Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5375
Revised: October 23, 2013
Accepted: November 3, 2013
Published online: May 14, 2014
Processing time: 229 Days and 0.8 Hours
Currently, nuclear imaging such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) is increasingly used in the management of liver malignancy. 18F-fluorodeoxyglucose (FDG)-PET is the most widely used nuclear imaging in liver malignancy as in other cancers, and has been reported to be effective in diagnosis, response monitoring, recurrence evaluation, and prognosis prediction. Other PET imaging such as 11C-acetate PET is also used complementarily to FDG-PET in diagnosis of liver malignancy. Additionally, image-based evaluation of regional hepatic function can be performed using nuclear imaging. Those imaging modalities are also effective for candidate selection, treatment planning, and perioperative evaluation in liver surgery and transplantation. Recently, nuclear imaging has been actively adopted in the transarterial radioembolization therapy of liver malignancy, according to the concept of theragnosis. With the development of new hybrid imaging technologies such as PET/magnetic resonance imaging and SPECT/CT, nuclear imaging is expected to be more useful in the management of liver malignancy, particularly regarding liver surgery and transplantation. In this review, the efficacy and roles of nuclear imaging methods in diagnosis, transplantation and theragnosis are discussed.
Core tip: Nuclear imaging methods including single photon emission computed tomography (SPECT) and positron emission tomography (PET) are increasingly being used in the management of liver malignancy. In this review, the efficacy and clinical role of nuclear imaging methods are discussed with regard to fluorodeoxyglucose PET and other PET or SPECT imaging methods. In particular, the application of nuclear imaging for theragnosis and surgical intervention including transplantation is discussed in detail. This review may be helpful for understanding current trends of nuclear imaging for liver malignancy.
- Citation: Eo JS, Paeng JC, Lee DS. Nuclear imaging for functional evaluation and theragnosis in liver malignancy and transplantation. World J Gastroenterol 2014; 20(18): 5375-5388
- URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5375
Liver cancer is one of the leading causes of cancer death; particularly in men and developing countries. In 2008, the worldwide incidence and the number of deaths from liver cancer were estimated to be 748300 and 695900, respectively[1]. Additionally, the liver is a frequent metastatic site of almost all cancers, and metastatic liver cancer is much more common than primary liver cancer[2].
For primary liver cancer, the curative treatment is surgical resection and/or interventional treatment when the disease is in an early stage. Thus, early diagnosis, accurate staging, and appropriate evaluation of tumor characteristics are of utmost importance to cure the disease. In case of unresectable disease, liver transplantation can be another option for cure if the tumor is confined to the liver. However, because donor organ supply is limited, adequate recipients should be selected meticulously; currently the Milan criteria are most commonly used for candidate selection in liver transplantation[3]. In addition to accurate diagnosis and staging, pre- and postoperative functional evaluations are also required for successful transplantation.
In diagnosis and evaluation of liver malignancy, ultrasonography (USG) and computed tomography (CT) have been widely used as conventional imaging modalities, and recently, magnetic resonance imaging (MRI) is increasingly used with a strength of high image contrast in the soft tissue. These imaging methods are based on structural changes, and can show mass lesions in primary or metastatic sites. In contrast, nuclear imaging methods including gamma camera scanning, single photon emission computed tomography (SPECT), and positron emission tomography (PET) target specific physiological or molecular processes, and can show functional and biological features such as hepatobiliary function, viability, and metabolic activity of tumors.
Currently, 18F-fluorodeoxyglucose (FDG)-PET is the most widely used nuclear imaging for management of liver malignancy. FDG-PET shows cellular glucose metabolism, which is usually enhanced in malignant tissues, and it can be used for sensitive detection and characterization of tumors. Additionally, FDG-PET can cover the whole body with a single scan, therefore, it is valuable in detection of metastatic lesions throughout the body. As well as initial staging and characterization, FDG-PET is now widely used in response evaluation after transarterial chemoembolization (TACE), radiofrequency ablation (RFA) and chemotherapy. In addition to FDG, other imaging radiopharmaceuticals targeting fatty acid metabolism or nucleotide synthesis are also used in recent clinical practice for liver cancer.
Theragnosis, another new field of nuclear imaging, is a recently suggested concept that means simultaneous diagnosis and therapy with a common mechanism. In liver cancer, transarterial radioembolization (TARE) or selective internal radiotherapy (SIRT) is an example of theragnosis, in which SPECT or PET is directly used for planning treatment and evaluating response.
In this review, the clinical application of FDG and other PET imaging is discussed in terms of diagnostic efficacy in liver malignancy. Additionally, nuclear imaging is reviewed as a tool for candidate selection, and pre- and postoperative functional evaluation in liver surgery and transplantation. The theragnostic application of nuclear imaging and therapy is also discussed briefly.
FDG is an analog of glucose that is labeled with 18F. In actively growing tumor cells, glucose metabolism is enhanced under various conditions, which is known as the Warburg effect. FDG is taken up by cells with the same mechanism as that of glucose, depending on glucose transporters and hexokinases. FDG that is not taken up by cells is rapidly removed by renal excretion[4]. In addition to this biological decay, 18F decays physically with a half-life of 110 min. Thus, effective radiation doses from routine FDG-PET do not exceed 10 mSv, even with a combined low-dose CT scan. The radiation dose from FDG-PET/CT is usually not higher than that from a single whole-body diagnostic CT scan[5,6]. Also, FDG is a safe radiopharmaceutical that has caused no pharmacological adverse reaction in tens of thousands of cases of human administration[7]. As a result of the relatively long half-life of 18F, FDG can be delivered to an imaging center without an on-site cyclotron, within 1 or 2 h distance. Currently, most PET scans are performed using hybrid PET/CT scanners. The combined CT scan can compensate for some weaknesses of isolated PET scans, and faster scan and accurate localization of lesions are available with the CT scan. PET/CT images can provide both functional and anatomical information in a single study.
One of the most important strengths of PET is that it can provide quantitative information on metabolism or molecular processes. Standardized uptake value (SUV) is the most widely used semi-quantitative parameter on FDG-PET. SUV is defined as the ratio of tissue radioactivity concentration and injected dose of radioactivity per kilogram of the patient’s body weight. Some researchers adopt corrections for body surface area or serum glucose level. SUV can be easily measured and is commonly used for evaluation of glucose metabolism of normal or cancer tissues. However, some studies have suggested that the tumor-to-normal liver ratio is a more effective parameter than SUV[8,9], because FDG uptake is affected by underlying liver diseases or serum glucose level[10] and the ratio can reflect variations in liver glucose metabolism better than tumor SUV itself.
Despite the many advantages, FDG-PET also has some limitations in liver imaging. First of all, the liver is involved in the physiological glucose metabolism and normally shows considerable FDG uptake. The SUV of normal liver on FDG-PET is 2.0-3.0, which may interfere with detection of some tumors that have low glucose metabolism. Additionally, the liver is adjacent to the diaphragm and the liver dome area is prone to motion artifacts caused by breathing or swallowing.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. FDG-PET is effective in differential diagnosis between malignant and benign liver lesions such as hepatic adenoma, harmatoma, hemangioma, and nodular hyperplasia[11,12]. However, the sensitivity of FDG-PET in diagnosis of primary HCC is relatively limited and has been reported to be 50%-70%; particularly in small tumors[11,13,14]. In one of these studies, detection rate was as low as 27.2% for tumors of -2 cm and 47.8% for those of 2-5 cm[13]. One probable cause is the relatively high background uptake in the normal liver. Another cause is speculated to be glucose-6-phosphatase, which is highly expressed in HCC cells as well as normal hepatic cells, because dephosphorylation by glucose-6-phosphatase enables FDG to escape from cells. However, FDG uptake depends on malignancy grade of HCC; poorly differentiated HCC showed higher SUV and SUV ratio than moderately or well-differentiated HCC[9]. Thus, FDG-PET should be considered not only for lesion detection, but also for characterization or prognosis prediction. FDG-PET is also related to other characteristics of tumor phenotypes such as P-glycoprotein expression or aggressive biological properties[9,15].
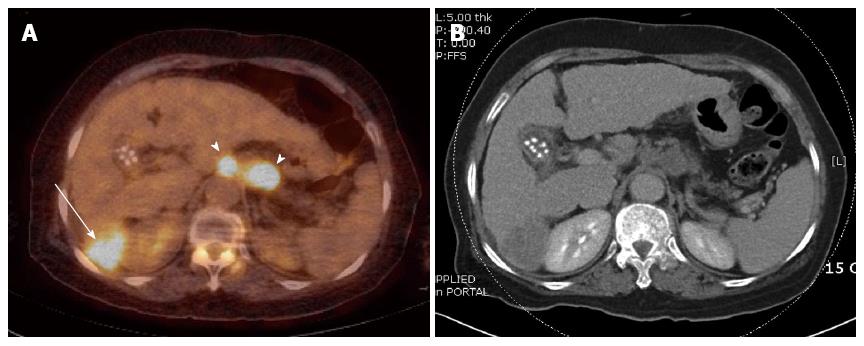
Despite relatively low sensitivities for primary liver lesions, FDG-PET plays an important role in finding extrahepatic or distant metastasis (Figure 1). A recent meta-analysis reported that PET or PET/CT has notable performances in diagnosis of extrahepatic metastasis or recurrent lesions of HCC[16]. In this meta-analysis, pooled sensitivity and specificity for metastasis were 76.6% and 98.0%, and those for recurrent lesions were 81.7% and 88.9%, respectively. Although PET has a lower sensitivity than CT in detection of small (< 1 cm) lung metastasis[17], PET is superior to other imaging modalities in detection of extrahepatic lesions, particularly bone lesions[18].
Selective local treatments are widely performed for unresectable HCCs, along with chemotherapy. In planning of treatment, response evaluation is crucial for adequate selection of treatment methods. Currently used criteria in HCC are the modified Response Evaluation Criteria in Solid Tumors (RECIST)[19], and the European Association for the Study of the Liver criteria[20], which depend on measurements of size or enhancing portion on contrast CT. However, FDG-PET has advantages in the evaluation of treatment response, in that it can reflect metabolic activity of cancer cells and is less affected by structural distortion after treatment than CT. Torizuka et al[21] reported that FDG uptake is increased in viable HCC tissue, whereas it is decreased or absent in the necrotic tissue in > 90% of cases.
Thus, FDG-PET can be used with high efficacy to detect residual or recurrent lesions. Kim et al[22] reported that sensitivity, specificity and accuracy of FDG PET/CT were 87.5%, 71.4%, and 80.0%, respectively, in evaluation of residual disease 1 mo after interventional therapy. In another study, diagnostic sensitivity and specificity were 100% and 63%, for residual lesions 3 mo after TACE[23]. Paudyal et al[24] reported that FDG-PET detected recurrence after RFA at least 4 mo earlier than CT in 33% of patients. In their study, overall detection rate of recurrence on FDG-PET was 92%, which was higher than 75% for CT. As a result, FDG-PET could change management plans in 18%-28% of HCC patients[25,26]. Additionally, pretreatment FDG-PET per se is an effective prognostic predictor for treatment response in HCC. Song et al[8] have reported that SUV ratio measured on pretreatment FDG-PET/CT is an independent predictor of response to TACE in patient with intermediate-stage HCC. Lee et al[27] have reported that HCC patients with lower SUV (maximum SUV < 5.0) on FDG-PET showed longer overall and progression-free survival than those with higher SUV, after sorafenib treatment. Intriguingly, Kucuk et al[28] have reported a longer progression-free survival rate in HCC patients with higher FDG uptake, in TARE treatment with 90Y.
Cholangiocarcinoma (CCA) is the second most common hepatobiliary malignancy. Prognosis of CCA is generally poor because of difficulty in early diagnosis, delayed clinical manifestation and lack of effective non-surgical therapeutic options.
In diagnosis of bile duct cancer, sensitivity, specificity and accuracy of FDG-PET were reported to be 92.3%, 92.9%, and 92.6%, respectively[29]. In another study, a maximum SUV of 3.9 was suggested as a cutoff for differential diagnosis between CCA and primary sclerosing cholangitis, and sensitivity, specificity, and accuracy were reported to be 94%, 83%, and 91%, respectively[30]. However, in contrast to intrahepatic CCA, diagnostic sensitivities of FDG-PET were relatively low in extrahepatic CCA, for which MRI or magnetic resonance cholangiopancreatography was more effective than FDG-PET/CT[31-33]; probably due to small tumor size and uptake in adjacent organs such as the small bowel. The detection of regional lymph node metastasis in CCA by FDG-PET also depends on size and metastatic tumor burden, thus demonstrating different results according to the stage. The sensitivity of FDG-PET for regional lymph node metastasis was 11.7%-31.6% in the resectable stage and 82.1% in the advanced stage. However, the specificity of FDG-PET was as high as 88.2%-96.4%[31-33].
One strength of FDG-PET/CT is the diagnosis of metastasis in CCA. The accuracy of PET/CT for distant metastasis was reported to be 88.3%-100%, which was superior to that of CT[31-34]. With high diagnostic performances, FDG-PET/CT findings changed management plans in 16%-20% of cases deemed resectable after conventional imaging studies[31,33,35].
Liver metastasis is from many types of malignancies such as colorectal, stomach, breast and lung cancers, and is often found incidentally on FDG-PET during staging work-up. A meta-analysis revealed that FDG-PET is more sensitive than USG and CT for detection of liver metastasis from gastrointestinal cancers[36]. Another meta-analysis including 39 studies, in which diagnostic performances of FDG-PET for liver metastasis from colorectal cancers were analyzed, reported sensitivities of CT, MRI and PET as 83.6%, 88.2% and 94.1%, respectively[37]. Additionally, PET/CT had a higher sensitivity and specificity (96.5% and 97.2%, respectively) than PET alone.
In colorectal cancers, isolated liver metastasis is a candidate for curative metastasectomy that can benefit long-term prognosis[38]. Thus, appropriate selection of resectable liver metastasis is of crucial importance for appropriate treatment and reducing unnecessary surgical procedures. Selzner et al[39] have reported that PET/CT is superior to contrast-enhanced CT for detection of local recurrences, and intra- and extrahepatic metastases in colorectal cancer patients who are candidates for liver metastasectomy. It has also been reported that adding FDG-PET/CT to the routine assessment of patients with liver metastases changes therapeutic plans in 28%-34% of cases by changing disease stage[40,41]. Eventually, patients with liver metastasis who were preoperatively screened by FDG PET/CT had a longer 5-year survival rate (58%) than patients who were not screened (30%)[38]. Thus, FDG-PET/CT is recommended by several guidelines as an appropriate and necessary imaging tool for initial staging of colorectal cancers[42,43].
FDG-PET is also effective for early response monitoring and follow-up after selective local treatment of liver metastasis. FDG-PET is reported to be more accurate for evaluation of treatment response and able to detect local relapse earlier than CT in RFA treatment of liver metastases[44,45]. Also in TARE, response evaluated by FDG-PET/CT is well correlated with changes in tumor markers and progression-free survival, whereas RECIST and tumor density criteria are not[46]. Haug et al[47] have reported that the change in maximal SUV at 3 mo after TARE is an independent prognostic factor in patients with liver metastasis from breast cancer. Regarding chemotherapy, Findlay et al[48] have reported that FDG-PET can be used for early response evaluation; > 15% reduction in tumor-to-liver ratio at 4-5 wk after chemotherapy was able to discriminate response from non-response with 100% sensitivity and 75% specificity. Parameters on metabolic volume have been widely investigated in evaluation of chemotherapeutic response. In a recent study, metabolic tumor volume and total lesion glycolysis measured on FDG-PET were shown to be effective in response evaluation[49]. However, it should be noted that sensitivity of FDG-PET is limited for small lesions with low uptake, particularly within 1 wk after chemotherapy[50-52].
Although FDG-PET is widely used in management of liver malignancy, the sensitivity of FDG-PET is limited because of relatively high background uptake in normal liver tissue. Additionally, FDG uptake is often lower in well-differentiated HCC. Thus, several alternative PET imaging agents have been tried in imaging of liver malignancy, including 11C-acetate, 11C-choline, 18F-choline and 18F-fluorothymidine (FLT).
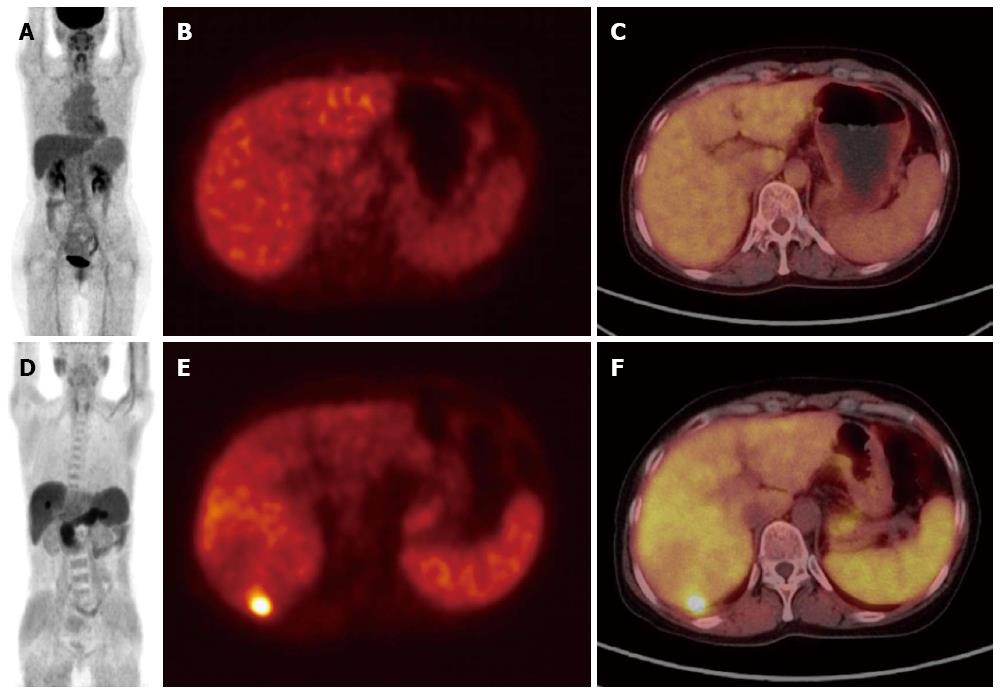
Acetate is a metabolic substance used for fatty acid synthesis and energy production via the Krebs cycle[53,54]. 11C-Acetate PET is approved for human use in many countries including the United States, European Union, and South Korea, and used for tumor imaging in various cancers including HCC, although establishment of an on-site cyclotron is required for use of 11C that has a short half-life of 20 min. In HCC, the uptake ratio between the lesion and the normal liver is usually much higher on 11C-acetate PET than on FDG-PET (Figure 2). Ho et al[55] have reported that 11C-acetate PET is more sensitive than FDG-PET for detection of HCC, particularly well-differentiated HCC. Intriguingly, uptake of 11C-acetate is not as high in CCA and metastatic liver tumors as in HCC, and thus, 11C-acetate PET is suggested as a complementary imaging method to FDG-PET in well-differentiated HCC. 11C-Acetate PET has also been reported to have a higher sensitivity than FDG-PET in detection of bone metastasis from HCC (93% and 62%, respectively)[56].
As a result of differences between FDG and 11C-acetate in effective half-life and metabolic characteristics, dual-tracer PET/CT that uses both FDG and 11C-acetate was suggested to be more accurate in imaging of HCC. A prospective study reported the overall sensitivity of dual-tracer PET/CT was 82.7% in primary HCC[13]. Additionally, sensitivity and specificity of dual-tracer PET/CT were significantly higher (96.8% and 91.7%, respectively) than those of contrast CT (41.9% and 33.0%, respectively), in selection of candidates for liver transplantation[57].
Choline is one of the essential components of phospholipids in the cellular membrane, and metabolism and uptake of choline are increased in actively proliferating tumor cells. As well as 11C-choline, several 18F-labeled choline analogs such as 18F-fluorocholine, 18F-fluoroethyl-choline and 18F-fluoromethyl-choline are used for clinical imaging of choline metabolism. These 18F-labeled tracers have longer half-lives and are more easily accessible in clinical practice[58]. A prospective study with 18F-fluorocholine PET in patients with chronic liver disease reported an overall sensitivity of 84% for HCC (including well-differentiated type), which was significantly higher than that of FDG (67%)[59]. Intriguingly, some HCCs presented as a photopenic pattern on choline PET, and both hyper- and hypometabolic lesions may be regarded as positive results. A pilot study reported that HCC with a photopenic pattern on 18F-fluorocholine PET was associated with the presence of microvascular invasion, high FDG uptake, and early recurrence after surgical resection, resulting in poor prognosis[60]. However, HCC with a photopenic pattern may be an obstacle in differential diagnosis between HCC and benign liver lesions. In a recent study, mean SUV ratios were 1.68 for focal nodular hyperplasia and 0.88 for hepatocellular adenoma[61].
18F-FLT is an analog of thymidine. FLT-PET is used for imaging of cellular proliferation, reflecting DNA synthesis. Although FLT-PET is effective in many cancers, its efficacy is limited in liver malignancy due to high physiological uptake in the normal liver. A pilot study reported higher FLT uptake than surrounding liver tissue in 11 of 16 HCC cases (69%), which was related to a proliferation marker, MIB-1[62]. However, in liver metastases of colorectal cancer, only 11 of 32 cases (34%) were discernible on FLT-PET[63].
PET/MRI is a recently developed hybrid imaging instrument that can provide both PET and MRI images simultaneously. MRI has excellent image contrast in the soft tissue including the liver, and shows a high diagnostic performance for liver malignancy using liver-specific contrast materials and diffusion-weighted imaging, particularly in small lesions[64,65]. The hybrid images of PET/MRI can yield benefits from the strengths of both PET and MRI, which are perfectly co-registered to each other. Thus, PET/MRI has potential for imaging of liver malignancy.
Several studies have investigated the efficacy of software-based image fusion between PET and MRI. One study reported that fusion images of FDG-PET/CT and MRI had a high sensitivity (93%) and specificity (87%-97%) for liver malignancy[66]. Recently, some clinical hybrid PET/MRI scanners became commercially available and results of initial studies on PET/MRI have been reported. PET/MRI provided better diagnostic confidence than PET/CT for both benign and malignant liver lesions[67]. Additionally, MRI can provide various information using different imaging sequences; diffusion-weighted imaging was reported to be related to histological grade of tumor, and dynamic contrast-enhancement imaging, to tumor viability[68,69]. The information from MRI combined with metabolic information from PET could be new imaging biomarker profiles for tumor characterization.
However, attenuation correction is performed by MRI-based methods in PET/MRI scanners, and there is a concern about the difference in SUV between PET/CT and PET/MRI[70,71]. Further studies are required to investigate quantitation methods for clinical application of PET/MRI in conjunction with PET/CT.
Liver transplantation is the best curative option in early but unresectable liver malignancy. However, because of limited sources of donor organs, careful candidate selection is of paramount importance. Currently, the Milan or University of California San Francisco criteria are widely used for candidate selection[3,72], in which size and number of tumors are considered. They are based on the concept that a lower tumor burden is related to lower probability of recurrence and better prognosis. However, size and number of tumors are not perfect markers for the tumor burden, and errors may exist in preoperative measurement of tumors on conventional CT.
FDG-PET has been used in pretransplantation evaluation of liver malignancy to detect extrahepatic metastases. Additionally, FDG-PET can show the metabolic activity of the primary liver lesion, which is related to the prognosis and tumor recurrence after transplantation. In a recent study, tumor-to-normal liver SUV ratio on preoperative FDG-PET was reported to be an independent and significant prognostic factor for tumor recurrence and survival in liver transplantation for HCC[73]. This agrees with the result that non-FDG-avid HCC showed a significantly lower rate of microvascular invasion, lower recurrence rate, and better 3-year recurrence-free survival (11.5%, 3.8% and 93%, respectively) than FDG-avid HCC (87.5%, 50% and 35%, respectively)[74]. In another study, even in cases exceeding the Milan criteria, the 5-year recurrence-free survival rate of patients with non-FDG-avid HCC was comparable (81%) to that of patients with tumors meeting the Milan criteria (81% and 86.2%, respectively)[75]. Pant et al[76] also have reported that patients with non-FDG-avid HCC largely had lower-stage disease and could be candidates for curative surgical resection and liver transplantation, whereas the majority of patients with FDG-avid HCC had advanced-stage disease, with a higher chance of metastases and vascular invasion. Similar results were reported in hilar CCA; patients with non-FDG-avid CCA had a significantly lower recurrence rate and higher 2-year recurrence-free survival rate after liver transplantation than patients with FDG-avid CCA[77]. Thus, FDG-PET can be recommended as an essential imaging modality for preoperative evaluation of liver transplantation.
In postoperative follow-up of liver transplantation, FDG-PET can also be used for detection of recurrence, although there is some limitation in detection of small lesions including intrahepatic and brain metastases[78]. Additionally, FDG-PET is effective for diagnosis of post-transplant lymphoproliferative disorder (PTLD). PTLD is the second most common malignancy in adult transplant recipients, and has a very high mortality rate of 50%. Despite a small number of subject cases, several studies reported that FDG-PET/CT may be a useful tool for detection, diagnosis, staging and therapy monitoring of PTLD[79,80].
In liver malignancy, radical treatment often requires extensive resection, which may impair hepatic function. In liver transplantation, resectability of tumor and feasibility of a living donor should be determined based on hepatic function. Thus, preoperative and postoperative residual hepatic function needs to be meticulously evaluated to prevent postoperative hepatic failure. Nuclear imaging can be used for image-based evaluation of liver function.
99mTc-labeled galactosyl human serum albumin (99mTc-GSA) is a radiopharmaceutical that targets asialoglycoprotein receptor of hepatocytes. 99mTc-GSA scanning can provide valuable parameters for determining hepatic functional reserve, which demonstrated a good relationship with other parameters of liver function such as Child-Pugh classification, indocyanine green clearance, serum bilirubin, prothrombin time, and histology[81,82]. On 99mTc-GSA scanning, the ratio of the heart activities at 15 and 3 min after injection (HH15) is used as a parameter for blood clearance, and the ratio of the liver activity and liver plus heart activity at 15 min after injection (LHL15) is used as a parameter for hepatic uptake. In patients with liver cirrhosis, high LHL15 or low HH15 is related to high survival rate[82].
Preoperatively measured LHL15 is reported to be related to postoperative complications after hepatectomy, with cutoff values of 0.875-0.90[83,84]. 99mTc-GSA scanning can also be used for postoperative evaluation of hepatic function; LHL15 measured at 2 wk after transplantation is correlated with other functional parameters such as model for end-stage liver disease score and graft-to-recipient weight ratio[85]. The modified receptor index, which is calculated as LHL15/HH15, was lower in the partial hepatectomy group than the control group in patients with fatty liver[86], reflecting residual hepatic function. In liver transplantation due to hepatitis, it is suggested that a decrease in the modified receptor index at 3 mo after transplantation could be assumed to be recurrent hepatitis affecting the graft[87].
The most notable advantage of image-based functional evaluation is that it can assess regional function easily. On 99mTc-GSA scanning, regional functions can be assessed using separate regions of interest target areas. In a study on auxiliary partial orthotopic liver transplantation, in which the donor liver and residual native liver coexist, 99mTc-GSA scanning can be used for monitoring both donor and native liver function after transplantation[88]. Additionally, more accurate evaluation of regional function and functional volume is available with SPECT or SPECT/CT imaging. Hwang et al[89] have reported that postoperative liver function and complications can be predicted using 99mTc-GSA dynamic SPECT in hepatectomy patients. 99mTc-GSA SPECT can also show hepatic function-volume relationship; functional recovery was reported to be more rapid than volumetric recovery after portal vein embolization or liver resection, in studies using 99mTc-GSA SPECT[90,91].
Hepatobiliary scanning has been used in clinical practice for several decades, using derivatives of 99mTc-labeled iminodiacetic acid (IDA) such as 99mTc-mebrofenin, 99mTc-dimethyl IDA and 99mTc-diisopropyl IDA. These radiotracers are transported into hepatocytes and go through the biliary system without being metabolized, and thus, hepatic excretion and biliary drainage can be visualized.
In liver transplantation, hepatobiliary scanning is used for diagnosis of postoperative biliary leakage or stricture, which is a frequent complication with incidences of 5%-32%[92]. Hepatobiliary scanning has a high specificity for diagnosis of post-transplantation biliary stricture, because passage of only a small amount of radiotracers can be visualized on the scan. In a study that investigated hepatobiliary scanning with regard to findings of endoscopic or percutaneous cholangiography, positive and negative predictive values were reported to be 92.6% and 22%, respectively[93], which presumably resulted from difference in imaging sensitivities between the modalities. Dynamic hepatobiliary scanning may be useful for diagnosis of complications such as biliary obstruction in liver transplantation[94].
Hepatobiliary scanning can also be used for evaluation of liver parenchymal function. On dynamic 99mTc-mebrofenin scanning, hepatic uptake rate expressed as %/min was well correlated with indocyanine green clearance test and residual liver function after major liver surgery[95]. The cutoff value of future remnant liver function to prevent postoperative liver failure was suggested as 2.5-2.7 %/min per m2 body surface area[96,97].
SPECT and SPECT/CT are also helpful for hepatobiliary scanning. Radiotracers are dynamically excreted through the hepatobiliary system, therefore, SPECT images are acquired at around the peak time of hepatic time-activity curve, when the amount of radioactivity within the liver is relatively stable and well correlated with hepatic function[98]. Fusion images of SPECT/CT are expected to be better for regional assessment, with the aid of anatomical reference images of CT.
Theragnosis is a term coined from therapy and diagnosis, which means simultaneous diagnosis and therapy sharing a common mechanism. Cancer-targeting tracers that have both imaging and therapeutic moieties are a typical example of theragnosis. In liver malignancy, TARE or SIRT has been investigated for more than a decade, as an effective local treatment. TARE is performed with radiopharmaceuticals emitting therapeutic radiations. Additionally, nuclear imaging can be acquired using the radiations and used as a theragnosis for treatment planning and monitoring.
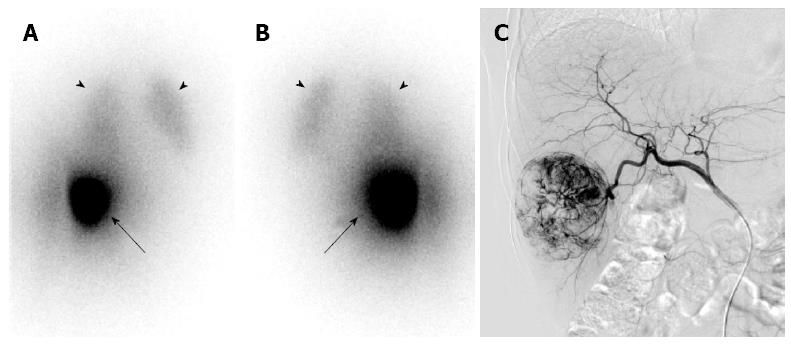
Currently, 90Y and 131I are widely used radioisotopes in TARE. Although 131I emits γ as well as β rays and can be imaged using a gamma camera, 90Y, more widely used than 131I, does not emit γ rays. However, 90Y can also be imaged using a gamma camera by the Bremsstrahlung X-ray, although image quality is relatively poor with it (Figure 3). Additionally, 90Y emits a small amount of positrons and can be imaged using a PET scanner. The images acquired from a gamma camera or a PET scanner show distribution of the radiopharmaceuticals, and are used for dosimetry, efficacy monitoring, and planning of next treatment.
TARE can be considered in a patient with unresectable and hepatic artery-dominant primary or metastatic cancer, who has adequate general condition, preserved liver function, and a life expectancy of at least 3 mo. 90Y-labeled microspheres are most widely used in clinical trials and practice of TARE. However, several other radiopharmaceuticals are also available for TARE, such as 131I-lipiodol[99], 166Ho-chitosan, and 188Re-lipiodol[100].
90Y-labeled microspheres are usually made of resin or glass with sizes of 20-40 μm, which enables optimal access into tumor preventing adverse effect by leakage through microcirculation. In addition to embolizing the tumor-feeding artery, β ray irradiation from injected microspheres destroys tumors. Dosimetry is a great benefit of theragnosis imaging. Radiation doses of the normal liver parenchyme and tumor can be calculated using partition models or body surface area models, based on images that are acquired from pilot or previous treatment. Dosimetry results are used for treatment planning so that the radiation dose for the normal liver parenchyme does not exceed 35 Gy and that of the tumor exceeds 70 Gy[101].
TARE with 90Y-labeled microspheres can be combined with other treatments. However, surgery immediately after TARE is recommended to be performed carefully considering the radiation safety for surgeons, although the risk of radiation exposure caused by a 90Y microsphere-administered patient is not high[102,103]. Additionally, discontinuation of antiangiogenic drugs such as sorafenib is recommended before pretreatment angiography, in order to avoid vascular complication and to optimize therapy.
Bremsstrahlung scanning and SPECT for 90Y-labeled microspheres are used for post-treatment imaging and confirmation of dose delivery. However, image quality of the Bremsstrahlung scan and SPECT is relatively poor and insufficient for quantitative analysis, although optimization of reconstruction algorithm has been attempted using a precalculated point-spread function of 90Y[104]. Recently, PET has been performed using positrons produced from minor decay branches of 90Y, which generate 32 electron-positron pairs per every 1 million decays of 90Y. As a result of a small branching fraction, 90Y PET has a limited image quality and requires long imaging time. However, with recent state-of-the-art PET scanners that have high sensitivity, high-quality 90Y PET images superior to those of Bremsstrahlung SPECT can be acquired[105]. More accurate measurements of tumor-absorbed dose and therapeutic response monitoring are provided by 90Y PET.
In planning treatment with TARE, 99mTc-labeled macroaggregated albumin (99mTc-MAA) scanning is obtained for simulation of microsphere distribution and dosimetry. The estimation from 99mTc-MAA scanning may not always be same as that of therapeutic microspheres, because of differences in particle size, specific gravity, injected particle load, microembolic effects, placement of microcatheter tip, and regional blood flow change from prophylactic coil embolization of non-target arteries[106,107]. However, 99mTc-MAA SPECT usually shows accurate registration with 90Y SPECT images[108,109].
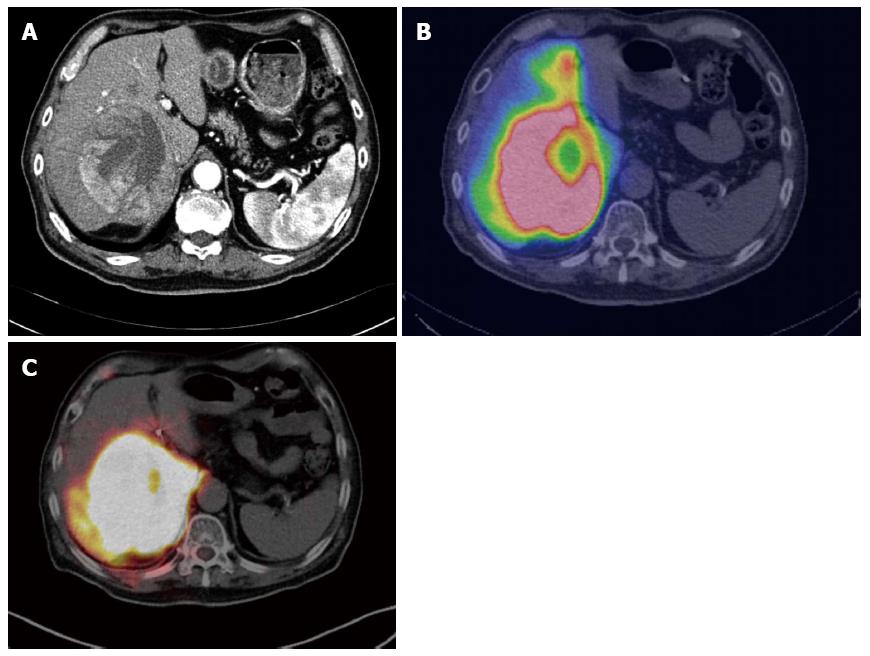
99mTc-MAA scanning is effective for detecting unexpectedly leaked activity in the gastrointestinal tract, measuring the amount of liver-to-lung shunt, and even predicting treatment response and survival[110]. In case of abnormally high gastrointestinal activity, changing the position of the microcatheter tip and re-evaluation by 99mTc-MAA scanning should be considered to minimize adverse effects on normal tissue. Furthermore, dose reduction of TARE or other treatment should be considered in patients with a large lung shunt, to prevent toxicity from systemic distribution of microspheres. 99mTc-MAA SPECT or SPECT/CT provides more valuable information than that provided by planar scans because cross-sectional SPECT images can show more accurate regional distribution, particularly with SPECT/CT (Figure 4). Radioactivity measured on SPECT or SPECT/CT can also be used for elaborate calculation of radiation dose, using anatomically correct partition models.
Response to TARE has been variable because of subject heterogeneity, different time points, and different methods of assessment. A recent prospective study including 52 HCC patients reported response, disease control and complete response rates of 40.4%, 78.8% and 9.6%, respectively[111]. In another prospective multicenter phase II trial of TARE in chemorefractory liver-dominant metastatic colorectal cancer, disease was controlled in 48% of patients with a median survival of 12.6 mo[112]. FDG-PET is also used for monitoring response in TARE, and interval-decreased intrahepatic tumoral uptake on post-treatment FDG-PET suggests better prognosis and longer survival[46,47,113].
FDG-PET/CT has demonstrated high diagnostic performances in liver malignancy, regarding diagnosis, treatment response monitoring and prognosis prediction. 11C-Acetate and radiolabeled choline PET is complementary to FDG-PET in liver malignancy with low FDG uptake, such as well-differentiated HCC. 99mTc-GSA and hepatobiliary scans can be used for regional evaluation of hepatic function. In liver resection and transplantation, those imaging methods are effectively used for candidate selection, treatment planning and perioperative evaluation of hepatic function. In recently developing treatment of TARE, nuclear imaging is used for planning and evaluation of treatment as theragnosis. With development of new hybrid imaging technologies such as PET/MRI and SPECT/CT, nuclear imaging is expected to be more useful in the management of liver malignancy, particularly regarding liver surgery and transplantation.
P- Reviewers: Gangl A, Llado L S- Editor: Gou SX L- Editor: Kerr C E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5306] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 4. | Jones SC, Alavi A, Christman D, Montanez I, Wolf AP, Reivich M. The radiation dosimetry of 2 [F-18]fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1982;23:613-617. [PubMed] |
| 5. | Brix G, Lechel U, Glatting G, Ziegler SI, Münzing W, Müller SP, Beyer T. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med. 2005;46:608-613. [PubMed] |
| 6. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6212] [Cited by in RCA: 5795] [Article Influence: 321.9] [Reference Citation Analysis (3)] |
| 7. | Silberstein EB. Prevalence of adverse reactions to positron emitting radiopharmaceuticals in nuclear medicine. Pharmacopeia Committee of the Society of Nuclear Medicine. J Nucl Med. 1998;39:2190-2192. [PubMed] |
| 8. | Song MJ, Bae SH, Lee SW, Song do S, Kim HY, Yoo IeR, Choi JI, Lee YJ, Chun HJ, Lee HG. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, Iwaisako K, Ikai I, Uemoto S. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Kubota K, Watanabe H, Murata Y, Yukihiro M, Ito K, Morooka M, Minamimoto R, Hori A, Shibuya H. Effects of blood glucose level on FDG uptake by liver: a FDG-PET/CT study. Nucl Med Biol. 2011;38:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510-515; discussion 515-516. [PubMed] |
| 12. | Nakajo M, Jinnouchi S, Hamada N, Sueyoshi K, Matukita S, Tanabe H, Tateno R, Nakajo M. FDG PET/CT findings of mesenchymal hamartoma of the liver in an adult. Clin Nucl Med. 2009;34:327-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, Kim SJ, Kim KS, Yang WI, Park YN. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging. 2004;31:1621-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Radiol. 2012;81:2417–2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Lee JE, Jang JY, Jeong SW, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J Gastroenterol. 2012;18:2979-2987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kawaoka T, Aikata H, Takaki S, Uka K, Azakami T, Saneto H, Jeong SC, Kawakami Y, Takahashi S, Toyota N. FDG positron emission tomography/computed tomography for the detection of extrahepatic metastases from hepatocellular carcinoma. Hepatol Res. 2009;39:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 20. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 21. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. Value of fluorine-18-FDG-PET to monitor hepatocellular carcinoma after interventional therapy. J Nucl Med. 1994;35:1965-1969. [PubMed] |
| 22. | Kim SH, Won KS, Choi BW, Jo I, Zeon SK, Chung WJ, Kwon JH. Usefulness of F-18 FDG PET/CT in the Evaluation of Early Treatment Response After Interventional Therapy for Hepatocellular Carcinoma. Nucl Med Mol Imaging. 2012;46:102-110. |
| 23. | Kim HO, Kim JS, Shin YM, Ryu JS, Lee YS, Lee SG. Evaluation of metabolic characteristics and viability of lipiodolized hepatocellular carcinomas using 18F-FDG PET/CT. J Nucl Med. 2010;51:1849-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Paudyal B, Oriuchi N, Paudyal P, Tsushima Y, Iida Y, Higuchi T, Hanaoka H, Miyakubo M, Takano A, Ishikita T. Early diagnosis of recurrent hepatocellular carcinoma with 18F-FDG PET after radiofrequency ablation therapy. Oncol Rep. 2007;18:1469-1473. [PubMed] |
| 25. | Wudel LJ, Delbeke D, Morris D, Rice M, Washington MK, Shyr Y, Pinson CW, Chapman WC. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg. 2003;69:117-124; discussion 124-126. [PubMed] |
| 26. | Böhm B, Voth M, Geoghegan J, Hellfritzsch H, Petrovich A, Scheele J, Gottschild D. Impact of positron emission tomography on strategy in liver resection for primary and secondary liver tumors. J Cancer Res Clin Oncol. 2004;130:266-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lee JH, Park JY, Kim do Y, Ahn SH, Han KH, Seo HJ, Lee JD, Choi HJ. Prognostic value of 18F-FDG PET for hepatocellular carcinoma patients treated with sorafenib. Liver Int. 2011;31:1144-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Kucuk ON, Soydal C, Araz M, Bilgic S, Ibis E. Prognostic importance of 18F-FDG uptake pattern of hepatocellular cancer patients who received SIRT. Clin Nucl Med. 2013;38:e283-e289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, Seese A, Huster D, Berr F. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Alkhawaldeh K, Faltten S, Biersack HJ, Ezziddin S. The value of F-18 FDG PET in patients with primary sclerosing cholangitis and cholangiocarcinoma using visual and semiquantitative analysis. Clin Nucl Med. 2011;36:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, Lee SS, Seo DW, Lee SK. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Jadvar H, Henderson RW, Conti PS. [F-18]fluorodeoxyglucose positron emission tomography and positron emission tomography: computed tomography in recurrent and metastatic cholangiocarcinoma. J Comput Assist Tomogr. 2007;31:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, Jarnagin WR. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748-756. [PubMed] |
| 37. | Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 393] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 38. | Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438-447; discussion 447-450. [PubMed] |
| 39. | Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027-1034; discussion 1035-1036. [PubMed] |
| 40. | Grassetto G, Fornasiero A, Bonciarelli G, Banti E, Rampin L, Marzola MC, Massaro A, Galeotti F, Del Favero G, Pasini F. Additional value of FDG-PET/CT in management of “solitary” liver metastases: preliminary results of a prospective multicenter study. Mol Imaging Biol. 2010;12:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Engledow AH, Skipworth JR, Pakzad F, Imber C, Ell PJ, Groves AM. The role of 18FDG PET/CT in the management of colorectal liver metastases. HPB (Oxford). 2012;14:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 753] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 43. | Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS. ACR Appropriateness Criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Donckier V, Van Laethem JL, Goldman S, Van Gansbeke D, Feron P, Ickx B, Wikler D, Gelin M. [F-18] fluorodeoxyglucose positron emission tomography as a tool for early recognition of incomplete tumor destruction after radiofrequency ablation for liver metastases. J Surg Oncol. 2003;84:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Travaini LL, Trifirò G, Ravasi L, Monfardini L, Della Vigna P, Bonomo G, Chiappa A, Mallia A, Ferrari M, Orsi F. Role of [18F]FDG-PET/CT after radiofrequency ablation of liver metastases: preliminary results. Eur J Nucl Med Mol Imaging. 2008;35:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Zerizer I, Al-Nahhas A, Towey D, Tait P, Ariff B, Wasan H, Hatice G, Habib N, Barwick T. The role of early ¹⁸F-FDG PET/CT in prediction of progression-free survival after ⁹⁰Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP, Uebleis C, Bartenstein P, Heinemann V, Hacker M. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Findlay M, Young H, Cunningham D, Iveson A, Cronin B, Hickish T, Pratt B, Husband J, Flower M, Ott R. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14:700-708. [PubMed] |
| 49. | Kim DH, Kim SH, Im SA, Han SW, Goo JM, Willmann JK, Lee ES, Eo JS, Paeng JC, Han JK. Intermodality comparison between 3D perfusion CT and 18F-FDG PET/CT imaging for predicting early tumor response in patients with liver metastasis after chemotherapy: preliminary results of a prospective study. Eur J Radiol. 2012;81:3542-3550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, Even-Sapir E, Figer A, Ben-Haim M. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg. 2007;11:472-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Carnaghi C, Tronconi MC, Rimassa L, Tondulli L, Zuradelli M, Rodari M, Doci R, Luttmann F, Torzilli G, Rubello D. Utility of 18F-FDG PET and contrast-enhanced CT scan in the assessment of residual liver metastasis from colorectal cancer following adjuvant chemotherapy. Nucl Med Rev Cent East Eur. 2007;10:12-15. [PubMed] |
| 52. | Glazer ES, Beaty K, Abdalla EK, Vauthey JN, Curley SA. Effectiveness of positron emission tomography for predicting chemotherapy response in colorectal cancer liver metastases. Arch Surg. 2010;145:340-345; discussion 345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Song WS, Nielson BR, Banks KP, Bradley YC. Normal organ standard uptake values in carbon-11 acetate PET imaging. Nucl Med Commun. 2009;30:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med. 2009;50:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 56. | Ho CL, Chen S, Cheng TK, Leung YL. PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma. Radiology. 2011;258:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon’s perspective. J Nucl Med. 2013;54:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 58. | Talbot JN, Gutman F, Fartoux L, Grange JD, Ganne N, Kerrou K, Grahek D, Montravers F, Poupon R, Rosmorduc O. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2006;33:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 60. | Fartoux L, Balogova S, Nataf V, Kerrou K, Huchet V, Rosmorduc O, Talbot JN. A pilot comparison of 18F-fluorodeoxyglucose and 18F-fluorocholine PET/CT to predict early recurrence of unifocal hepatocellular carcinoma after surgical resection. Nucl Med Commun. 2012;33:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | van den Esschert JW, Bieze M, Beuers UH, van Gulik TM, Bennink RJ. Differentiation of hepatocellular adenoma and focal nodular hyperplasia using 18F-fluorocholine PET/CT. Eur J Nucl Med Mol Imaging. 2011;38:436-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Eckel F, Herrmann K, Schmidt S, Hillerer C, Wieder HA, Krause BJ, Schuster T, Langer R, Wester HJ, Schmid RM. Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. J Nucl Med. 2009;50:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Francis DL, Visvikis D, Costa DC, Arulampalam TH, Townsend C, Luthra SK, Taylor I, Ell PJ. Potential impact of [18F]3’-deoxy-3’-fluorothymidine versus [18F]fluoro-2-deoxy-D-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging. 2003;30:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, Brozzetti S, Masciangelo R, Passariello R, Catalano C. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 65. | Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol. 2011;46:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Donati OF, Hany TF, Reiner CS, von Schulthess GK, Marincek B, Seifert B, Weishaupt D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Beiderwellen K, Gomez B, Buchbender C, Hartung V, Poeppel TD, Nensa F, Kuehl H, Bockisch A, Lauenstein TC. Depiction and characterization of liver lesions in whole body [¹⁸F]-FDG PET/MRI. Eur J Radiol. 2013;82:e669-e675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, Koh YS, Cho CK, Kang HK. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010;11:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Bolog N, Pfammatter T, Müllhaupt B, Andreisek G, Weishaupt D. Double-contrast magnetic resonance imaging of hepatocellular carcinoma after transarterial chemoembolization. Abdom Imaging. 2008;33:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Kershah S, Partovi S, Traughber BJ, Muzic RF, Schluchter MD, O’Donnell JK, Faulhaber P. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in an oncology patient population. Mol Imaging Biol. 2013;15:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Kim JH, Lee JS, Song IC, Lee DS. Comparison of segmentation-based attenuation correction methods for PET/MRI: evaluation of bone and liver standardized uptake value with oncologic PET/CT data. J Nucl Med. 2012;53:1878-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1694] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 73. | Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, Lee MC, Lee DS. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D, Settmacher U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, Gottschild D, Friess H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012;18:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Pant V, Sen IB, Soin AS. Role of ¹⁸F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Kornberg A, Küpper B, Thrum K, Wilberg J, Sappler A, Gottschild D. Recurrence-free long-term survival after liver transplantation in patients with 18F-FDG non-avid hilar cholangiocarcinoma on PET. Am J Transplant. 2009;9:2631-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Kim YK, Lee KW, Cho SY, Han SS, Kim SH, Kim SK, Park SJ. Usefulness 18F-FDG positron emission tomography/computed tomography for detecting recurrence of hepatocellular carcinoma in posttransplant patients. Liver Transpl. 2010;16:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Panagiotidis E, Quigley AM, Pencharz D, Ardeshna K, Syed R, Sajjan R, Bomanji J. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis of post-transplant lymphoproliferative disorder. Leuk Lymphoma. 2014;55:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | McCormack L, Hany TI, Hübner M, Petrowsky H, Mullhaupt B, Knuth A, Stenner F, Clavien PA. How useful is PET/CT imaging in the management of post-transplant lymphoproliferative disease after liver transplantation? Am J Transplant. 2006;6:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Kwon AH, Ha-Kawa SK, Uetsuji S, Kamiyama Y, Tanaka Y. Use of technetium 99m diethylenetriamine-pentaacetic acid-galactosyl-human serum albumin liver scintigraphy in the evaluation of preoperative and postoperative hepatic functional reserve for hepatectomy. Surgery. 1995;117:429-434. [PubMed] |
| 82. | Sasaki N, Shiomi S, Iwata Y, Nishiguchi S, Kuroki T, Kawabe J, Ochi H. Clinical usefulness of scintigraphy with 99mTc-galactosyl-human serum albumin for prognosis of cirrhosis of the liver. J Nucl Med. 1999;40:1652-1656. [PubMed] |
| 83. | Kim YK, Nakano H, Yamaguchi M, Kumada K, Takeuchi S, Kitamura N, Takahashi H, Hasebe S, Midorikawa T, Sanada Y. Prediction of postoperative decompensated liver function by technetium-99m galactosyl-human serum albumin liver scintigraphy in patients with hepatocellular carcinoma complicating chronic liver disease. Br J Surg. 1997;84:793-796. [PubMed] |
| 84. | Nanashima A, Yamaguchi H, Shibasaki S, Morino S, Ide N, Takeshita H, Sawai T, Nakagoe T, Nagayasu T, Ogawa Y. Relationship between indocyanine green test and technetium-99m galactosyl serum albumin scintigraphy in patients scheduled for hepatectomy: Clinical evaluation and patient outcome. Hepatol Res. 2004;28:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Iida T, Isaji S, Yagi S, Hori T, Taniguchi K, Ohsawa I, Mizuno S, Usui M, Sakurai H, Yamagiwa K. Assessment of liver graft function and regeneration by galactosyl-human serum albumin (99mTc-GSA) liver scintigraphy in adult living-donor liver transplantation. Clin Transplant. 2009;23:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Kaibori M, Ha-Kawa SK, Matsui K, Saito T, Kamiyama Y. Usefulness of TC-99M GSA liver scintigraphy for the evaluation of liver regeneration in donors after living-donor liver transplantation. Transplant Proc. 2008;40:2457-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Kaibori M, Ha-Kawa SK, Uchida Y, Ishizaki M, Hijikawa T, Saito T, Imamura A, Hirohara J, Uemura Y, Tanaka K. Recurrent hepatitis C after living donor liver transplantation detected by Tc-99m GSA liver scintigraphy. Dig Dis Sci. 2006;51:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Sakahara H, Kiuchi T, Nishizawa S, Saga T, Nakamoto Y, Sato N, Higashi T, Tanaka K, Konishi J. Asialoglycoprotein receptor scintigraphy in evaluation of auxiliary partial orthotopic liver transplantation. J Nucl Med. 1999;40:1463-1467. [PubMed] |
| 89. | Hwang EH, Taki J, Shuke N, Nakajima K, Kinuya S, Konishi S, Michigishi T, Aburano T, Tonami N. Preoperative assessment of residual hepatic functional reserve using 99mTc-DTPA-galactosyl-human serum albumin dynamic SPECT. J Nucl Med. 1999;40:1644-1651. [PubMed] |
| 90. | Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Kwon AH, Matsui Y, Ha-Kawa SK, Kamiyama Y. Functional hepatic volume measured by technetium-99m-galactosyl-human serum albumin liver scintigraphy: comparison between hepatocyte volume and liver volume by computed tomography. Am J Gastroenterol. 2001;96:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 93. | Kim YJ, Lee KT, Jo YC, Lee KH, Lee JK, Joh JW, Kwon CH. Hepatobiliary scintigraphy for detecting biliary strictures after living donor liver transplantation. World J Gastroenterol. 2011;17:2626-2631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Lee JY, Hu W, Lee KH, Kwon CH, Lee EJ, Choi JY, Kim BT. Differentiation of liver transplantation complications by quantitative analysis of dynamic hepatobiliary scintigraphy. Nucl Med Commun. 2012;33:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Bennink RJ, Dinant S, Erdogan D, Heijnen BH, Straatsburg IH, van Vliet AK, van Gulik TM. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med. 2004;45:965-971. [PubMed] |
| 96. | Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, van Vliet AK, van Gulik TM. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, Bennink RJ, van Gulik TM. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 98. | de Graaf W, van Lienden KP, van Gulik TM, Bennink RJ. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 99. | Raoul JL, Guyader D, Bretagne JF, Duvauferrier R, Bourguet P, Bekhechi D, Deugnier YM, Gosselin M. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J Nucl Med. 1994;35:1782-1787. [PubMed] |
| 100. | Bernal P, Raoul JL, Vidmar G, Sereegotov E, Sundram FX, Kumar A, Jeong JM, Pusuwan P, Divgi C, Zanzonico P. Intra-arterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int J Radiat Oncol Biol Phys. 2007;69:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 102. | Högberg J, Rizell M, Hultborn R, Svensson J, Henrikson O, Gjertsson P, Bernhardt P. Radiation exposure during liver surgery after treatment with (90)Y microspheres, evaluated with computer simulations and dosimeter measurements. J Radiol Prot. 2012;32:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Kim YC, Kim YH, Uhm SH, Seo YS, Park EK, Oh SY, Jeong E, Lee S, Choe JG. Radiation Safety Issues in Y-90 Microsphere Selective Hepatic Radioembolization Therapy: Possible Radiation Exposure from the Patients. Nucl Med Mol Imaging. 2010;44:252-260. |
| 104. | Rong X, Du Y, Ljungberg M, Rault E, Vandenberghe S, Frey EC. Development and evaluation of an improved quantitative (90)Y bremsstrahlung SPECT method. Med Phys. 2012;39:2346-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 105. | Elschot M, Vermolen BJ, Lam MG, de Keizer B, van den Bosch MA, de Jong HW. Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS One. 2013;8:e55742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 106. | Kao YH, Tan EH, Ng CE, Goh SW. Clinical implications of the body surface area method versus partition model dosimetry for yttrium-90 radioembolization using resin microspheres: a technical review. Ann Nucl Med. 2011;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 107. | Wondergem M, Smits ML, Elschot M, de Jong HW, Verkooijen HM, van den Bosch MA, Nijsen JF, Lam MG. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 108. | Knesaurek K, Machac J, Muzinic M, DaCosta M, Zhang Z, Heiba S. Quantitative comparison of yttrium-90 (90Y)-microspheres and technetium-99m (99mTc)-macroaggregated albumin SPECT images for planning 90Y therapy of liver cancer. Technol Cancer Res Treat. 2010;9:253-262. [PubMed] |
| 109. | Kim YC, Kim YH, Um SH, Seo YS, Park EK, Oh SY, Han YM, Choe JG. Usefulness of Bremsstrahlung Images after Intra-arterial Y-90 Resin Microphere Radioembolization for Hepatic Tumors. Nucl Med Mol Imaging. 2011;45:59-67. |
| 110. | Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, Porée P, Clément B, Raoul JL, Boucher E. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 111. | Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 112. | Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 113. | Haug AR, Heinemann V, Bruns CJ, Hoffmann R, Jakobs T, Bartenstein P, Hacker M. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |