Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13911
Revised: April 29, 2014
Accepted: June 13, 2014
Published online: October 14, 2014
Processing time: 247 Days and 16.4 Hours
AIM: To investigate the colocalization, density and profile of neuronal areas of enteric neurons in the ileum of male obese mice.
METHODS: The small intestinal samples of male mice in an obese group (OG) (C57BL/6J ob/ob) and a control group (CG) (+/+) were used. The tissues were analyzed using a double immunostaining technique for immunoreactivity (ir) of the P2X2 receptor, nitric oxide synthase (NOS), choline acetyl transferase (ChAT) and calretinin (Calr). Also, we investigated the density and profile of neuronal areas of the NOS-, ChAT- and Calr-ir neurons in the myenteric plexus. Myenteric neurons were labeled using an NADH-diaphorase histochemical staining method.
RESULTS: The analysis demonstrated that the P2X2 receptor was expressed in the cytoplasm and in the nuclear and cytoplasmic membranes only in the CG. Neuronal density values (neuron/cm2) decreased 31% (CG: 6579 ± 837; OG: 4556 ± 407) and 16.5% (CG: 7796 ± 528; OG: 6513 ± 610) in the NOS-ir and calretinin-ir neurons in the OG, respectively (P < 0.05). Density of ChAT-ir (CG: 6200 ± 310; OG: 8125 ± 749) neurons significantly increased 31% in the OG (P < 0.05). Neuron size studies demonstrated that NOS, ChAT, and Calr-ir neurons did not differ significantly between the CG and OG groups. The examination of NADH-diaphorase-positive myenteric neurons revealed an overall similarity between the OG and CG.
CONCLUSION: Obesity may exert its effects by promoting a decrease in P2X2 receptor expression and modifications in the density of the NOS-ir, ChAT-ir and CalR-ir myenteric neurons.
Core tip: The neuronal density (neuron/cm2) of nitric oxide synthase- and calretinin-ir neurons are decreased and the density of choline acetyl transferase-ir neurons is significantly increased in male ob/ob mice. In addition, the P2X2 receptor is only expressed in the enteric neurons of healthy mice. These findings have clinical relevance for understanding alterations in gastrointestinal motility in obesity.
-
Citation: Mizuno MS, Crisma AR, Borelli P, Schäfer BT, Silveira MP, Castelucci P. Distribution of the P2X2 receptor and chemical coding in ileal enteric neurons of obese male mice (
ob/ob ). World J Gastroenterol 2014; 20(38): 13911-13919 - URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13911
Currently, obesity is not considered a single disorder but a multifactorial disease, with a complex and often polygenic nature[1]. Many physiological and pathological processes of the gastrointestinal tract rely on the integrity of the enteric nervous system, and this integrity is essential for normal functioning. The submucosal plexus and the myenteric plexus are components of the enteric nervous system and are distributed throughout the gastrointestinal tract[2]. The functions of enteric neurons in the mouse enteric system have been elucidated by studying the chemical coding of these neurons[3-5]. Previous studies in obese mice have revealed decreased intestinal motility[6] and alterations in the morphology of the myenteric plexus[7-9]. The authors of these studies have proposed that changes in the enteric nervous system can be regarded as factors for the development and maintenance of obesity.
In the nervous system, adenosine 5’-triphosphate (ATP) is a neurotransmitter that binds to the P2X family of receptors, consisting of P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X7[10]. Electrophysiological studies of the myenteric plexus have shown that 80%-90% of neurons have P2X receptors[11]. Immunohistochemical studies have also shown that P2X receptors are expressed in the myenteric and submucosal plexuses of the guinea pig[12-16], rat[17,18] and mouse[19,20]. The presence of the P2X2 receptor in the enteric neurons of female mice has also been shown[9]. However, the presence of the P2X2 receptor in the myenteric plexus of male obese mice has not yet been studied.
In the present work, we used immunohistochemical and histochemical methods to analyze the effects of obesity on the chemical coding of the P2X2 receptor, choline acetyltransferase (ChAT), calretinin and nitric oxide synthase (NOS) in the small intestinal myenteric ganglia of obese male mice. We also analyzed the effects of obesity on neuronal density and the profile area of these neurons.
Eleven-month-old male mice were used. Six ob/ob mice were assigned to an obese group (OG) and six C57BL/6J mice comprised a control group (CG). The animals were obtained from the Breeding Center at the State University of Campinas (CEMIB). The mice were maintained in a cage with six mice per cage in an artificially lit room with a 12-h light/dark cycle and were supplied ad libitum with water and a standard pellet diet (Nuvilab, São Paulo, Brazil). For experiments, animals were euthanized in a CO2 chamber. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Biomedical Science Institute of the University of São Paulo. The biochemical analysis of the blood was done as described by Mizuno et al[9].
Ileum samples were taken and placed in PBS (phosphate-buffered saline, pH 7.2) to which nicardipine (Sigma, United States) was added. The samples were prepared for double-label immunohistochemistry as described by Mizuno et al[9] (Table 1). Quantitative analyses were conducted with a Nikon 80i epifluorescence microscope, and the samples were analyzed using a Zeiss confocal scanning laser system. The analyses of neuronal double labeling; the density of myenteric neurons positive for the P2X2 receptor, ChAT, calretinin and NOS; and the cell body area profiles were obtained as described by Mizuno et al[9].
| Tissue antigen | Host | Dilution | Code and reference |
| P2X2 receptor | Rabbit | 1:120 | AB5244, Chemicon |
| Nitric oxide synthase | Sheep | 1:2000 | H205[38] |
| Choline acetyltransferase | Goat | 1:50 | AB144P, Chemicon |
| Calretinin | Goat | 1:100 | CG1, Swant |
| Donkey anti-rabbit IgG Alexa 488 | 1:500 | Molecular probes | |
| Donkey anti-sheep IgG Alexa 594 | 1:100 | Molecular probes |
The histochemical method was performed as described by Mizuno et al[9]. Using NADH-diaphorase staining, the myenteric plexus was observed by the presence of a formazan reaction product filling the perikaryon in addition to large, round, unstained nuclei. The profile areas of the nerve cell bodies and the numbers of neurons were measured as described by Mizuno et al[9].
Differences between the studied groups were analyzed using one-way analysis of variance (ANOVA) and Student’s t-test. The results were considered significant when P < 0.05[9].
Body weight of the OG animals was significantly increased (by 90%) compared to the CG (Table 2). The ileum area was increased by 40% in the obese group compared to the control group (Table 2). The plasma glucose levels were 224.7 ± 50 mg/dL and 195.5 ± 93.5 mg/dL in the CG and OG mice, respectively (Table 2).
| Control group | Obese group | |
| Body weight (g) | 32.5 ± 0.7 | 64.7 ± 1a |
| Small intestine area (cm2) | 28.4 ± 5.2 | 40 ± 3.8a |
| Glucose (mg/dL) | 224.7 ± 50.1 | 195.5 ± 93.5 |
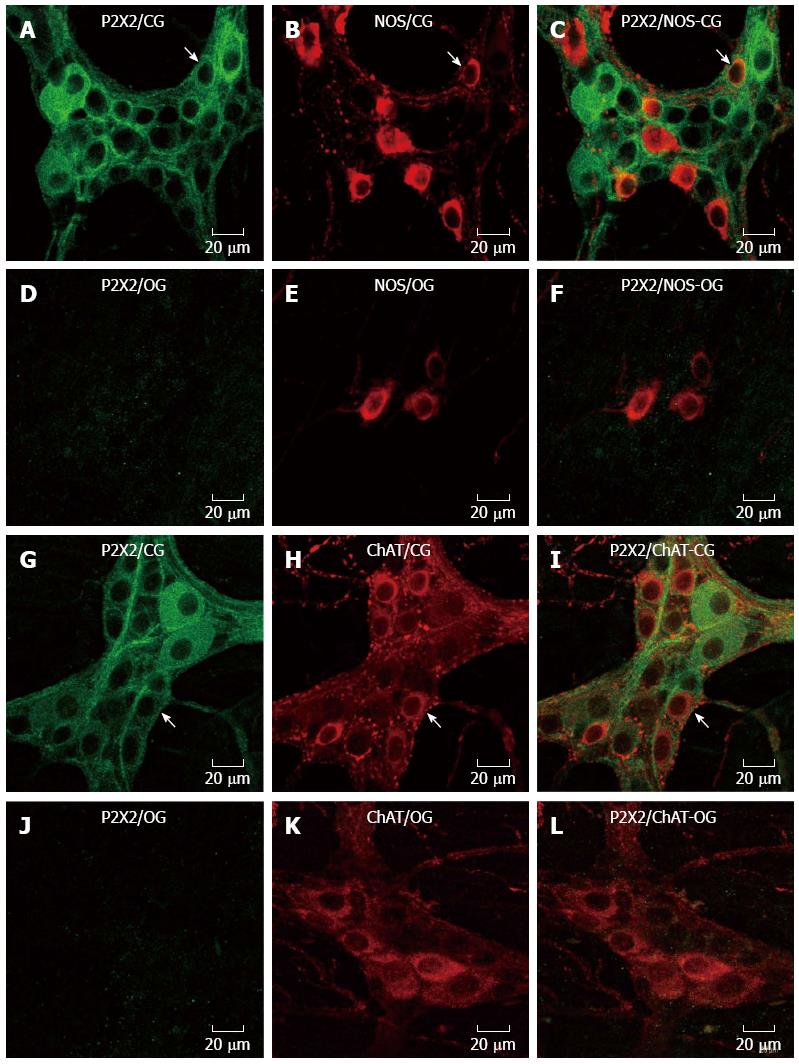
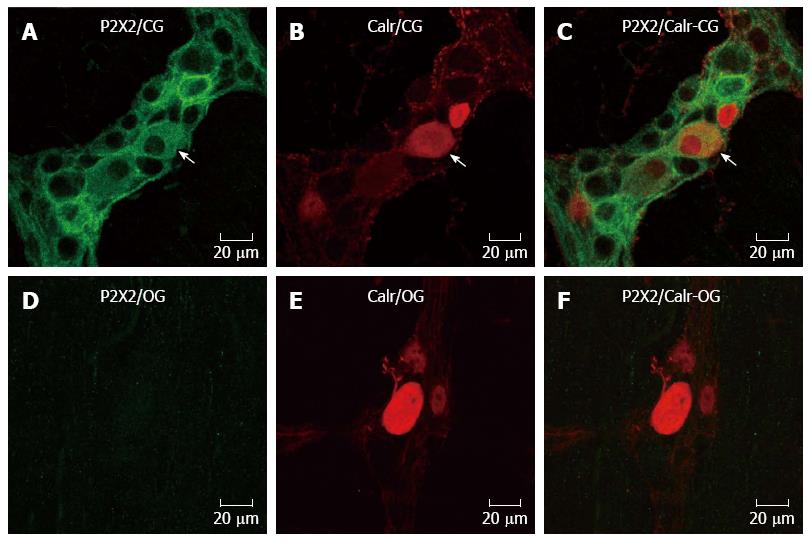
Immunohistochemistry for the P2X2 receptor, NOS, ChAT and calretinin was performed in myenteric neurons in the CG and OG (Figures 1 and 2). The expression of the P2X2 receptor was decreased in the neurons of the myenteric plexus of the OG mice (Figures 1 and 2). The P2X2 receptor immunoreactivity was evident throughout the cytoplasm and on the membrane and nuclear surfaces of the nerve cells in the CG (Figures 1 and 2). NOS immunoreactive (-ir) neurons were observed in both the CG and OG groups. Some of the NOS neurons exhibited Dogiel type I morphology (Figure 1). ChAT-ir neurons were also observed; ChAT immunoreactivity was observed in the cytoplasmic membrane of neurons in the myenteric ganglia of both the CG and OG (Figure 1). The calretinin immunoreactivity was similar to that observed in the ChAT neurons and showed Dogiel types I and II morphology (Figure 2).
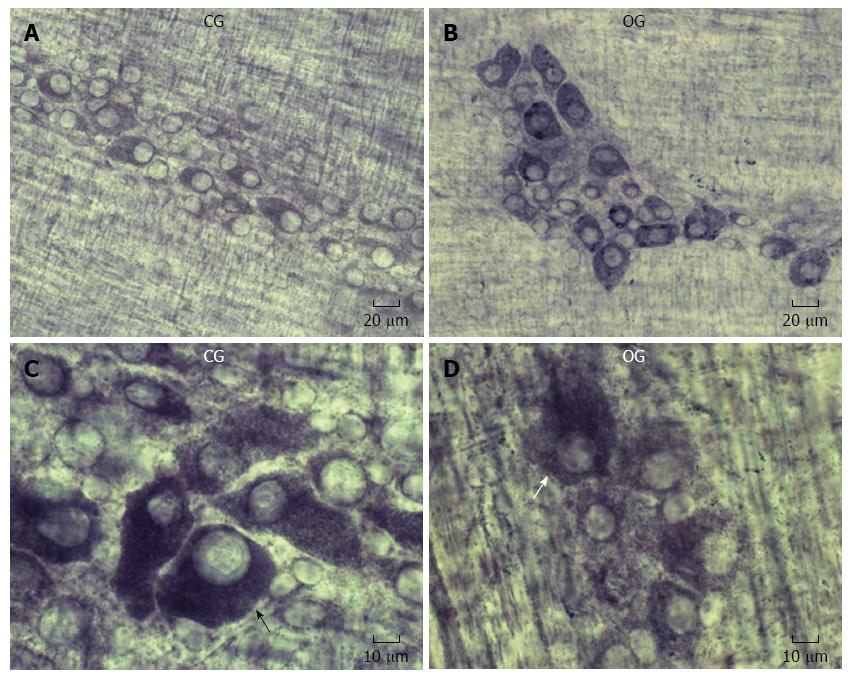
NADH-diaphorase histochemistry provided general information about the myenteric neuron population; our qualitative examination showed a similarity between the CG and OG. The formazan reaction product stained the neuronal cytoplasm, while the neuronal nuclei appear unstained (Figure 3).
Colocalization: Double labeling for the P2X2 receptor with ChAT, NOS, or calretinin was performed to examine the chemical coding of the myenteric neurons of the CG and OG. P2X2 receptor-ir was not evident in the neurons of the obese group; thus, this analysis was not possible in this group. In the CG, the percentage of P2X2-ir neurons and NOS immunoreactive neurons was 23.4 ± 2.6. Conversely, 99% ± 1% of NOS-ir neurons were colocalized with the P2X2 receptor. The percentage of P2X2-ir receptor neurons that were colocalized with ChAT-ir neurons was 34.1% ± 5.3%. In contrast, the percentage of ChAT-ir neurons that were also positive for the P2X2 receptor was 100%. The colocalization of the P2X2-ir receptor with Calr-ir neurons was 31.9% ± 6%, and the Calr-ir neurons were colocalized 100% with the P2X2-ir receptor neurons.
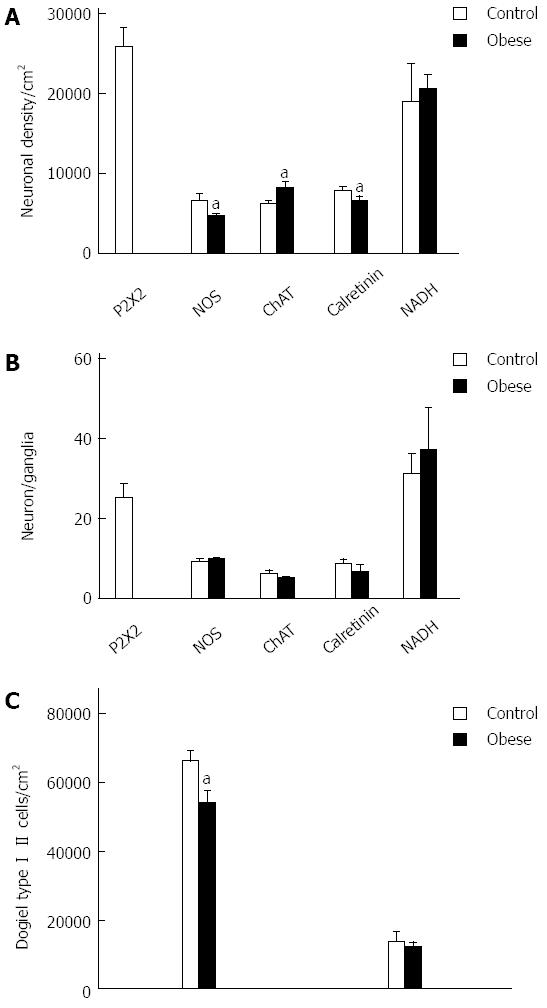
Neuronal density and number of neurons per ganglion: We observed a decrease of 31% and 16.5% in the density of NOS-ir and Calr-ir neurons, respectively (Figure 4). The density of ChAT-ir neurons significantly increased by 31%. In addition, the neuronal density of P2X2-ir neurons in the CG was 25800 ± 2300 cm2 (Figure 4). The quantitative analysis of the NADH-diaphorase density showed an increase of 8% in the OB group; however, the statistical analysis detected no significant difference (P > 0.05) (Figure 4). The number of neurons per ganglion was also assessed; there were no significant differences in the numbers of NOS-ir neurons per ganglion, ChAT-ir neurons per ganglion, or calretinin-ir neurons per ganglion between the CG and the OG (Figure 4). Evaluation of the neuron number per ganglion in the NADH-diaphorase-positive neurons did not show a significant difference (P > 0.05). The values of neurons/ganglia were 31.3 ± 4.9 (CG) and 37.1 ± 10.6 (OG) (Figure 4).
Dogiel Type I and Dogiel Type II neurons were detected in the calretinin-ir neurons (Figure 4). The number of the Dogiel type I/cm2 in control and obese groups was 6595 ± 300/cm2 and 5477 ± 345/cm2, respectively, showing a significant decrease of 20.4% (P < 0.05). There were 1365 ± 270/cm2 and 1184 ± 142/cm2 Dogiel type II/cm2 in the control group and the obese group, respectively, with no significant difference between the groups (P > 0.05) (Figure 4).
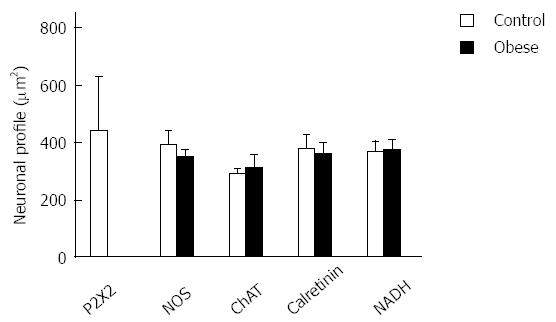
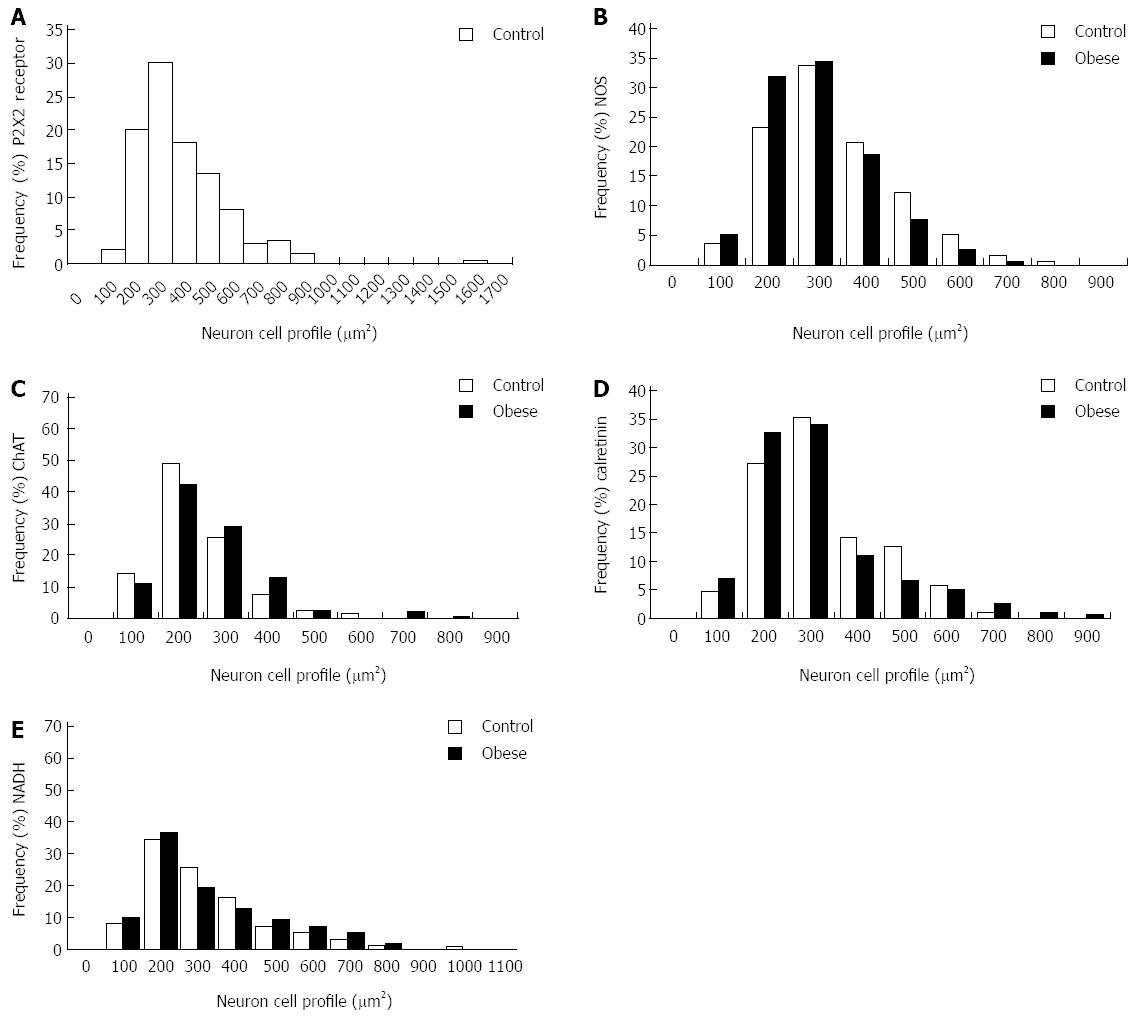
Neuronal size: The average size of the cell perikary in the P2X2 receptor-ir, NOS-ir, ChAT-ir, and Calr-ir neurons in the myenteric plexus did not differ significantly between the CG and OG (P > 0.05) (Figure 5). Evaluations of the size of NADH-diaphorase-positive neurons also did not show a significant difference (P > 0.05); the profile areas of the cell body were 364.8 ± 37.6 μm2 (CG) and 372.8 ± 34.6 μm2 (OG) (Figure 5). The size distributions of the myenteric neurons of the CG and OG groups are shown in Figure 6.
Disorders of intestinal motility have been documented in obese individuals[21]. The present study revealed that the body weight of obese mice was twice that of control mice (P < 0.05). The ileum area in the OG increased by 29.6%, which is consistent with the results of Kiely et al[6] (2005), who showed that the level of an obesity-associated gene correlated with increased body weight in mice and that the mean overall length of the small intestine of obese mice was 33.9 cm compared to 28.0 cm in control mice.
In the present work, we used ob/ob mice, which are an excellent model to study obesity[9,22] and have been characterized by motor and sensory nerve conduction deficits, which are also characteristics of humans with obesity and diabetes[23].
We analyzed both excitatory and inhibitory motor neurons, along with interneurons, using antibodies against NOS, ChAT and calretinin, which are markers for many of the neuronal subtypes in the mouse small intestine[3-5] (Table 1). Mizuno et al[9] (2012) studied female obese mice and also found similar levels of ChAT-ir, NOS-ir and calretinin-ir neurons.
In the present work, ChAT-ir neurons were present in the myenteric plexus of the CG and OG, as in other species[24]. Furthermore, all calretinin-ir neurons were also ChAT-ir, confirming that they were cholinergic excitatory motor neurons[3,4].
Previous studies have demonstrated the distribution of the P2X2 receptor in the guinea pig[12-16], rat[17,18], and mouse[19,20]. We observed that the P2X2 receptor was present in the cytoplasm of myenteric neurons only in the CG; in the OG, the P2X2 receptor expression decreased in the myenteric neurons. The colocalization of the various neuronal markers we analyzed confirms the distribution of P2X2 receptors in NOS, ChAT and calretinin-ir myenteric neurons in the CG. Mizuno et al[9] (2012) demonstrated the presence of the P2X2 receptor in the female ob/ob mouse myenteric plexus. These data suggest that the P2X2 receptor has differential staining in ob/ob female and male animals and that it did not change the neurochemical coding in female mice. Alterations in the P2X2 receptor and P2X7 receptor have been demonstrated in undernourished and refeeding rats[25,26] and ischemia/reperfusion (I/R)[27,28]. A variety of different activities in the gut involve purinergic signaling, such as synaptic transmission and peristalsis in the myenteric plexus. Studies on purinoceptors as therapeutic targets for gut disorders are currently underway[29,30].
Alterations in the distribution of P2X receptors not only occur over the course of maturation and differentiation of neurons but also result from insults such as hypoxia, ischemia, mechanical stress, and inflammation. Many neurodegenerative conditions involve purinergic mechanisms, which may cause pathophysiological effects[31,32].
Alterations in the number of neurons per unit of area (density) have been reported in the small and large intestine as a result of malnutrition protocols[25,26,33,34] and following intestinal I/R[27,28,35].
In the male ob/ob group, NOS-ir and calretinin-ir neurons per area decreased by 30% and 16%, respectively. This result is explained by a 40% increase in the area of the small intestine in the OG. ChAT-ir neurons increased by 31%. In addition, the increased ChAT-ir neurons may be due to neuronal plasticity in the male ob/ob mice. In female ob/ob mice, Mizuno et al[9] (2012) have shown a decrease of 49% and 57% in the density of NOS-ir neurons and ChAT-ir neurons, respectively. Using immunohistochemistry methods, Surendram and Kondapaka (2005)[8] also observed a decreasing trend in the inhibitory motor neurons in the duodenum of obese diabetic mice. These authors also demonstrated a decrease in nNOS expression in male obese diabetic animals compared to male obese controls by RT-PCR; in contrast, female obese diabetic animals had higher levels of nNOS compared to female obese controls[8]. The difference observed in the myenteric neurons may also be due to gender differences.
The NADH-diaphorase is a marker for enteric neurons and is commonly used in protocols studying the effects of undernourishment, refeeding and aging on enteric neurons[33,34,36]. In the present study results showed that the density of the NADH-diaphorase-positive neurons increased 8% in the ileum of male obese mice, but this difference was not significant. In a previous study, the NADH-diaphorase-positive female myenteric neurons in obese mice showed a tendency toward a decrease in density[9]. The difference between the male and female ob/ob mice indicates that gender may affect the results.
Studies have shown that undernutrition[25,26,33,34] and I/R[27,28,35] affect the neuron size profile of the gastrointestinal tract. In the present work, the results of neuronal staining for NOS, ChAT, calretinin and NADH did not show a change in the cell profile of male obese mice compared to the control group. In female ob/ob mice, Mizuno et al[9] showed that the areas in the cellular profiles were larger in the obese group by 34%, 35% and 17% for ChAT-ir, NOS-ir and calretinin-ir neurons, respectively. Previous studies have also reported alterations in the neuronal areas of diabetic animals, such as an increase in the profile area of the myenteric plexus in rats with diabetes[37,38]. This suggests that obesity may differentially affect males and females. In the present work, the distribution of the neurons was between 100-600 μm2, which is in agreement with the literature[9].
The extensive innervation of the gut makes elucidating the processes involved in obesity difficult. The present study demonstrates that obesity can differently affect neuronal subtypes. These findings have clinical relevance for understanding alterations in gastrointestinal motility in obesity.
We thank Professor Edson Aparecido Liberti, Professor Jackson Cioni Bittencourt and Associate Professor Carol Fuzeti Elias for use of their microscopes and Professor Lício A. Velloso for providing the ob/ob mice. Additionally, we thank Rosana Prisco for the statistical analysis.
Changes in intestinal motility have been observed in obese individuals and may be linked to their myenteric neurons. Alterations in enteric neurons have also been demonstrated in obese mice, including variations in intestinal motility.
In the present work, the authors used immunohistochemical and histochemical methods to analyze the effects of obesity on the distribution and chemical coding, neuronal density and the area of the perikaryon of the P2X2 receptor, nitric oxide synthase (NOS), choline acetyl transferase (ChAT) and calretinin in the ileal myenteric plexus of male mice.
The present work demonstrated that P2X2-ir, NOS-ir, calretinin-ir and ChAT-ir neurons in the ileum myenteric plexus are affected by obesity in the ob/ob mouse model.
The present study demonstrates that obesity can differentially affect neuronal subtypes. These findings have clinical relevance for understanding alterations in gastrointestinal motility in obesity.
The myenteric and submucosal plexuses belong to the enteric nervous system, whose function is to control motility. ATP is a transmitter and ligand for the P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X7 receptors.
This is an excellent paper. It is well presented and easy to read and follow. This study compares the distribution and chemical coding of the P2X2 receptor, NOS, ChAT and calretinin in myenteric neurons of the small intestine in obese (ob/ob) vs control mice.
P- Reviewer: Blennerhassett MG, Southwell BR S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Furness JB. The enteric nervous system. Blackwell publishing. 2006; Available from: http://www.doc88.com/p-3834317712448.html. |
| 3. | Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185-199. [PubMed] |
| 4. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Spångéus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes). Histol Histopathol. 2001;16:159-165. [PubMed] |
| 8. | Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005;338:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Mizuno MS, Crisma AR, Borelli P, Castelucci P. Expression of the P2X₂ receptor in different classes of ileum myenteric neurons in the female obese ob/ob mouse. World J Gastroenterol. 2012;18:4693-4703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 630] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 11. | Barajas-López C, Huizinga JD, Collins SM, Gerzanich V, Espinosa-Luna R, Peres AL. P2x-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Br J Pharmacol. 1996;119:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063-8067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 327] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 16. | Xiang Z, Burnstock G. Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol. 2005;124:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Yu Q, Zhao Z, Sun J, Guo W, Fu J, Burnstock G, He C, Xiang Z. Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol. 2010;133:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Ruan HZ, Burnstock G. The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res. 2005;319:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 23. | Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Chiocchetti R, Poole DP, Kimura H, Aimi Y, Robbins HL, Castelucci P, Furness JB. Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons. Cell Tissue Res. 2003;311:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Misawa R, Girotti PA, Mizuno MS, Liberti EA, Furness JB, Castelucci P. Effects of protein deprivation and re-feeding on P2X2 receptors in enteric neurons. World J Gastroenterol. 2010;16:3651-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Girotti PA, Misawa R, Palombit K, Mendes CE, Bittencourt JC, Castelucci P. Differential effects of undernourishment on the differentiation and maturation of rat enteric neurons. Cell Tissue Res. 2013;353:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Paulino AS, Palombit K, Cavriani G, Tavares-de-Lima W, Mizuno MS, Marosti AR, da Silva MV, Girotti PA, Liberti EA, Castelucci P. Effects of ischemia and reperfusion on P2X2 receptor expressing neurons of the rat ileum enteric nervous system. Dig Dis Sci. 2011;56:2262-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Palombit K, Mendes CE, Tavares-de-Lima W, Silveira MP, Castelucci P. Effects of ischemia and reperfusion on subpopulations of rat enteric neurons expressing the P2X7 receptor. Dig Dis Sci. 2013;58:3429-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20 Suppl 1:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109:297-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Franke H, Krügel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Castelucci P, de Souza RR, de Angelis RC, Furness JB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat large intestine: a quantitative morphological study. Cell Tissue Res. 2002;310:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Gomes OA, Castelucci P, de Vasconcellos Fontes RB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat small intestine: a quantitative morphological study. Auton Neurosci. 2006;126-127:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Rivera LR, Thacker M, Castelucci P, Bron R, Furness JB. The reactions of specific neuron types to intestinal ischemia in the guinea pig enteric nervous system. Acta Neuropathol. 2009;118:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | De Freitas P, Natali MR, Pereira RV, Miranda Neto MH, Zanoni JN. Myenteric neurons and intestinal mucosa of diabetic rats after ascorbic acid supplementation. World J Gastroenterol. 2008;14:6518-6524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Williamson S, Pompolo S, Furness JB. GABA and nitric oxide synthase immunoreactivities are colocalized in a subset of inhibitory motor neurons of the guinea-pig small intestine. Cell Tissue Res. 1996;284:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |