Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17132
Revised: May 16, 2014
Accepted: July 22, 2014
Published online: December 7, 2014
Processing time: 271 Days and 4 Hours
AIM: To select appropriate patients before surgical resection for hepatocellular carcinoma (HCC), especially those with advanced tumors.
METHODS: From January 2000 to December 2012, we retrospectively analyzed the medical records of 298 patients who had undergone surgical resections for HCC with curative intent at our hospital. We evaluated preoperative prognostic factors associated with histologic grade of tumor, recurrence and survival, especially the findings of pre-operative imaging studies such as positron emission tomography-computed tomography (PET-CT) and magnetic resonance imaging (MRI). And then, we established a scoring system to predict recurrence and survival after surgery dividing the patients into two groups based on a tumor size of 5 cm.
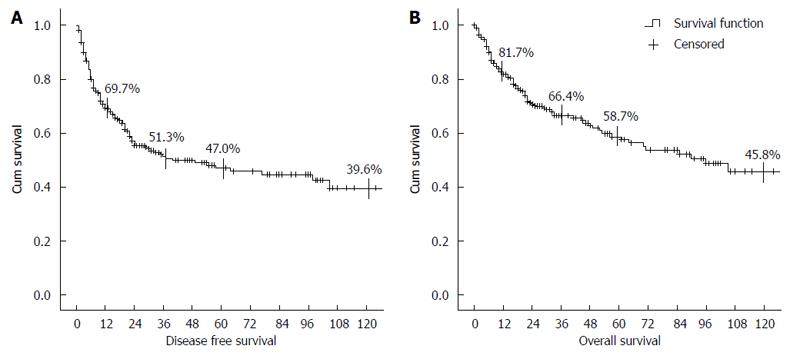
RESULTS: Of the 298 patients, 129 (43.3%) developed recurrence during the follow-up period. The 5 year disease free survival and overall survival were 47.0% and 58.7% respectively. In multivariate analysis, a serum alpha-fetoprotein (AFP) level of > 100 ng/mL and a standardized uptake value (SUV) of PET-CT of > 3.5 were predictive factors for histologic grade of tumor, recurrence, and survival. Tumor size of > 5 cm and a relative enhancement ratio (RER) calculated from preoperative MRI were also significantly associated with prognosis in univariate analysis. We established a scoring system to predict prognosis using AFP, SUV, and RER. In those with tumors of > 5 cm, it showed predicted both recurrence (P = 0.005) and survival (P = 0.001).
CONCLUSION: The AFP, tumor size, SUV and RER are useful for prognosis preoperatively. An accurate prediction of prognosis is possible using our scoring system in large size tumors.
Core tip: Tumor recurrence after surgical resection for hepatocellular carcinoma is an obstacle to long-term survival. Thus, selection of appropriate patients is important, especially those with advanced tumors. Several factors responsible for the high recurrence and poor survival rates after surgical resection have been described. We evaluate the preoperative clinical factors such as serum alpha fetoprotein, protein induced by vitamin K absence or angiotensin-II and the findings of pre-operative imaging studies such as positron emission tomography-computed tomography and magnetic resonance imaging. And then, we established a scoring system to predict recurrence and survival after surgery.
- Citation: Han JH, Kim DG, Na GH, Kim EY, Lee SH, Hong TH, You YK. Evaluation of prognostic factors on recurrence after curative resections for hepatocellular carcinoma. World J Gastroenterol 2014; 20(45): 17132-17140
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17132
Most cases of hepatocellular carcinoma (HCC) are accompanied by liver disease induced by viral hepatitis or alcohol. Therefore, it is necessary to consider both the tumor characteristics and hepatic function to determine the most appropriate treatment method. There exist two treatment options with curative intention: liver resection and liver transplantation. However, other treatment modalities have also been reported to show survival advantages, such as radiofrequency ablation and transcatheter arterial chemoembolization[1-3]. Despite this fact, there is no evidence of any treatment more effective than surgical resection, and surgery has thus been considered the gold standard treatment for HCC[4,5]. Regardless, tumor recurrence after surgical resection has been reported in over half of affected patients[6,7], and is the major obstacle to long-term survival. Therefore, it is important to select appropriate patients and evaluate preoperative predictive factors for recurrence and survival, to ensure that liver resection is performed in those in the high-risk group who would be most likely to benefit from surgery.
Several factors responsible for the high recurrence and poor survival rates after surgical resection have been described by investigators. One is tumor characteristics, which are divided into morphological and biological factors. Biological factors, especially tumor cell differentiation, are known to be more accurate in predicting the prognosis than tumor morphology. However, preoperative needle biopsy is not generally recommended because of the possibility of tumor seeding[8]. Therefore, it is important to identify biological factors using an indirect method prior to surgery. Serum alpha fetoprotein (AFP) and protein induced by vitamin K absence or angiotensin-II (PIVKA-II) can be analyzed using indirect methods. Some investigators reported the usefulness of positron emission tomography-computed tomography (PET-CT) to detect HCC using standardized uptake values (SUVs)[9]. A noninvasive method using gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) magnetic resonance imaging (MRI) has also been reported that the differentiation of enhancement between the tumors and surrounding liver parenchyma using Gd-EOB-DTPA-enhanced MRI can predict the histological grade of HCC[10,11].
The aim of the present study was to determine the disease-free and overall survival rates and evaluate the preoperative clinical factors associated with histologic grade of tumor, recurrence and survival, especially the findings of pre-operative imaging studies such as PET-CT and MRI.
We retrospectively reviewed the medical records of 335 patients who had undergone surgical resection for HCC with curative intent from January 2000 to December 2012 at our hospital. We excluded 31 patients with confirmed extrahepatic metastasis, vascular invasion, and positive resection margins intraoperatively or postoperatively. Six patients who died of liver failure or postoperative bleeding within 1 mo after surgery were also excluded. This study was approved by the Institutional Review Board of our center.
The indocyanine green (ICG) clearance test was performed in all patients to evaluate residual hepatic function. We measured the Child-Turcotte-Pugh (CTP) and Model for End-stage Liver Disease (MELD) scores prior to surgery. AFP and PIVKA-II were assessed as tumor markers, and all patients were preoperatively evaluated by CT of the abdomen and chest. MRI, PET-CT, and a bone scan were also conducted to identify metastases. Gd-EOB-DTPA-enhanced MRI and PET-CT was routinely performed after March 2008 for all HCC patients. 100 patients were assessed MRI and PET-CT in this study. If extrahepatic metastases or tumor thrombi in the main portal vein were identified, the patient was excluded from curative resection. Patients with a large amount of ascites or hyperbilirubinemia as well as those who corresponded to Child class C were also excluded; however, partial hepatectomy was performed in Child class B patients. In the ICG clearance test, we set the safe limit for the ICG retention value as < 15% at 15 min for major hepatectomy. For patients with an ICG retention value of > 15%, we performed a partial hepatectomy[10]. Patient age, sex, and underlying disease were collected as demographic data.
Tumor number and size were determined preoperatively by CT or MRI, and the size was based on the largest diameter of the tumor. The histological grade of tumor was recorded using the Edmondson-Steiner classification based on the data in the pathology reports. 18F-fluoro-2-deoxy-D-glucose (18F-FDG) PET-CT was routinely performed for almost all patients with HCC. The SUV is the tumor FDG uptake value that can effectively correct the variation in relative tissue FDG uptake. The majority of all candidates for HCC resection had been assessed MRI prior to surgery using Gd-EOB-DTPA-enhanced MRI using the same MRI system (Magnetom Verio; Siemens Healthcare, Germany). Unenhanced, arterial, portal, and hepatobiliary phase images were assessed. We calculated the relative signal intensity ratio (RIR) of the tumor and the surrounding liver parenchyma on the unenhanced and hepatobiliary phase images, respectively. We then compared these RIR values and calculated the relative enhancement ratio (RER) of the tumor as follows[11]: RIR = Signal intensity of the tumor/Signal intensity of the liver parenchyma; RER = RIR on hepatobiliary phase image/RIR on unenhanced image.
The signal intensity (SI) of the tumor and the surrounding liver parenchyma were measured in the section with the largest tumor diameter using regions of interests (ROIs). The ROIs of each tumor were set to avoid necrotic areas, and the SI of the liver parenchyma was assessed using the same ROI size used for the tumor, including only the liver parenchyma as much as possible[11-13]. When the patients had a multicentric HCC, we used the largest size of tumor to measure the ROIs. We also evaluated the recurrence rate, disease-free survival (DFS) rate, and survival rate. The factors associated with recurrence and survival were identified. The predictive factors of the tumor biology such as histological grade were also evaluated.
All patients were managed with a standardized treatment protocol. On the seventh day after surgery, follow-up abdominal CT was performed for evaluation of the intra-abdominal status. After discharge, we assessed tumor markers, such as AFP and PIVKA II, in the outpatient clinic every 2 mo, and abdominal and chest CT was performed at intervals of 4 mo for the first year after surgery. During the second year after surgery, tumor markers were evaluated at intervals of 3 mo, and CT was performed every 6 mo for the next year and then annually. If recurrence was suspected or other abnormal findings were noted, liver MRI and PET-CT were performed.
Mean, standard deviation, and ranges were used to present numerical variables. Continuous variables were compared by Student’s t-test. Differences in categorical variables were analyzed with the χ2 test. The continuous variables which have large standard variation were converted to categorical variables using the ROC curve. The logistic regression analysis was used for multivariate analysis to identify the preoperative prediction factors of tumor biology. The Cox proportional hazard regression model was used for multivariate analysis to identify risk factors independently associated with recurrence or survival. Survival data were analyzed using the Kaplan-Meier method to describe the DFS and 5- and 10-year survival rates. The survival time of the groups was compared using the log-rank test. P-values of < 0.05 were considered to indicate statistical significance.
The mean age of all patients was 56.4 ± 9.7 (33-77) years; among them, 232 (77.9%) were male. The most common underlying disease was hepatitis B virus (HBV) infection (71.5%), followed by hepatitis C virus (HCV) infection (6.0%), and alcohol-related disease (5.4%). An ICG clearance test was routinely performed prior to surgery, and the mean ICG retention value was 11.64% ± 13.95%; 82.5% of patients did not exceed 15%. The mean CTP score was 5.40 ± 0.72, and 90.6% of patients corresponded to Child class A; no patients were Child class C. The mean MELD score was 6.36 ± 2.93. We routinely assessed tumor markers prior to surgery. The mean AFP level was 1459.90 ± 10124.9 ng/mL, and the mean PIVKA II was 499.1 ± 1434.7 mAU/mL. The preoperative mean tumor size was 4.49 ± 3.12 (0.5-16.0) cm, and the mean number of tumors identified in preoperative imaging studies was 1.27 ± 0.85. Among the 298 patients enrolled in the present study, 112 (37.6%) underwent major liver resection, such as hemi-hepatectomy; 124 (41.6%) underwent segmental liver resection; and 62 (20.8%) underwent partial resection. The median follow-up duration was 32 mo (3-163 mo) (Table 1).
| Total | Patients (n = 298) |
| Age (yr) | 56.4 ± 9.7 |
| Sex (male) | 232 (77.9) |
| Disease | |
| Hepatitis B | 213 (71.5) |
| Hepatitis C | 18 (6.0) |
| Alcohol | 16 (5.4) |
| Others | 51 (17.1) |
| PLT (× 103/μL) | 151.93 ± 70.11 (36.0-417.0) |
| Total bilirubin (mg/dL) | 1.17 ± 5.97 (0.11-7.88) |
| ICG at 15 min (%) | 11.64 ± 13.95 |
| CTP score | 5.40 ± 0.72 |
| MELD score | 6.36 ± 2.93 |
| AFP (ng/mL) | 1459.90 ± 10124.9 |
| 19.50 (4.30-268.31)1 | |
| PIVKA II (mAU/mL) | 499.1 ± 1434.7 |
| 44.50 (21.00-255.00)1 | |
| Tumor number | 1.27 ± 0.85 |
| Tumor size (cm) | 4.49 ± 3.12 |
The tumor grade identified after surgery by pathology was divided into low grade group (grade I, II) and high grade group (grade III, IV) based on the Edmondson-Steiner’s classification (E and S grade). And then, it was compared to the preoperative factors. In univariate analysis, factors related to tumor biology were the mean tumor size (P < 0.001), tumor size of > 5 cm (P = 0.003), mean AFP (P = 0.023), AFP level of > 100 ng/mL (P < 0.001) and mean PIVKA II level (P = 0.001). The mean SUVs obtained by preoperative PET-CT was also significantly higher in the high grade group (P = 0.004). When compared on the basis of 3.5, there was most significantly difference between two groups (P < 0.001). The value of RER was statistically significantly lower in high grade group than low grade group (P < 0.001). When compared on the basis of 0.6, it showed most significant difference between two groups (P = 0.001).
To identify factors influencing high grade tumor, a multivariate analysis of factors which was statistically significance in univariate analysis was done. Among them, AFP of > 100 ng/mL [Exp(B) = 2.897; 95%CI: 1.080-7.775; P = 0.035], SUVs on PET-CT > 3.5 [Exp(B) = 3.305; 95%CI: 1.124-8.996; P = 0.019] and RER on MRI < 0.6 [Exp(B) = 1.888; 95%CI: 1.094-3.588; P = 0.050] showed significant influence on high grade tumor (Table 2).
| Variables | Low grade (I, II)(n = 134) | High grade (III, IV)(n = 105) | P value | Multivariate | |
| Relative risk | P value | ||||
| Tumor size (cm) | 3.83 ± 2.35 | 5.46 ± 3.75 | < 0.001 | ||
| > 5 (n = 59) | 22 (16.4) | 37 (35.2) | 0.003 | 1.695 (0.594–4.836) | 0.324 |
| AFP (ng/mL) | 254.4 ± 771.0 | 3644.9 ± 16856.5 | 0.023 | ||
| > 100 (n = 91) | 37 (27.6) | 54 (51.4) | < 0.001 | 2.897 (1.080-7.775) | 0.035 |
| PIVKA II (mAU/mL) | 214.2 ± 355.6 | 1079.1 ± 2248.3 | 0.001 | ||
| > 300 (n = 36) | 17 (12.7) | 19 (18.1) | 0.173 | 1.000 (1.000-1.001) | 0.303 |
| SUVs on PET | 3.80 ± 2.21 | 5.83 ± 3.93 | 0.004 | ||
| > 3.5 (n = 44) | 15 (11.2) | 29 (27.6) | < 0.001 | 3.305 (1.214-8.996) | 0.019 |
| RER on MRI | 0.80 ± 0.14 | 0.68 ± 0.12 | < 0.001 | ||
| < 0.6 (n = 11) | 1 (0.7) | 10 (9.5) | 0.001 | 1.888 (1.094–3.588) | 0.050 |
Of the 298 patients, 129 (43.3%) developed recurrence during the follow-up period. 114 (38.3%) developed recurrence within 2 years after surgery, and 86 (28.9%) developed recurrence within 1 year. In the survival analysis, the cumulative proportion of recurrence in the first year after surgery was 29.2%, that within 3 years was 48.7%, and that within 5 years was 53.0%. The 5- and 10-year DFS rates were 47.0% and 39.6%, respectively (Figure 1). Patient demographics and tumor characteristics were compared between the recurrence and nonrecurrence groups. In univariate analysis, factors related to tumor recurrence were the percentage of males (P = 0.026), AFP level of > 100 ng/mL (P = 0.004), mean PIVKA II level (P = 0.048), mean tumor size, tumor size of > 5 cm (P < 0.001), and mean tumor number (P = 0.046). The mean SUV obtained by preoperative PET-CT was significantly higher in the recurrence group (P = 0.001). When the SUVs of both groups were compared to the average of 3.5, a significant difference was noted (P = 0.005). We also calculated the RER from the preoperative MRI and compared it within both groups. The average RERs was higher in the nonrecurrence group, but the difference was not statistically significant (P = 0.295). However, when the cutoff value was 0.6, RER was significantly higher in the nonrecurrence group (P = 0.011).
To identify factors influencing recurrence, the factors that showed significance in the univariate analysis were subjected to a multivariate analysis. In the multivariate analysis, male sex [Exp(B) = 2.192; 95%CI: 1.060-4.532; P = 0.034], AFP of > 100 ng/mL [Exp(B) = 1.888; 95%CI: 1.094-3.588; P = 0.050], and an SUV on PET-CT of > 3.5 [Exp(B) = 2.025; 95%CI: 1.046-3.921; P = 0.036] showed significant influences on recurrence (Table 3).
| Variables | Non recurrence(n = 169) | Recurrence(n = 129) | P value | Multivariate | |
| Relative risk | P value | ||||
| Age (yr) | 56.4 ± 9.6 | 56.2 ± 9.9 | 0.717 | ||
| Gender (male) | 123 (72.8) | 109 (84.5) | 0.026 | 2.192 (1.060–4.532) | 0.034 |
| Cause of HCC (HBV:HCV:Alcohol:Others) | 116:11:8:34 | 97:7:8:17 | 0.402 | ||
| PLT (× 103/μL) | 155.07 ± 80.10 | 149.53 ± 61.57 | 0.500 | ||
| Bilirubin (mg/dL) | 1.38 ± 7.88 | 0.90 ± 0.97 | 0.491 | ||
| ICG | 12.54 ± 16.83 | 10.43 ± 8.68 | 0.271 | ||
| CTP scores | 5.37 ± 0.72 | 5.44 ± 0.71 | 0.409 | ||
| MELD scores | 6.26 ± 3.03 | 6.48 ± 2.79 | 0.521 | ||
| AFP (ng/mL) | 868.10 ± 4358.4 | 2212.3 ± 14588.5 | 0.264 | ||
| > 100 (n = 101) | 48 (28.4) | 53 (41.1) | 0.004 | 1.888 (1.094–3.588) | 0.050 |
| PIVKA II (mAU/mL) | 327.4 ± 886.0 | 795.1 ± 2040.9 | 0.048 | ||
| > 100 (n = 55) | 32 (18.9) | 23 (17.8) | 0.466 | ||
| Tumor size (cm) | 3.84 ± 2.66 | 5.28 ± 3.46 | < 0.001 | ||
| > 5 (n = 72) | 27 (16.0) | 45 (34.9) | < 0.001 | 1.516 (0.797–2.882) | 0.204 |
| Tumor number | 1.18 ± 0.57 | 1.39 ± 1.10 | 0.046 | ||
| Multiple (n = 40) | 19 (11.2) | 21 (16.2) | 0.124 | ||
| SUVs on PET CT | 3.84 ± 1.80 | 5.94 ± 4.20 | 0.001 | ||
| > 3.5 (n = 47) | 22 (13.0) | 25 (19.4) | 0.005 | 2.025 (1.046–3.921) | 0.036 |
| RER (n = 100) | 0.75 ± 0.16 | 0.72 ± 0.12 | 0.295 | ||
| < 0.6 (n = 11) | 4 (2.4) | 7 (5.4) | 0.011 | ||
Of the 298 patients, 96 (32.2%) died during the follow-up period. Fifty (52.1%) patients died within 1 year after surgery, and 86 (89.6%) died within 5 years. The cause of death was HCC recurrence in 76 (79.2%) patients, hepatic failure in 11 (11.5%), sepsis in 3 (3.1%), and other causes in 6 (6.2%). The overall mean survival period was 38.6 ± 3.0 mo in the nonrecurrence group and 24.6 ± 2.2 mo in the recurrence group, respectively (P < 0.001). The 5-year survival rates were 83.0% and 27.6%, respectively, and the 10-year survival rates were 72.3% and 21.7%, respectively, in each group (P < 0.001) (Figure 1). We compared the patient demographics and tumor characteristics between the death and survival groups. In univariate analysis, factors related to patient survival were CTP score (P = 0.003), MELD score (P = 0.020), and an AFP level of > 100 ng/mL (P = 0.005). The mean tumor size (P = 0.002), size of > 5 cm (P < 0.001), mean tumor number (P = 0.005), and multiple tumors (P = 0.010). The SUV obtained from the preoperative PET-CT was significantly higher in the death group (P = 0.001). When the SUVs of both groups were compared to the average of 3.5, a statistically significant difference was noted (P < 0.001). The average RERs was higher in the survival group, but the difference was not significant (P = 0.295). However, when the cutoff value was 0.6, the RER was significantly higher in the survival group (P = 0.003).
To identify factors influencing survival, the factors that had shown significance in the univariate analysis were subjected to multivariate analysis. In the multivariate analysis, an AFP of > 100 ng/mL [Exp(B) = 3.061; 95%CI: 1.183-7.922; P = 0.021] and SUV on PET-CT of > 3.5 [Exp(B) = 7.331; 95%CI: 2.182-24.630; P = 0.001] showed significant influences on survival (Table 4).
| Variable | Survival(n = 202) | Death(n = 96) | P value | Multivariate | |
| Relative risk | P value | ||||
| Age (yr) | 57.0 ± 9.6 | 55.2 ± 9.8 | 0.131 | ||
| Gender (male) | 150 (74.3) | 82 (85.4) | 0.102 | ||
| Cause of HCC (HBV:HCV:Alcohol:Others) | 143:14:9:36 | 70:4:7:15 | 0.568 | ||
| PLT (× 103/μL) | 151.84 ± 62.56 | 152.11 ± 84.19 | 0.975 | ||
| Bilirubin (mg/dL) | 1.24 ± 7.21 | 1.04 ± 1.15 | 0.783 | ||
| ICG | 12.05 ± 15.45 | 10.61 ± 9.21 | 0.496 | ||
| CTP scores | 5.31 ± 0.63 | 5.59 ± 0.84 | 0.003 | 1.682 (0.310-9.116) | 0.066 |
| MELD scores | 6.08 ± 2.51 | 6.93 ± 3.76 | 0.020 | 5.413 (0.895-32.718) | 0.547 |
| AFP (ng/mL) | 667.2 ± 3733.2 | 3086.7 ± 16970.2 | 0.057 | ||
| > 100 (n = 101) | 58 (28.7) | 43 (44.8) | 0.005 | 3.061 (1.183-7.922) | 0.021 |
| PIVKA II (mAU/mL) | 399.6 ± 1200.3 | 941.6 ± 2169.4 | 0.066 | ||
| > 100 (n = 55) | 42 (20.8) | 13 (13.5) | 0.159 | ||
| Tumor size (cm) | 4.08 ± 2.86 | 5.30 ± 3.47 | 0.002 | ||
| > 5 (n = 72) | 36 (17.8) | 36 (37.5) | < 0.001 | 1.796 (0.705-4.574) | 0.220 |
| Tumor number | 1.17 ± 0.59 | 1.48 ± 1.19 | 0.005 | ||
| multiple (n = 40) | 20 (9.9) | 20 (20.8) | 0.010 | 2.475 (0.505-12.137) | 0.264 |
| SUVs on PET CT | 4.09 ± 2.45 | 6.57 ± 4.35 | 0.001 | ||
| > 3.5 (n = 47) | 28 (13.9) | 19 (19.8) | < 0.001 | 7.331 (2.182-24.630) | 0.001 |
| RER (n = 100) | 0.75 ± 0.15 | 0.70 ± 0.13 | 0.295 | ||
| < 0.6 (n = 11) | 7 (3.5) | 4 (4.2) | 0.003 | ||
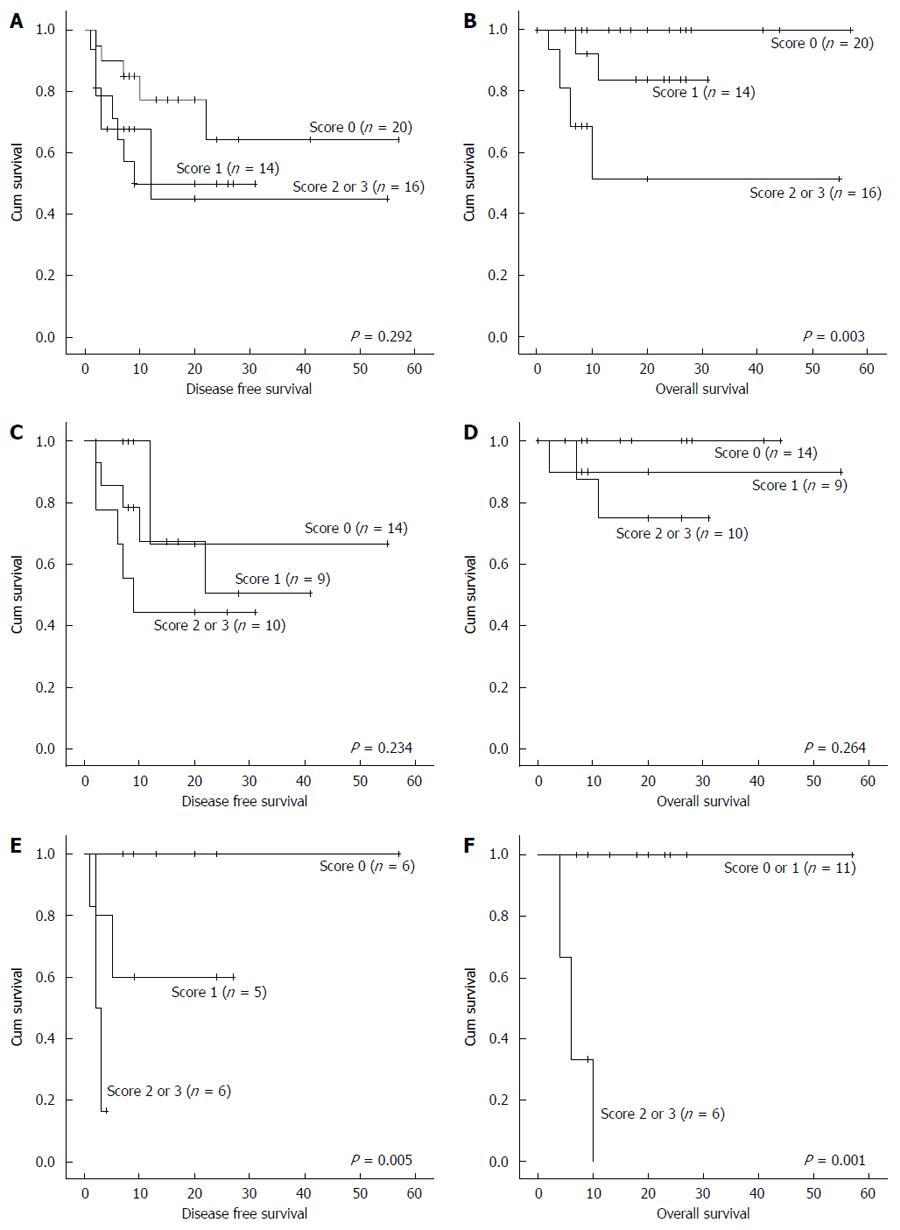
Based on these data, we established a scoring system to predict recurrence and survival after surgery. We assigned 0 and 1 points on the basis of an AFP of 100 ng/mL, SUV on PET-CT of 3.5, and RER on MRI of 0.6. We then summed the scores. 50 patients met these criteria; 20 patients (40.0%) had 0 point, 14 (28.0%) patients had 1 point, and 16 (32.0%) patients had 2 or 3 points. This scoring system was significantly predictive of survival (P = 0.003) but not recurrence (P = 0.292) in all patients. However, when the patients were divided into two groups based on a tumor size of 5 cm, the score was not significantly predictive of recurrence (P = 0.234) or survival (P = 0.264) in the group with a tumor size < 5 cm. However, the score was significantly predictive of both recurrence (P = 0.005) and survival (P = 0.001) in the group with a tumor size > 5 cm (Figure 2). The sensitivity of this scoring system was 71.4%, specificity was 90.0%, and the positive predictive value was 83.3% for the patients with a tumor size > 5 cm.
Surgical resection is a curative treatment modality for HCC. However, the major obstacle to surgical resection is the high recurrence rate after surgery. Thus, appropriate patient selection and analysis of preoperative factors predictive of prognosis are important to improve the recurrence and survival rates. We analyzed tumor cell differentiation, recurrence and survival using preoperative factors to select the most appropriate patients for surgical resection, then established a novel scoring system that incorporates the most important influencing factors.
In previous reports of prognosis after surgery for HCC, the 3- and 5-year survival rates were 46%-47% and 26%-68%, respectively. The 5-year disease-free survival rate was 24%-56% in those studies[7,14,15]. In our study, the 3- and 5-year survival rates and 5-year disease-free survival rate were 66.4%, 58.7% and 47.0%, respectively. These results are better than those reported previously. Some investigators have reported that several prognostic factors are related to recurrence and survival rates after surgical resection for HCC. Chen et al[16] reported that a tumor size > 5 cm was significantly related to a worse prognosis after surgery, and Miyaaki et al[17] reported that high levels of tumor markers such as AFP or PIVKA II were closely related to a poor prognosis. Preoperative MELD or CTP scores are also well known predictive factors for the prognosis of HCC[18,19]. Tumor biology, such as tumor differentiation, was reported to be closely related to prognosis after surgery[20,21]; thus, this should also be predicted prior to surgery to reduce the recurrence and survival rates. Needle biopsy can be performed to confirm the histological grade of the tumor prior to surgery[22]; however, this is not generally recommended because of the complications associated with the procedure itself, such as tumor seeding or bleeding. Furthermore, it is not possible to obtain an precise result by needle biopsy alone if the tumor is of a heterogeneous nature[8]. Therefore, it is important to evaluate tumor differentiation using an indirect method prior to surgery; evaluation of serum AFP and PIVKA-II levels is one such method[23,24].
Talbot et al[9] reported that the degree of SUVs on PET-CT can predict tumor differentiation. Kitamura et al[25] assessed the difference in SUVs between the tumor and the surrounding tissue and reported that the recurrence rate increased significantly with an increasing difference in the SUVs. In our study, the higher SUVs of the tumors were correlated with poor tumor cell differentiation, higher recurrence rates and lower survival rates. The most significant difference was between the SUVs of both groups and the average of 3.5. Some reports have stated that the histological grade of a tumor can be predicted by evaluating the signal intensity of the tumor on MRI using hepatocyte-specific contrast media such as Gd-EOB-DTPA[12,26]. Kim et al[11] reported that the difference in enhancement between the tumor and the surrounding liver parenchyma is predictive of the histological grade of HCC. However, whether this difference in enhancement is directly correlated with the prognosis after surgery has not been investigated to date. Our findings showed that this difference (RER) was significantly correlated with tumor cell differentiation, the recurrence and survival rates. A higher recurrence rate and lower survival rate were identified in the group with an RER of < 0.6. However, multivariate analysis with RER was not possible because an insufficient number of patients underwent routine MRI. Thus, further investigation of RER is necessary. In addition to SUVs on PET-CT, RER may be predictive of the prognosis after surgical resection.
Upon multivariate analysis, an AFP of > 100 ng/mL and SUVs on PET-CT of > 3.5 were significantly associated with the recurrence and survival rates as well as the tumor cell differentiation. The mean tumor size and tumors of > 5 cm, mean PIVKA II level, and RER of < 0.6 showed significant results in the univariate analysis. Based on these data, we established a scoring system to predict the prognosis after surgical resection. We scored patients based on an AFP of 100 ng/mL, SUVs on PET-CT of 3.5, and RER of 0.6, and analyzed the correlation between the summed scores and the recurrence and survival rates. We divided the patients into two groups based on a mean tumor size of 5 cm. In the group with a mean tumor size > 5 cm, the score was significantly associated with both the recurrence and survival rates; however, this was not so in the group with a mean tumor size < 5 cm. There is consensus that surgical resection is the gold standard treatment for small HCC, but not large HCC. However, surgical resection also can be recommended to patients with large tumors who show a good prognosis (0 or 1 point) using this scoring system. The patients with large HCC and high score (2 or 3 point) should be needed careful, close monitoring and adjuvant therapy after surgery. Our study had a number of limitations. It was neither prospective nor case-controlled, and relatively few cases were included. Thus, the factors that showed significant results in this study should be confirmed in a prospective study that includes a larger number of cases.
In conclusion, in cases with an AFP of > 100 ng/mL and SUVs on PET-CT of > 3.5, it is possible to predict high histologic grade of tumor, the recurrence and survival rates preoperatively. The tumor size, serum PIVKA II level, and RER were also predictive of the prognosis after surgery. Furthermore, based on these data, it is possible to predict a good prognosis after surgical resection using our scoring system even in high-risk patients with a mean tumor size of > 5 cm.
Tumor recurrence after surgical resection for hepatocellular carcinoma is an obstacle to long term survival. For this reason, selection of appropriate patients before surgery is important, especially those with advanced tumors. Many studies reported that the importance of the clinical factors such as tumor size, histologic grade of tumor, serum alpha fetoprotein and the protein induced by vitamin K absence or angiotensin-II. In the present study, the authors evaluated these clinical factors associated with histologic grade of tumor, recurrence and survival, especially the findings of pre-operative imaging studies such as positron emission tomography-computed tomography (PET-CT) and magnetic resonance imaging (MRI). And then, they established a scoring system to predict recurrence and survival after surgery.
This study is based on the large, single center experience of curative resection for hepatocellular carcinoma (HCC). The authors investigated the multiple factors and the correlation between them, simultaneously.
The histologic grade of HCC is one of the important predictor of postoperative prognosis; however, preoperative assessment has been limited. The authors investigated the pre-operative imaging studies such as PET-CT and MRI can be correlated to the histologic grade of HCC.
The result showed that SUV of PET-CT and RER from MRI were significantly associated with prognosis. An accurate prediction of prognosis is possible using their scoring system in large size tumors.
This manuscript retrospectively reports a large monocentric experience of curative hepatic resection after HCC. It’s a very useful study on clinical treatment for HCC.
P- Reviewer: Cao GW, Detry O, Hayano K S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y, Miao Y. Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol. 2012;10:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, Lu JH, Yang GS, Wu MC. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC, Chen MF, Tsai CN. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol. 2012;19 Suppl 3:S455-S463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, Meyer G, Frilling A, Paul A, Malagó M, Broelsch CE. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Talbot JN, Gutman F, Fartoux L, Grange JD, Ganne N, Kerrou K, Grahek D, Montravers F, Poupon R, Rosmorduc O. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2006;33:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Lam CM, Fan ST, Lo CM, Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Kim HY, Choi JY, Kim CW, Bae SH, Yoon SK, Lee YJ, Rha SE, You YK, Kim DG, Jung ES. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging predicts the histological grade of hepatocellular carcinoma only in patients with Child-Pugh class A cirrhosis. Liver Transpl. 2012;18:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Choi JY, Kim MJ, Park YN, Lee JM, Yoo SK, Rha SY, Seok JY. Gadoxetate disodium-enhanced hepatobiliary phase MRI of hepatocellular carcinoma: correlation with histological characteristics. AJR Am J Roentgenol. 2011;197:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Forbes F, Peyrard N, Fraley C, Georgian-Smith D, Goldhaber DM, Raftery AE. Model-based region-of-interest selection in dynamic breast MRI. J Comput Assist Tomogr. 2006;30:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Laurent C, Blanc JF, Nobili S, Sa Cunha A, le Bail B, Bioulac-Sage P, Balabaud C, Capdepont M, Saric J. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, Uchida M. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Chen YL, Ko CJ, Chien SY, Chen LS, Chen ML, Chi CW, Lai HW. Tumor size as a prognostic factor in resected small hepatocellular carcinoma: a controversy revisited. J Gastroenterol Hepatol. 2011;26:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Miyaaki H, Nakashima O, Kurogi M, Eguchi K, Kojiro M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol. 2007;42:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Brown DB, Fundakowski CE, Lisker-Melman M, Crippin JS, Pilgram TK, Chapman W, Darcy MD. Comparison of MELD and Child-Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2004;15:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Lee DH, Son JH, Kim TW. [New scoring systems for severity outcome of liver cirrhosis and hepatocellular carcinoma: current issues concerning the Child-Turcotte-Pugh score and the Model of End-Stage Liver Disease (MELD) score]. Taehan Kan Hakhoe Chi. 2003;9:167-179. [PubMed] |
| 20. | Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, Regimbeau JM, Nagorney DM, Do KA, Ellis LM. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722-730; discussion 730-731. [PubMed] |
| 21. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 705] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 22. | Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Kim do Y, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, Lee KS, Chon CY, Han KH. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72 Suppl 1:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Yaprak O, Akyildiz M, Dayangac M, Demirbas BT, Guler N, Dogusoy GB, Yuzer Y, Tokat Y. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:256-261. [PubMed] |
| 25. | Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, Iida T, Taura K, Yasuchika K, Uemoto S. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012;19:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Sutcliffe RP, Lewis D, Kane PA, Portmann BC, O’Grady JG, Karani JB, Rela M, Heaton ND. Manganese-enhanced MRI predicts the histological grade of hepatocellular carcinoma in potential surgical candidates. Clin Radiol. 2011;66:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |