12 | No difference |
| La Torre | 2012 | 204 | 16% | 100% | Yes | 5yDFS, 5yCsS, and 5yOS | > 12 | 5yOS 78.5% vs 53.1%, P = 0.001 |
| Iachetta | 2013 | 657 | 22% | 100% | Yes | CsS/PFS | < 12 vs≥ 20 | HR = 0.49 (CI: 0.30-0.79), P = 0.003 |
| Xingmao | 2013 | 729 | NA | 100% | Yes | OS | > 12 | 88.7% vs 64.9%, P = 0.000 |
Node negative and positive colorectal cancers were investigated in 17 studies between 1998 and 2014 (Table 5)[19,26,75,96-109]. Again all but one study reported better clinical courses for cases with higher lymph node counts in stage II cancers. The half of the studies, however, found no significant difference in stage III cancers. An explanation for this discrepancy to the findings in other constellations could be that fact locally advanced rectal cancers are usually treated by neoadjuvant radiochemotherapy which itself is associated with decreased lymph node yields[13]. Govindarajan et al[110] found that lymph node harvests beyond the 12 lymph node rule in neoadjuvantly treated rectal cancers were not associated with understaging or inferior survival.
| First author | Year | n | N+ | Insuff-rate | pT3/4 | Endpoints | Cut off | Stage II | Stage III |
| Caplin | 1998 | 377 | NA | NA | NA | OS | > 6 | Yes | No |
| Sarli | 2005 | 1040 | NA | NA | 100% | 5yOS | < 10 | Yes | No |
| Wong | 2005 | 21491 | 37% | NA | 67% | OS | > 13 | Yes | 1 |
| George | 2006 | 3592 | NA | 79% | NA | 5yOS | 0-4; 5-10; > 10 | Yes | Yes |
| Edler | 2007 | 125 | 51% | 87% | NA | OS | 0-11 vs > 11 | Yes | Yes1 |
| Evans | 2008 | 381 | 45.3% | 47%1 | 82% | 5yOS | ≥ 9 | Yes | 2 |
| Choi | 2010 | 664 | NA | NA | 100% | DFS | > 20 | Yes | No |
| Desolneux | 2010 | 362 | NA | NA | 72.4% | OS | < 8 vs≥ 8 and < 12 vs≥ 12 | Yes | No |
| Ogino | 2010 | 716 | 38% | 63%1 | 68.3% | CsS/OS | 0-3 negative LN, 7-12 and > or = 13 negative LN | Yes | Yes |
| Fretwell | 2010 | 351 | 48% | min 20% | 95% | 5yOS | ≥ 9 (Dukes B); > 9 (Dukes C) | Yes | Yes |
| Wong | 2011 | 8521 | About 30% | 32% | 66% | CsS | medians: 4 vs 8 vs 10 | Yes | No |
| Kotake | 2011 | 16865 | 46% | 24%1 | 100% | 5yOS | < 10 vs > 27 | Yes | Yes |
| Kritsanasakul | 2012 | 533 | 43% | 59.1% | 82% | 5yOS | ≥ 12 | Yes | 1 |
| Moro-Valdezate | 2013 | 11662 | 39.7% | 65%1 | 79.7% | 5yOS/5yCsS | ≥ 12 | No | No |
| Zhang | 2013 | 265 | 42.3% | 75.1% | 79.2% | OS | < 12 | Yes | Yes |
| Onitilo | 2013 | 1397 | 37% | 26% | 67% | OS | ≥ 12 | Yes | Yes |
| Duraker | 2014 | 461 | NA | 51% | 74% | CsS | ≥ 12 | Yes | No1 |
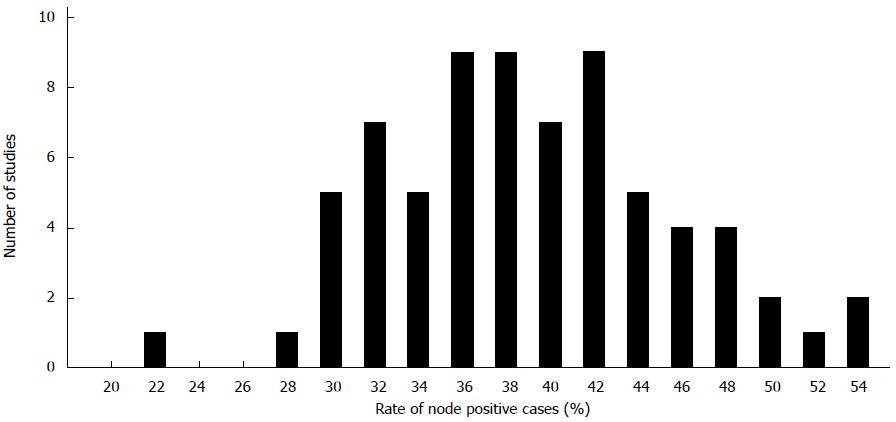
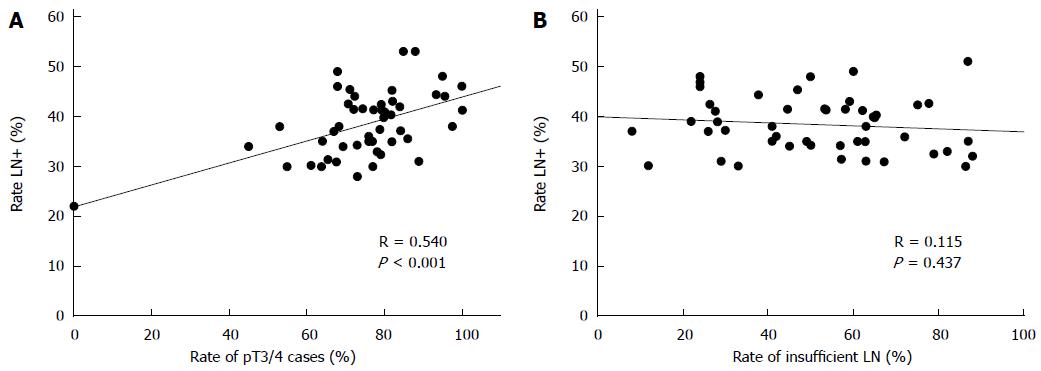
For an estimation of the lymph node positivity rate that can be expected by conventional pathological examination technique 57 studies including about 750000 cases of colon and colorectal cancers were analyzed published between 1987 and 2015[5,6,10,14,15,17,24,26,40-44,46,48,53,55,57,59,61,63,64,66,71,73,75,83,84,95,100,101,103-109,111-127]. The mean and median rates of lymph node positivity rate on the basis of the selected studies were 39% and 38% (range: 28-53) (Figure 2). Based on patients the mean percentage of node positive cases was 37%. There was a significant correlation between the portion of pT3/4 cancers and the occurrence of lymph node metastases (Figure 3A). The rate of inadequately staged cancers, however, had no influence on the rate node positive cases (Figure 3B). The results of the five largest studies are given in Table 6.
| First author | Year | n | Register | N+ rate | Insuff rate | Rate T3/4 |
| Gleisner | 2013 | 154208 | SEER | 34% | NA | 69.4% |
| Baxter | 2010 | 110444 | SEER | 41% | 53.6% | 100% |
| Ricciardi | 2006 | 106900 | SEER | 34% | 57% | 73% |
| Gonsalves | 2011 | 19240 | VACCR | 30% | NA | 61.1% |
| Chang | 2012 | 9644 | Taiwan Cancer Database | 41% | 27.7% | 80.2% |
Fourteen studies were identified that evaluated the effect of secondary or even tertiary lymph node dissection in colorectal cancer most often with aid of clearance techniques (Table 7)[22,23,48,128-137]. Only the authors study was restricted to colon cancers. All these studies are limited by relatively small case numbers ranging from 15 to 188. Nevertheless, nine studies report upstaging from N0 to N+ after re-evaluation of the specimens in up to 31%. All studies reporting relatively high upstaging rates between 5%-31%, however, show poor harvest results after the initial dissection step with mean numbers between 3 and 10 lymph nodes. In studies with adequate or high primary lymph node counts upstaging occurs almost exclusively in single cases.
| First author | Year | n | Mean LN before | Mean LN after | UpstagingN0/N+ | Up-rate | Location | Technique | Comment |
| Scott | 1989 | 103 | 6.2 | 12.4 | Yes | 8.6% | CR | Fat clearing | 5yFU available |
| Haboubi | 1992 | 41 | 6.7 | 58.2 | Yes | 28%1 | CR | Fat clearing | Based on HE; higher up-staging with ICH1 |
| Cohen | 1994 | 41 | 13 | 17 | ?1 | 1 | CR | Xylene | Upstaging in 1 single case; primary N-stage (N0/1) not given; %tage N+ not given1 |
| Koren | 1997 | 30 | 2.6 | 8.6 | Yes | 31% | CR | Fat clearing | |
| Brown | 2005 | 15 | 20.8 | 89.6 | Yes | 1 | CR | ESMT | 1 of 7; however unclear wether it was a LN metastasis or a deposit1 |
| Kim | 2007 | 48 | 19.4 | 43 | No | / | CR | ESMT | |
| Richter | 2007 | 188 | n.m. | n.m. | Yes | min 4% | CR | Fat clearing | Initinal insuff rate 59; after 9 |
| Vogel | 2008 | 80 | 6.9 | 11.3 | Yes | 2% | CR | Fat clearing | |
| Märkl | 2008 | 30 | 17 | 25 | Yes | 3% | C | Fat clearing | Primarily conventional technique |
| Märkl | 2008 | 30 | 35 | 40 | No | / | C | Fat clearing | Primarily methylen technique |
| Fan | 2010 | 115 | 9.1 | 14.2 | Yes | 5%-10%1 | CR | Re-evaluation | Insuff Rate 79%; Up Staging rate not exactly calculatable |
| Hernanz | 2010 | 50 | 13.9 | 23.9 | Yes | 4%1 | CR | Fat clearing | based on own calculation |
| Chapman | 2012 | 94 | 22.5 | 29 | Yes | 1 | CR | Schwartz-clearing | 1 single case upstaged1 |
| Chen | 2014 | 83 | 7.2 | 14.1 | No | / | CR | Re-evaluation: partly Fat clearing | |
| Ma | 2014 | 55 | 9.8 | 18.4 | Yes1 | 1 | CR | GEWF | Upstaging in cases with primary insufficient LNY; 3 cases N0 to N+1 |
Eleven studies published between 1999 and 2015 which compared the result of advanced techniques like fat clearing or methylene blue injection with conventional manual dissection were selected to investigate the influence of these techniques on staging (Table 8)[16,138-147]. Only three of these studies[141,145,146] report significant higher node positivity rates in the study groups compared to the control groups. Despite acceptable or even excellent lymph node yields the rates of node involvement was considerable low in the control groups of these studies. The study groups showed results comparable to the values achieved by standard technique as shown in the section above. This indicates that the reported differences might by caused more by the especially low metastatic rates in the control arms than by the effect of the advanced technique. A study performed by the authors group including more than 1300 cases revealed no differences regarding the local metastatic rate[16].
| First author | Year | n | Mean/median LN-Conv | Mean/median LN-Spec | N+ Konv | N+ Spec | T3/4 Konv | T3/4 Spec | Technique | P value |
| N+ rates | ||||||||||
| Ratto | 1999 | 801 | 11.4% | 29.4% | 30.2% | 37.5% | 76.9% | 84.5% | Fixing Technique | < 0.05 |
| Newell | 2001 | 67 | 6.8% | 10.2% | 31% | 46% | 81% | 85% | GEWF | NS |
| Kukreja | 2009 | 701 | 12.8% | 17.3% | 36.9% | 32.4% | 65.8% | 62.8% | Fat clearance | NS |
| Törnroos | 2009 | 32 | 22% | 61% | 56.3% | 37.5% | 100% | 100% | MB | NS |
| van Steenbergen | 2010 | 170 | 11% | 14% | 42% | 41% | 80% | 79% | mesent. Patent Blue Injection | ND |
| Frasson | 2012 | 473 | 20.6% | 37.1%/47.6% | 38.9% | 48% | 80.9% | 72% | MB | NS |
| Jepsen | 2012 | 428 | 24% | 37% | 9.4%1 | 26.7%1 | 82% | 81% | MB | 0.040 |
| Märkl | 2013 | 1332 | 13% | 34% | 37% | 37% | 65% | 63% | MB | ND |
| Kir | 2014 | 180 | 21.5% | 24.5% | 28% | 47.9% | 91.6% | 84.9% | MB | 0.006 |
| Borowski | 2014 | 100 | 15% | 23% | 34%12 | 40%12 | NA | NA | MB | NS |
| Iversen | 2015 | 120 | 9.5% | 16.5% | 44% | 36% | 81% | 71% | GEWF | NS |
Several studies report an improvement concerning the lymph node yields over time with increasing mean/median lymph node numbers per case and increasing rates of adequately staged cases. Twelve studies were identified investigating the development from the 50ies to present[9,15,17,95,119,120,126,148-151]. An increase in evaluated lymph nodes per case is shown in all these investigations. However, only three report an associated increase of the lymph node positivity rate. Analyzing data from 750 patients with pT3 colorectal cancers from the SEER database Goldstein et al[149] found an almost continuous increase concerning the mean lymph node count from 3.3 in the 1950ies to 19.4 in 1990ies. Reaching a mean count of 8.4 lymph nodes the metastases rate increased relatively abruptly from rates ≤ 35% to 38%-53%. A rate of 70% found in the latest investigation period is very likely a statistical outlier. In 2002 Goldstein published an analysis of an enlarged group from the SEER database including 2427 pT3 cases[9]. Again, the author found a similar association. Noteworthy, despite a temporary decrease of the lymph node yield in the 1980ies the trend of an increasing rate of lymph node metastases was not affected. Wong et al[95] investigated a cohort of total 345 patient between 1995 and 1999. They found an inverse association between increasing numbers of investigated lymph nodes and the percentage of node negative cases. However, similar mean lymph node numbers in 1995 and 1997 corresponded to considerable differing node negative rates of 65% and 55.4%, respectively. This indicates that random changes could play a major role. All other studies including total about 250000 patients found no change in the rate of lymph node metastases over time although the lymph node yield could be improved significantly.
Hospitals belong to the factors that inhere with the lymph node harvest in colon cancers. Several investigations addressed this issue particular with respect on its impact on the detection of lymph node metastases. Nine such studies published between 2004 and 2014 were identified[68,80,105,109,122,125,129,152,153]. Miller et al[152] evaluated the performance of low-, medium and high volume hospitals and found significant differences concerning the rate of poor lymph node harvest (< 7 lymph nodes) and lymph node positivity rates of 15.2% vs 35.6% and 42.6%, respectively between the low volume hospitals and the other hospital categories. Chen et al[129] analyzed two branches of the same institution and found significant differences with rates of inadequate staging in 20% vs 75% with corresponding lymph node positivity rates of 40.5% vs 30.6%. This was also associated with an increased long term survival. In contrast, all other seven studies, did not identify an association between the number of identified lymph nodes and the rate of stage III cancers on the hospital level. Despite the lacking impact on staging, an influence of lymph node count on survival could be shown. Wong et al[68] showed a favorable outcome for cases with ≥ 12 evaluated lymph nodes on the patients’ level. Survival difference for N0 patients between differently performing hospital despite similar rates of lymph node positivity were reported by Wong et al[105].
Facing limitations of the current explanation of the prognostic impact of lymph node count a possible link between immune response and the number of detected lymph nodes was proposed by a number of authors discussing their results or commenting other’s papers[19,42,68,102,150,154]. These authors suggest changes of the lymph nodes - either enlargement or a diminishing - that alter its detectability. The associated differences in the number of identified number of lymph node would display a surrogate marker of the immune response against the tumor. The important role of the immune system for the patient’s prognosis is unquestionably. For instance tumor infiltrating lymphocytes are associated with a favorable prognosis[155]. The same is true for crohn-like reactions in colon cancer[156].
To the authors knowledge, however, there are only a few studies published that investigated a direct connection between parameters representing the extent of an immune response and the numbers of investigated lymph nodes. Recently, Kim et al[18] as well as George et al[102] found an association between tumor infiltration lymphocytes and the number of retrieved lymph nodes. In 1980 Pihl et al[157] described favorable outcomes in colorectal cancer cases with germinal center- or paracortical hyperplasia in Dukes B and C stages. Dworak[158] reported 1991 the incidence of germinal center- and paracortical hyperplasia in non-involved lymph nodes in rectal cancers. The author, however, did not perform a survival analysis. An association between the occurrence of ≥ 7 lymph nodes larger than 5 mm with the total number of dissected lymph node and with favorable outcome was shown by the authors group[62]. As mentioned above microsatellite instability is found to be associated with lymph node harvest by a several authors[14,53,64]. Moreover, it is a well-known predictor for a favorable prognosis and immunologic factors are believed to be the reason for that[159].
Lymph node harvest has a substantial impact on the prognosis in colon cancer and has been proven in many investigations as could be shown in this review and also in a systematic review by Chang et al[7].
The number of harvested lymph nodes in colon cancers is influenced by a number of modifiable and unmodifiable factors. The pathologist, surgeon and the hospital volume belong to the modifiable factors. Age, tumor stage, location and genetic alterations of the tumor are unchangeable. Stage migration also known as Will-Rogers-phenomenon is believed to be the result of understaging of stage I/II cases caused by poor lymph node retrieval. If this is true surgeons and/or pathologist would be to blame for it.
Depending on the used technique and the extent of the operation surgeons doubtless can influence the number of identifiable lymph nodes in colonic specimens. Therefore, it is to assume that more restricted excisions are prone to miss involved lymph nodes. To the author’s knowledge, however, there is no evidence for that. On the other hand there are some arguments at least against a relevant frequency of its occurrence. Complete mesocolic excision has shown to be associated with reduced local recurrence and superior overall and disease-free survival in stage II and III cancers[160,161]. Nevertheless, despite improved lymph node yields the rates of nodal positive cases did not increase by this technique and seem therefore unrelated to the improved outcome results. Moreover, the reported rates of local recurrence after curatively intended resections are low which seem not compatible with a relevant rate of missed positive lymph during surgical excision[162]. Law et al[81] reported a higher incidence of recurrence in stage II colon cancers with inadequate lymph node harvest. Interestingly, this was caused by a higher rate of distant metastases. The rates of local recurrence did not differ between well and poorly lymph node harvested cases.
On the other hand it seems obvious that pathologist in deed have a big influence on the number of reported lymph nodes. This can be stated based on the author’s experience in daily practice and many reports in the literature. The fact that pathology assistants and young residents do a better job than pathologist by spending more time and diligence[35,37] as well as the fact that the same surgeons achieve different results when he is collaborating with different pathology department[67] are only two examples. If pathologist missed positive lymph nodes by inadequate dissection of the specimens in a significant number differences regarding the lymph node positivity rates should be determinable. However, the analysis of the data provided in the literature shows no association between the rate of inadequate lymph node yields and the rate of lymph node positivity (Figure 2). Upstaging occurs after reevaluation, however, this is mainly restricted to cases with poor lymph node harvests or single cases as shown above. Techniques to improve lymph node harvest are highly effective but not associated with higher rates of stage III cancers[38]. This is remarkable. However, pathologists seem to be highly effective in picking the relevant lymph nodes from the correct area. In an experimental model the authors group could show that there is 63% chance to detect the first lymph node metastases within the first five dissected lymph node. This increases to 86% when analyzing the first nine lymph nodes[163]. The work of Mainprize et al[164] point in the same direction.
These arguments raise doubts that understaging is actually a relevant problem in the management of colon cancer. The comparison of differently performing hospitals as well as the analysis of the continuously improved lymph node harvest results over time show in the vast majority of studies no association between the numbers of investigated lymph nodes and the rate of lymph node positivity.
Another point is that lymph node count was shown to be prognostic in stage III cancers at least in a part of studies. Stage migration in these cases is ruled out, naturally. Based on these arguments stage migration as reason for the well investigated prognostic impact can be excluded in the author’s point of view. If at all stage migration effects seem to be restricted to the very poorly staged node negative cases. Such cases show a prognosis similar to stage III cancers. If false negative diagnoses would be the reason for that logically 100% of these cases actually had to be node positive, which seems very unlikely.
In concordance with other authors[19,42,68,102,150,154] one can state that a confounder which is related to the both lymph node harvest and to outcome has to be searched. It seems very likely that immune response is this confounder. A strong reaction can cause lymphatic hyperplasia with enlargement of lymph nodes and enhanced detectability. On the other hand an intensive immune reaction can prevent the patient from tumor progression. This hypothesis, however, is not proven yet. Nevertheless, there are indications pointing in this direction. Emerging evidence is provided by studies addressing the impact of lymph node reactions on survival as well as the prognostic relevance of lymph node size and tumor infiltrating lymphocytes[16,18,102,157,158]. Microsatellite instable tumors seem to be especially immunogenic associated with both high lymph node counts and reduce risk of progression. They could, therefore, serve as model helping to understand what happens.
This is of high clinical relevance. The role of lymph node number as reliable quality marker becomes more and more questionable. More important a low lymph node count is currently accepted as a risk factor and often prompts the administration of adjuvant chemotherapy[165]. In many cases such poor lymph node harvests are probably not the result of poorly performing physicians but the expression of an impaired immune response. With growing success of quality initiatives these cases will escape from a possibly necessary adjuvant therapy by forcing the 12 lymph nodes. It is therefore of crucial importance to close the existing knowledge gaps and reconsider the concerned recommendations.
P- Reviewer: Wang S S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008;CD005390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Benson AB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1017] [Cited by in RCA: 1066] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 3. | Benson AB; AWMF. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Kolorektales Karzinom, Langversion 1. 1, 2014, AWMF Registrierungsnummer: 021-007OL. Accessed April 21 2015; Available from: http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html. |
| 4. | Benson AB; NCCN-Guideline. Accessed April 21, 2015. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. |
| 5. | Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daudé H, Raverdy N, Faivre J. Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer. 1998;82:1482-1486. [PubMed] |
| 6. | Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 780] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 8. | Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1144] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 9. | Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179-189. [PubMed] |
| 10. | Leibl S, Tsybrovskyy O, Denk H. How many lymph nodes are necessary to stage early and advanced adenocarcinoma of the sigmoid colon and upper rectum? Virchows Arch. 2003;443:133-138. [PubMed] |
| 11. | Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, Catalano PJ, Haller DG. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol. 2003;10:213-218. [PubMed] |
| 12. | Bilimoria KY, Bentrem DJ, Stewart AK, Talamonti MS, Winchester DP, Russell TR, Ko CY. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst. 2008;100:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Mekenkamp LJ, van Krieken JH, Marijnen CA, van de Velde CJ, Nagtegaal ID. Lymph node retrieval in rectal cancer is dependent on many factors--the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Søreide K, Nedrebø BS, Søreide JA, Slewa A, Kørner H. Lymph node harvest in colon cancer: influence of microsatellite instability and proximal tumor location. World J Surg. 2009;33:2695-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Porter GA, Urquhart R, Bu J, Johnson P, Rayson D, Grunfeld E. Improving nodal harvest in colorectal cancer: so what? Ann Surg Oncol. 2012;19:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Märkl B, Schaller T, Krammer I, Cacchi C, Arnholdt HM, Schenkirsch G, Kretsinger H, Anthuber M, Spatz H. Methylene blue-assisted lymph node dissection technique is not associated with an increased detection of lymph node metastases in colorectal cancer. Mod Pathol. 2013;26:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | van Erning FN, Crolla RM, Rutten HJ, Beerepoot LV, van Krieken JH, Lemmens VE. No change in lymph node positivity rate despite increased lymph node yield and improved survival in colon cancer. Eur J Cancer. 2014;50:3221-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kim YW, Jan KM, Jung DH, Cho MY, Kim NK. Histological inflammatory cell infiltration is associated with the number of lymph nodes retrieved in colorectal cancer. Anticancer Res. 2013;33:5143-5150. [PubMed] |
| 19. | Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, Regina G, Roncoroni L. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol. 2013;19:8515-8526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 21. | Li Destri G, Di Carlo I, Scilletta R, Scilletta B, Puleo S. Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol. 2014;20:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Brown HG, Luckasevic TM, Medich DS, Celebrezze JP, Jones SM. Efficacy of manual dissection of lymph nodes in colon cancer resections. Mod Pathol. 2004;17:402-406. [PubMed] |
| 23. | Kim YM, Suh JH, Cha HJ, Jang SJ, Kim MJ, Yoon S, Kim B, Chang H, Kwon Y, Hong EK. Additional lymph node examination from entire submission of residual mesenteric tissue in colorectal cancer specimens may not add clinical and pathologic relevance. Hum Pathol. 2007;38:762-767. [PubMed] |
| 24. | Dejardin O, Ruault E, Jooste V, Pornet C, Bouvier V, Bouvier AM, Launoy G. Volume of surgical activity and lymph node evaluation for patients with colorectal cancer in France. Dig Liver Dis. 2012;44:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Leung AM, Scharf AW, Vu HN. Factors affecting number of lymph nodes harvested in colorectal cancer. J Surg Res. 2011;168:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Moro-Valdezate D, Pla-Martí V, Martín-Arévalo J, Belenguer-Rodrigo J, Aragó-Chofre P, Ruiz-Carmona MD, Checa-Ayet F. Factors related to lymph node harvest: does a recovery of more than 12 improve the outcome of colorectal cancer? Colorectal Dis. 2013;15:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Sinan H, Demirbas S, Ersoz N, Ozerhan IH, Yagci G, Akyol M, Cetiner S. Who is responsible for inadequate lymph node retrieval after colorectal surgery: surgeon or pathologist? Acta Chir Belg. 2012;112:200-208. [PubMed] |
| 28. | Stocchi L, Fazio VW, Lavery I, Hammel J. Individual surgeon, pathologist, and other factors affecting lymph node harvest in stage II colon carcinoma. is a minimum of 12 examined lymph nodes sufficient? Ann Surg Oncol. 2011;18:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Valsecchi ME, Leighton J, Tester W. Modifiable factors that influence colon cancer lymph node sampling and examination. Clin Colorectal Cancer. 2010;9:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Lagoudianakis E, Pappas A, Koronakis N, Tsekouras D, Dallianoudis J, Kontogianni P, Papanikolaou D, Chrysikos J, Karavitis G, Markogiannakis H. Lymph node harvesting in colorectal carcinoma specimens. Tumori. 2011;97:74-78. [PubMed] |
| 31. | Hsu CW, Lin CH, Wang JH, Wang HT, Ou WC, King TM. Factors that influence 12 or more harvested lymph nodes in early-stage colorectal cancer. World J Surg. 2009;33:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Scabini S, Rimini E, Romairone E, Scordamaglia R, Pertile D, Testino G, Ferrando V. Factors that influence 12 or more harvested lymph nodes in resective R0 colorectal cancer. Hepatogastroenterology. 2010;57:728-733. [PubMed] |
| 33. | Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 34. | Wu Z, Zhang S, Aung LH, Ouyang J, Wei L. Lymph node harvested in laparoscopic versus open colorectal cancer approaches: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Bamboat ZM, Deperalta D, Dursun A, Berger DL, Bordeianou L. Factors affecting lymph node yield from patients undergoing colectomy for cancer. Int J Colorectal Dis. 2011;26:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Minhas JS, Igali L. Lymph node correlations and thresholds in colorectal cancer specimens. Int J Surg Pathol. 2011;19:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Kuijpers CC, van Slooten HJ, Schreurs WH, Moormann GR, Abtahi MA, Slappendel A, Cliteur V, van Diest PJ, Jiwa NM. Better retrieval of lymph nodes in colorectal resection specimens by pathologists’ assistants. J Clin Pathol. 2013;66:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Abbassi-Ghadi N, Boshier PR, Goldin R, Hanna GB. Techniques to increase lymph node harvest from gastrointestinal cancer specimens: a systematic review and meta-analysis. Histopathology. 2012;61:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Ahmadi O, Stringer MD, Black MA, McCall JL. Influence of age and site of disease on lymph node yield in colorectal cancer. N Z Med J. 2014;127:31-40. [PubMed] |
| 40. | Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 41. | Field K, Platell C, Rieger N, Skinner I, Wattchow D, Jones I, Chen F, Kosmider S, Wohlers T, Hibbert M. Lymph node yield following colorectal cancer surgery. ANZ J Surg. 2011;81:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Gonsalves WI, Kanuri S, Tashi T, Aldoss I, Sama A, Al-Howaidi I, Ganta A, Kalaiah M, Thota R, Krishnamurthy J. Clinicopathologic factors associated with lymph node retrieval in resectable colon cancer: a Veterans’ Affairs Central Cancer Registry (VACCR) database analysis. J Surg Oncol. 2011;104:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Jakub JW, Russell G, Tillman CL, Lariscy C. Colon cancer and low lymph node count: who is to blame? Arch Surg. 2009;144:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Kelder W, Inberg B, Schaapveld M, Karrenbeld A, Grond J, Wiggers T, Plukker JT. Impact of the number of histologically examined lymph nodes on prognosis in colon cancer: a population-based study in the Netherlands. Dis Colon Rectum. 2009;52:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Khan H, Olszewski AJ, Somasundar P. Lymph node involvement in colon cancer patients decreases with age; a population based analysis. Eur J Surg Oncol. 2014;40:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Nedrebø BS, Søreide K, Nesbakken A, Eriksen MT, Søreide JA, Kørner H. Risk factors associated with poor lymph node harvest after colon cancer surgery in a national cohort. Colorectal Dis. 2013;15:e301-e308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Norwood MG, Sutton AJ, West K, Sharpe DP, Hemingway D, Kelly MJ. Lymph node retrieval in colorectal cancer resection specimens: national standards are achievable, and low numbers are associated with reduced survival. Colorectal Dis. 2010;12:304-309. [PubMed] |
| 48. | Fan L, Levy M, Aguilar CE, Mertens RB, Dhall D, Frishberg DP, Wang HL. Lymph node retrieval from colorectal resection specimens for adenocarcinoma: is it worth the extra effort to find at least 12 nodes? Colorectal Dis. 2011;13:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Horzic M, Kopljar M. Minimal number of lymph nodes that need to be examined for adequate staging of colorectal cancer--factors influencing lymph node harvest. Hepatogastroenterology. 2005;52:86-89. [PubMed] |
| 50. | Kuo YH, Lee KF, Chin CC, Huang WS, Yeh CH, Wang JY. Does body mass index impact the number of LNs harvested and influence long-term survival rate in patients with stage III colon cancer? Int J Colorectal Dis. 2012;27:1625-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Damadi AA, Julien L, Arrangoiz R, Raiji M, Weise D, Saxe AW. Does obesity influence lymph node harvest among patients undergoing colectomy for colon cancer? Am Surg. 2008;74:1073-1077. [PubMed] |
| 52. | Linebarger JH, Mathiason MA, Kallies KJ, Shapiro SB. Does obesity impact lymph node retrieval in colon cancer surgery? Am J Surg. 2010;200:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Berg M, Guriby M, Nordgård O, Nedrebø BS, Ahlquist TC, Smaaland R, Oltedal S, Søreide JA, Kørner H, Lothe RA. Influence of microsatellite instability and KRAS and BRAF mutations on lymph node harvest in stage I-III colon cancers. Mol Med. 2013;19:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Dillman RO, Aaron K, Heinemann FS, McClure SE. Identification of 12 or more lymph nodes in resected colon cancer specimens as an indicator of quality performance. Cancer. 2009;115:1840-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Gelos M, Gelhaus J, Mehnert P, Bonhag G, Sand M, Philippou S, Mann B. Factors influencing lymph node harvest in colorectal surgery. Int J Colorectal Dis. 2008;23:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Morikawa T, Tanaka N, Kuchiba A, Nosho K, Yamauchi M, Hornick JL, Swanson RS, Chan AT, Meyerhardt JA, Huttenhower C. Predictors of lymph node count in colorectal cancer resections: data from US nationwide prospective cohort studies. Arch Surg. 2012;147:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Nash GM, Row D, Weiss A, Shia J, Guillem JG, Paty PB, Gonen M, Weiser MR, Temple LK, Fitzmaurice G. A predictive model for lymph node yield in colon cancer resection specimens. Ann Surg. 2011;253:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Porter GA, Urquhart R, Bu J, Johnson P, Grunfeld E. The impact of audit and feedback on nodal harvest in colorectal cancer. BMC Cancer. 2011;11:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Tekkis PP, Smith JJ, Heriot AG, Darzi AW, Thompson MR, Stamatakis JD. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum. 2006;49:1673-1683. [PubMed] |
| 60. | Wright FC, Law CH, Last L, Khalifa M, Arnaout A, Naseer Z, Klar N, Gallinger S, Smith AJ. Lymph node retrieval and assessment in stage II colorectal cancer: a population-based study. Ann Surg Oncol. 2003;10:903-909. [PubMed] |
| 61. | Lee S, Hofmann LJ, Davis KG, Waddell BE. Lymph node evaluation of colon cancer and its association with improved staging and survival in the Department of Defense Health Care System. Ann Surg Oncol. 2009;16:3080-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Märkl B, Rößle J, Arnholdt HM, Schaller T, Krammer I, Cacchi C, Jähnig H, Schenkirsch G, Spatz H, Anthuber M. The clinical significance of lymph node size in colon cancer. Mod Pathol. 2012;25:1413-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Sloothaak DA, Grewal S, Doornewaard H, van Duijvendijk P, Tanis PJ, Bemelman WA, van der Zaag ES, Buskens CJ. Lymph node size as a predictor of lymphatic staging in colonic cancer. Br J Surg. 2014;101:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Belt EJ, te Velde EA, Krijgsman O, Brosens RP, Tijssen M, van Essen HF, Stockmann HB, Bril H, Carvalho B, Ylstra B. High lymph node yield is related to microsatellite instability in colon cancer. Ann Surg Oncol. 2012;19:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | MacQuarrie E, Arnason T, Gruchy J, Yan S, Drucker A, Huang WY. Microsatellite instability status does not predict total lymph node or negative lymph node retrieval in stage III colon cancer. Hum Pathol. 2012;43:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Rhoads KF, Ackerson LK, Ngo JV, Gray-Hazard FK, Subramanian SV, Dudley RA. Adequacy of lymph node examination in colorectal surgery: contribution of the hospital versus the surgeon. Med Care. 2013;51:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Senthil M, Trisal V, Paz IB, Lai LL. Prediction of the adequacy of lymph node retrieval in colon cancer by hospital type. Arch Surg. 2010;145:840-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149-2154. [PubMed] |
| 69. | Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 848] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 70. | Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, Ballario E, Becchi G, Bonilauri S, Carobbi A. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002;235:458-463. [PubMed] |
| 71. | Chang YJ, Chang YJ, Chen LJ, Chung KP, Lai MS. Evaluation of lymph nodes in patients with colon cancer undergoing colon resection: a population-based study. World J Surg. 2012;36:1906-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Cserni G, Vinh-Hung V, Burzykowski T. Is there a minimum number of lymph nodes that should be histologically assessed for a reliable nodal staging of T3N0M0 colorectal carcinomas? J Surg Oncol. 2002;81:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Gleisner AL, Mogal H, Dodson R, Efron J, Gearhart S, Wick E, Lidor A, Herman JM, Pawlik TM. Nodal status, number of lymph nodes examined, and lymph node ratio: what defines prognosis after resection of colon adenocarcinoma? J Am Coll Surg. 2013;217:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 75. | Kotake K, Honjo S, Sugihara K, Hashiguchi Y, Kato T, Kodaira S, Muto T, Koyama Y. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. 2012;42:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Maggard MA, Yermilov I, Tomlinson JS, Ko CY. Are 12 nodes needed to accurately stage T1 and T2 colon cancers? Dig Dis Sci. 2009;54:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65-71. [PubMed] |
| 78. | Vather R, Sammour T, Kahokehr A, Connolly AB, Hill AG. Lymph node evaluation and long-term survival in Stage II and Stage III colon cancer: a national study. Ann Surg Oncol. 2009;16:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Bilimoria KY, Palis B, Stewart AK, Bentrem DJ, Freel AC, Sigurdson ER, Talamonti MS, Ko CY. Impact of tumor location on nodal evaluation for colon cancer. Dis Colon Rectum. 2008;51:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Bui L, Rempel E, Reeson D, Simunovic M. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: a population-based study. J Surg Oncol. 2006;93:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Law CH, Wright FC, Rapanos T, Alzahrani M, Hanna SS, Khalifa M, Smith AJ. Impact of lymph node retrieval and pathological ultra-staging on the prognosis of stage II colon cancer. J Surg Oncol. 2003;84:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 82. | Sato H, Maeda K, Sugihara K, Mochizuki H, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hirai T. High-risk stage II colon cancer after curative resection. J Surg Oncol. 2011;104:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Jestin P, Påhlman L, Glimelius B, Gunnarsson U. Cancer staging and survival in colon cancer is dependent on the quality of the pathologists’ specimen examination. Eur J Cancer. 2005;41:2071-2078. [PubMed] |
| 84. | Shanmugam C, Hines RB, Jhala NC, Katkoori VR, Zhang B, Posey JA, Bumpers HL, Grizzle WE, Eltoum IE, Siegal GP. Evaluation of lymph node numbers for adequate staging of Stage II and III colon cancer. J Hematol Oncol. 2011;4:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Tsikitis VL, Larson DL, Wolff BG, Kennedy G, Diehl N, Qin R, Dozois EJ, Cima RR. Survival in stage III colon cancer is independent of the total number of lymph nodes retrieved. J Am Coll Surg. 2009;208:42-47. [PubMed] |
| 86. | Berberoglu U. Prognostic significance of total lymph node number in patients with T1-4N0M0 colorectal cancer. Hepatogastroenterology. 2004;51:1689-1693. [PubMed] |
| 87. | Cianchi F, Palomba A, Boddi V, Messerini L, Pucciani F, Perigli G, Bechi P, Cortesini C. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg. 2002;26:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Iachetta F, Reggiani Bonetti L, Marcheselli L, Di Gregorio C, Cirilli C, Messinese S, Cervo GL, Postiglione R, Di Emidio K, Pedroni M. Lymph node evaluation in stage IIA colorectal cancer and its impact on patient prognosis: a population-based study. Acta Oncol. 2013;52:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Ishizuka M, Nagata H, Takagi K, Kubota K. Insufficient lymph node dissection is an independent risk factor for postoperative cancer death in patients undergoing surgery for stage II colorectal cancer. Eur Surg Res. 2011;46:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | La Torre M, Lorenzon L, Pilozzi E, Barucca V, Cavallini M, Ziparo V, Ferri M. Number of harvested lymph nodes is the main prognostic factor in Stage IIa colorectal cancer patients. J Surg Oncol. 2012;106:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Nir S, Greenberg R, Shacham-Shmueli E, White I, Schneebaum S, Avital S. Number of retrieved lymph nodes and survival in node-negative patients undergoing laparoscopic colorectal surgery for cancer. Tech Coloproctol. 2010;14:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Tsai HL, Lu CY, Hsieh JS, Wu DC, Jan CM, Chai CY, Chu KS, Chan HM, Wang JY. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg. 2007;11:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Xingmao Z, Hongying W, Zhixiang Z, Zheng W. Analysis on the correlation between number of lymph nodes examined and prognosis in patients with stage II colorectal cancer. Med Oncol. 2013;30:371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Yoshimatsu K, Ishibashi K, Umehara A, Yokomizo H, Yoshida K, Fujimoto T, Watanabe K, Ogawa K. How many lymph nodes should be examined in Dukes’ B colorectal cancer? Determination on the basis of cumulative survival rate. Hepatogastroenterology. 2005;52:1703-1706. [PubMed] |
| 95. | Wong JH, Bowles BJ, Bueno R, Shimizu D. Impact of the number of negative nodes on disease-free survival in colorectal cancer patients. Dis Colon Rectum. 2002;45:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 96. | Caplin S, Cerottini JP, Bosman FT, Constanda MT, Givel JC. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer. 1998;83:666-672. [PubMed] |
| 97. | Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Desolneux G, Burtin P, Lermite E, Bergamaschi R, Hamy A, Arnaud JP. Prognostic factors in node-negative colorectal cancer: a retrospective study from a prospective database. Int J Colorectal Dis. 2010;25:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Duraker N, Civelek Çaynak Z, Hot S. The prognostic value of the number of lymph nodes removed in patients with node-negative colorectal cancer. Int J Surg. 2014;12:1324-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 100. | Edler D, Ohrling K, Hallström M, Karlberg M, Ragnhammar P. The number of analyzed lymph nodes - a prognostic factor in colorectal cancer. Acta Oncol. 2007;46:975-981. [PubMed] |
| 101. | Fretwell VL, Ang CW, Tweedle EM, Rooney PS. The impact of lymph node yield on Duke’s B and C colorectal cancer survival. Colorectal Dis. 2010;12:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | George S, Primrose J, Talbot R, Smith J, Mullee M, Bailey D, du Boulay C, Jordan H. Will Rogers revisited: prospective observational study of survival of 3592 patients with colorectal cancer according to number of nodes examined by pathologists. Br J Cancer. 2006;95:841-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Mino-Kenudson M, Giovannucci EL, Meyerhardt JA, Fuchs CS. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. 2010;105:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 104. | Onitilo AA, Stankowski RV, Engel JM, Doi SA. Adequate lymph node recovery improves survival in colorectal cancer patients. J Surg Oncol. 2013;107:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Wong JH, Lum SS, Morgan JW. Lymph node counts as an indicator of quality at the hospital level in colorectal surgery. J Am Coll Surg. 2011;213:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Zhang B, Lv M, Chen T, Wei Q, Wang G, Tian J, Chen B. The association between lymph node resection and postoperative survival in patients with colorectal cancer. Hepatogastroenterology. 2013;60:1922-1926. [PubMed] |
| 107. | Evans MD, Barton K, Rees A, Stamatakis JD, Karandikar SS. The impact of surgeon and pathologist on lymph node retrieval in colorectal cancer and its impact on survival for patients with Dukes’ stage B disease. Colorectal Dis. 2008;10:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Kritsanasakul A, Boonpipattanapong T, Wanitsuwan W, Phukaoloun M, Prechawittayakul P, Sangkhathat S. Impact of lymph node retrieval on surgical outcomes in colorectal cancers. J Surg Oncol. 2012;106:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Wong JH, Johnson DS, Hemmings D, Hsu A, Imai T, Tominaga GT. Assessing the quality of colorectal cancer staging: documenting the process in improving the staging of node-negative colorectal cancer. Arch Surg. 2005;140:881-886; discussion 886-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Baxter NN, Ricciardi R, Simunovic M, Urbach DR, Virnig BA. An evaluation of the relationship between lymph node number and staging in pT3 colon cancer using population-based data. Dis Colon Rectum. 2010;53:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Bernhoff R, Holm T, Sjövall A, Granath F, Ekbom A, Martling A. Increased lymph node harvest in patients operated on for right-sided colon cancer: a population-based study. Colorectal Dis. 2012;14:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Cserni G, Bori R, Sejben I. Limited lymph-node recovery based on lymph-node localisation is sufficient for accurate staging. J Clin Pathol. 2011;64:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Cserni G, Tarján M, Bori R. Distance of lymph nodes from the tumor: an important feature in colorectal cancer specimens. Arch Pathol Lab Med. 2001;125:246-249. [PubMed] |
| 115. | Cserni G, Vajda K, Tarján M, Bori R, Svébis M, Baltás B. Nodal staging of colorectal carcinomas from quantitative and qualitative aspects. Can lymphatic mapping help staging? Pathol Oncol Res. 1999;5:291-296. [PubMed] |
| 116. | Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122:1253-1256. [PubMed] |
| 117. | Huh JW, Kim YJ, Kim HR. Distribution of lymph node metastases is an independent predictor of survival for sigmoid colon and rectal cancer. Ann Surg. 2012;255:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 118. | Kajiwara Y, Ueno H, Hashiguchi Y, Mochizuki H, Hase K. Risk factors of nodal involvement in T2 colorectal cancer. Dis Colon Rectum. 2010;53:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Lindboe CF. Lymph node harvest in colorectal adenocarcinoma specimens: the impact of improved fixation and examination procedures. APMIS. 2011;119:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Parsons HM, Tuttle TM, Kuntz KM, Begun JW, McGovern PM, Virnig BA. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 121. | Perrakis A, Weber K, Merkel S, Matzel K, Agaimy A, Gebbert C, Hohenberger W. Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int J Colorectal Dis. 2014;29:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Rajput A, Romanus D, Weiser MR, ter Veer A, Niland J, Wilson J, Skibber JM, Wong YN, Benson A, Earle CC. Meeting the 12 lymph node (LN) benchmark in colon cancer. J Surg Oncol. 2010;102:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 124. | Song YX, Gao P, Wang ZN, Tong LL, Xu YY, Sun Z, Xing CZ, Xu HM. Which is the most suitable classification for colorectal cancer, log odds, the number or the ratio of positive lymph nodes? PLoS One. 2011;6:e28937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Stanisavljević L, Søndenaa K, Storli KE, Leh S, Nesvik I, Gudlaugsson E, Bukholm I, Eide GE. The total number of lymph nodes in resected colon cancer specimens is affected by several factors but the lymph node ratio is independent of these. APMIS. 2014;122:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Storli K, Søndenaa K, Furnes B, Leh S, Nesvik I, Bru T, Gudlaugsson E, Bukholm I, Norheim-Andersen S, Eide G. Improved lymph node harvest from resected colon cancer specimens did not cause upstaging from TNM stage II to III. World J Surg. 2011;35:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Wang H, Wei XZ, Fu CG, Zhao RH, Cao FA. Patterns of lymph node metastasis are different in colon and rectal carcinomas. World J Gastroenterol. 2010;16:5375-5379. [PubMed] |
| 128. | Chapman B, Paquette C, Tooke C, Schwartz M, Osler T, Weaver D, Wilcox R, Hyman N. Impact of Schwartz enhanced visualization solution on staging colorectal cancer and clinicopathological features associated with lymph node count. Dis Colon Rectum. 2013;56:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 129. | Chen HH, Chakravarty K D, Wang JY, Changchien CR, Tang R. Pathological examination of 12 regional lymph nodes and long-term survival in stages I-III colon cancer patients: an analysis of 2,056 consecutive patients in two branches of same institution. Int J Colorectal Dis. 2010;25:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Cohen SM, Wexner SD, Schmitt SL, Nogueras JJ, Lucas FV. Effect of xylene clearance of mesenteric fat on harvest of lymph nodes after colonic resection. Eur J Surg. 1994;160:693-697. [PubMed] |
| 131. | Haboubi NY, Clark P, Kaftan SM, Schofield PF. The importance of combining xylene clearance and immunohistochemistry in the accurate staging of colorectal carcinoma. J R Soc Med. 1992;85:386-388. [PubMed] |
| 132. | Hernanz F, García-Somacarrera E, Fernández F. The assessment of lymph nodes missed in mesenteric tissue after standard dissection of colorectal cancer specimens. Colorectal Dis. 2010;12:e57-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Koren R, Kyzer S, Paz A, Veltman V, Klein B, Gal R. Lymph node revealing solution: a new method for detection of minute axillary lymph nodes in breast cancer specimens. Am J Surg Pathol. 1997;21:1387-1390. [PubMed] |
| 134. | Ma XL, Ye JX, Su J, Qi FF, Meng QY, Shi XY. A modified GEWF solution is cost-saving and effective for lymph node retrieval in resected colorectal carcinoma specimens. Pathol Res Pract. 2014;210:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Richter D, Lorenz D, Isemer FE, Braun S, Fisseler-Eckhoff A. [Acetone treatment of lymph node preparations in staging colorectal specimens]. Pathologe. 2007;28:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg. 1989;76:1165-1167. [PubMed] |
| 137. | Vogel C, Kirtil T, Oellig F, Stolte M. Lymph node preparation in resected colorectal carcinoma specimens employing the acetone clearing method. Pathol Res Pract. 2008;204:11-15. [PubMed] |
| 138. | Iversen LH, Laurberg S, Hagemann-Madsen R, Dybdahl H. Increased lymph node harvest from colorectal cancer resections using GEWF solution: a randomised study. J Clin Pathol. 2008;61:1203-1208. [PubMed] |
| 139. | Kukreja SS, Esteban-Agusti E, Velasco JM, Hieken TJ. Increased lymph node evaluation with colorectal cancer resection: does it improve detection of stage III disease? Arch Surg. 2009;144:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Newell KJ, Sawka BW, Rudrick BF, Driman DK. GEWF solution. Arch Pathol Lab Med. 2001;125:642-645. [PubMed] |
| 141. | Ratto C, Sofo L, Ippoliti M, Merico M, Bossola M, Vecchio FM, Doglietto GB, Crucitti F. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum. 1999;42:143-154; discussion 154-158. [PubMed] |
| 142. | van Steenbergen LN, van Lijnschoten G, Rutten HJ, Lemmens VE, Coebergh JW. Improving lymph node detection in colon cancer in community hospitals and their pathology department in southern Netherlands. Eur J Surg Oncol. 2010;36:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Borowski DW, Banky B, Banerjee AK, Agarwal AK, Tabaqchali MA, Garg DK, Hobday C, Hegab M, Gill TS. Intra-arterial methylene blue injection into ex vivo colorectal cancer specimens improves lymph node staging accuracy: a randomized controlled trial. Colorectal Dis. 2014;16:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Frasson M, Faus C, Garcia-Granero A, Puga R, Flor-Lorente B, Cervantes A, Navarro S, Garcia-Granero E. Pathological evaluation of mesocolic resection quality and ex vivo methylene blue injection: what is the impact on lymph node harvest after colon resection for cancer? Dis Colon Rectum. 2012;55:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 145. | Jepsen RK, Ingeholm P, Lund EL. Upstaging of early colorectal cancers following improved lymph node yield after methylene blue injection. Histopathology. 2012;61:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 146. | Kır G, Alimoglu O, Sarbay BC, Bas G. Ex vivo intra-arterial methylene blue injection in the operation theater may improve the detection of lymph node metastases in colorectal cancer. Pathol Res Pract. 2014;210:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Törnroos A, Shabo I, Druvefors B, Arbman G, Olsson H. Postoperative intra-arterial methylene blue injection of colorectal cancer specimens increases the number of lymph nodes recovered. Histopathology. 2011;58:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Budde CN, Tsikitis VL, Deveney KE, Diggs BS, Lu KC, Herzig DO. Increasing the number of lymph nodes examined after colectomy does not improve colon cancer staging. J Am Coll Surg. 2014;218:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol. 1996;106:209-216. [PubMed] |
| 150. | Hogan NM, Winter DC. A nodal positivity constant: new perspectives in lymph node evaluation and colorectal cancer. World J Surg. 2013;37:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Stojadinovic A, Nissan A, Wainberg Z, Shen P, McCarter M, Protic M, Howard RS, Steele SR, Peoples GE, Bilchik A. Time-dependent trends in lymph node yield and impact on adjuvant therapy decisions in colon cancer surgery: an international multi-institutional study. Ann Surg Oncol. 2012;19:4178-4185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Miller EA, Woosley J, Martin CF, Sandler RS. Hospital-to-hospital variation in lymph node detection after colorectal resection. Cancer. 2004;101:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 153. | Mitchell PJ, Ravi S, Grifftiths B, Reid F, Speake D, Midgley C, Mapstone N. Multicentre review of lymph node harvest in colorectal cancer: are we understaging colorectal cancer patients? Int J Colorectal Dis. 2009;24:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Simunovic M, Baxter NN. Lymph node counts in colon cancer surgery: lessons for users of quality indicators. JAMA. 2007;298:2194-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 155. | Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1121] [Cited by in RCA: 1075] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 156. | Jass JR, O’Brien MJ, Riddell RH, Snover DC. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Virchows Arch. 2007;450:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Pihl E, Nairn RC, Milne BJ, Cuthbertson AM, Hughes ES, Rollo A. Lymphoid hyperplasia: a major prognostic feature in 519 cases of colorectal carcinoma. Am J Pathol. 1980;100:469-480. [PubMed] |
| 158. | Dworak O. Morphology of lymph nodes in the resected rectum of patients with rectal carcinoma. Pathol Res Pract. 1991;187:1020-1024. [PubMed] |
| 159. | Saridaki Z, Souglakos J, Georgoulias V. Prognostic and predictive significance of MSI in stages II/III colon cancer. World J Gastroenterol. 2014;20:6809-6814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 160. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1106] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 161. | Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gögenur I; Danish Colorectal Cancer Group. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 162. | Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 163. | Märkl B, Kerwel TG, Jähnig HG, Oruzio D, Arnholdt HM, Schöler C, Anthuber M, Spatz H. Methylene blue-assisted lymph node dissection in colon specimens: a prospective, randomized study. Am J Clin Pathol. 2008;130:913-919. [PubMed] |
| 164. | Mainprize KS, Kulacoglu H, Hewavisinthe J, Savage A, Mortensen N, Warren BF. How many lymph nodes to stage colorectal carcinoma? J Clin Pathol. 1998;51:165-166. [PubMed] |
| 165. | Earle CC, Weiser MR, Ter Veer A, Skibber JM, Wilson J, Rajput A, Wong YN, Benson AB, Shibata S, Romanus D. Effect of lymph node retrieval rates on the utilization of adjuvant chemotherapy in stage II colon cancer. J Surg Oncol. 2009;100:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |