Published online Mar 7, 2013. doi: 10.3748/wjg.v19.i9.1396
Revised: January 10, 2013
Accepted: January 18, 2013
Published online: March 7, 2013
Processing time: 110 Days and 14 Hours
AIM: To investigate the effect of mild steatotic liver on ischemia-reperfusion injury by focusing on Kupffer cells (KCs) and platelets.
METHODS: Wistar rats were divided into a normal liver group (N group) and a mild steatotic liver group (S group) induced by feeding a choline-deficient diet for 2 wk. Both groups were subjected to 20 min of warm ischemia followed by 120 min of reperfusion. The number of labeled KCs and platelets in sinusoids and the blood perfusion in sinusoids were observed by intravital microscopy (IVM), which was performed at 30, 60 and 120 min after reperfusion. To evaluate serum alanine aminotransferase as a marker of liver deterioration, blood samples were taken at the same time as IVM.
RESULTS: In the S group, the number of platelets adhering to KCs decreased significantly compared with the N group (120 after reperfusion; 2.9 ± 1.1 cells/acinus vs 4.8 ± 1.2 cells/acinus, P < 0.01). The number of KCs in sinusoids was significantly less in the S group than in the N group throughout the observation periods (before ischemia, 19.6 ± 3.3 cells/acinus vs 28.2 ± 4.1 cells/acinus, P < 0.01 and 120 min after reperfusion, 29.0 ± 4.3 cells/acinus vs 40.2 ± 3.3 cells/acinus, P < 0.01). The blood perfusion of sinusoids 120 min after reperfusion was maintained in the S group more than in the N group. Furthermore, elevation of serum alanine aminotransferase was lower in the S group than in the N group 120 min after reperfusion (99.7 ± 19.8 IU/L vs 166.3 ± 61.1 IU/L, P = 0.041), and histological impairment of hepatocyte structure was prevented in the S group.
CONCLUSION: Ischemia-reperfusion injury in mild steatotic liver was attenuated compared with normal liver due to the decreased number of KCs and the reduction of the KC-platelet interaction.
- Citation: Ogawa K, Kondo T, Tamura T, Matsumura H, Fukunaga K, Oda T, Ohkohchi N. Influence of Kupffer cells and platelets on ischemia-reperfusion injury in mild steatotic liver. World J Gastroenterol 2013; 19(9): 1396-1404
- URL: https://www.wjgnet.com/1007-9327/full/v19/i9/1396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i9.1396
It is widely accepted that the steatotic liver is more susceptible to hepatic ischemia-reperfusion (IR) injury. Increased microcirculatory deterioration is suggested as a reason for this increased liver damage in the steatotic liver[1-3]. Morphologically, the steatotic liver is characterized by a demonstrable deposition of large, macronodular fatty droplets in liver parenchymal cells[4]. In addition, narrow and distorted lumens of sinusoids, resulting from the swelling of hepatic parenchymal cells due to accumulated lipid, cause a decrease in sinusoidal perfusion[2]. These changes to microcirculation are exacerbated by leukocytes, either mechanically trapped in the narrowed sinusoids or adhering to activated Kupffer cells (KCs) with the release of cytokines, in addition to free radicals[2,5].
Thirty percent of the population in Japan and Western countries suffers from steatotic liver, and the percentage is still increasing[3,6]. Most of these patients have been diagnosed by abdominal ultrasonography screening, even though elevation of serum liver enzyme levels was detected in a lesser percentage[6]. Steatosis of the liver is classified clinically into three grades according to the proportion of hepatocytes with fatty droplets: mild (< 30%), moderate (30%-60%) and massive (> 60%)[7]. Fishbein et al[8] reported that liver enzyme levels were not elevated in the steatotic liver in which the proportion of hepatocytes with fat deposition was less than 18%. Steatotic liver even in a mild degree is a risk factor for complication after liver resection[9]. Most of the reports describing IR injury of the steatotic liver were investigations of moderate and severe steatosis. It is unclear whether the intensity of the hepatic IR injury depends on the degree of fatty change.
Previously, we reported that liver ischemia induced the adhesion of platelets to KCs in the early period after reperfusion, and that interaction between KCs and platelets played a key role in reperfusion injury of the liver[10,11]. In this study, we have focused on the interaction between KCs and platelets in the mild steatotic liver with intravital microscopy (IVM). We hypothesized that tolerance to hepatic IR injury differs according to the degree of steatosis. The aim of this study was to clarify the hepatic dysfunction after IR in the mild steatotic liver compared with the normal liver.
Male Wistar rats were obtained from CLEA Japan, Inc. (Tokyo, Japan). We prepared two model types, normal liver and mild steatotic liver. In the normal liver model, rats weighting 250 g to 300 g were used. In the steatotic model, the rats’ weights were adjusted to the same weight range after they were fed a choline-deficient diet (CDD) (Oriental Bio Service Kanto Inc., Ibaraki, Japan) for 2 wk. Animal experiments were carried out in a humane manner after receiving approval from the Institutional University Experiment Committee of the University of Tsukuba, and in accordance with the Regulation for Animal Experiments in our university and the Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
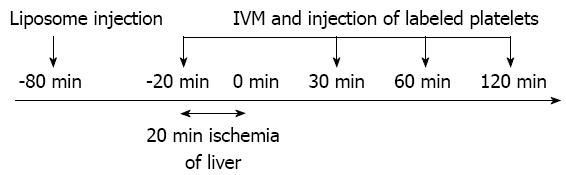
Animals were divided into two groups as follows: (1) the normal liver group (N group; n = 6); and (2) the mild steatotic liver group (S group; n = 6). In both groups, total normothermic hepatic ischemia was induced for 20 min by clamping the portal triad. The hepatic microcirculation and dynamics of platelets and KCs were observed just before ischemia and at 30, 60 and 120 min after reperfusion (Figure 1).
Under anesthesia using isoflurane, the animals were tracheotomized. To reduce spontaneous breathing, animals were ventilated mechanically (MK-V100; Muromachi Kikai Co. Ltd, Tokyo, Japan). The animals were placed in a supine position on a heated pad to maintain the rectal temperature at 37 °C. To monitor arterial blood pressure and allow continuous infusion of Ringer’s solution, polyethylene catheters (PE-50, 0.58/0.96mm internal/external diameter; Becton Dickinson, Sparks, MD) were inserted into the left carotid artery and left jugular vein, respectively. After performing laparotomy via a transverse incision, the ligaments around the liver were dissected to mobilize the left lobe. At the same time, the hepatoduodenal ligament was taped for clamping later. The left hepatic lobe was exteriorized on a plate specially designed to minimize movements caused by respiration and covered with cover glass. Surgical procedures were performed using sterile techniques. After 60 min of normal saline continuous infusion, IVM was performed as a pre ischemia study. Then, hepatic ischemia was induced by clamping the portal triad (the hepatic artery, portal vein, and bile duct) with a microclip (B. Braun Aesculap Japan Co., Ltd, Tokyo, Japan) for 20 min. IVM was performed at 30, 60 and 120 min after reperfusion. Blood samples were taken for analysis of enzyme activities from a catheter placed in the left carotid artery at the same time as IVM. Alanine aminotransferase (ALT) was evaluated as one of the liver enzyme. At 120 min of reperfusion, total body blood was taken for euthanasia. At the end of the experiments, liver tissue was taken for histological examination.
Platelets were isolated from whole blood samples of syngeneic rats and labeled with rhodamine-6G (50 μL/mL whole blood: R-4127; Sigma, St. Louis, MO, United States), as described by Massberg et al[12]. Briefly, the collected blood was diluted with buffer after the addition of prostaglandin E1 and rhodamine 6G. After two-cycle centrifugation, fluorescent platelets were resuspended in phosphate-buffered saline. In this study, a total of 1 × 108 fluorescence-labeled platelets, approximately 1% of all circulating platelets in the recipient rat, were injected through the left carotid artery at 5 min before IVM.
Fluorescently labeled phosphatidylcholine (PC) was incorporated into liposomes, as described by Watanabe et al[13]. The fluorescent pigment used was 2-(12-(7-nitrobenz-2-oxa 1,3-diazol-4-yl) amino) dodecanoyl-1-hexadecanoly-sn-glycero-3-phosphocholine (NBD-C12-HPC; Molecular Probes; Eugene, United States). After intra-arterial injection, KCs in the rat livers were stained and were clearly delineated under the fluorescence image in the IVM. Phagocytic activity of KCs after the administration of liposomes was reported by measuring the amount of hepatic uptake of intravenously administered fluorescent microspheres; no detrimental influence of the liposomes on the phagocytic activity was observed. Additionally, no histopathological changes were found in the livers from liposome-treated rats[13]. Sixty minutes before hepatic ischemia, liposome-encapsulated fluorescent liposomes (4 mL/kg) were administered via the carotid artery catheter.
IVM was performed using a modified microscope (BX30 FLA-SP; Olympus Co., Tokyo, Japan) with a 100 W mercury lamp attached to a filter block. The hepatic microcirculation was recorded by means of a CCD camera (C5810; Hamamatsu Photonics, Hamamatsu, Japan) and a digital video recorder (GV-HD700/1; Sony, Tokyo, Japan) for offline analysis. Using objective lenses (10 × 0.3 to 20 × 0.7; Olympus Co., Tokyo, Japan), a final magnification from x325 to x650 was achieved on the video screen. To assess sinusoidal perfusion, sodium fluorescein (2 × 10-3 M/kg, F-6377; Sigma, St. Louis, MO, United States) was injected via the jugular catheter. Rhodamine-6G labeled platelets were infused intra-arterially just before ischemia and at 30, 60 and 120 min after reperfusion, and 10 randomly chosen acini were visualized. Quantitative assessment of the microcirculatory parameters was performed offline using WinROOF imaging software (version 5.0; Mitani Shoji, Tokyo, Japan).
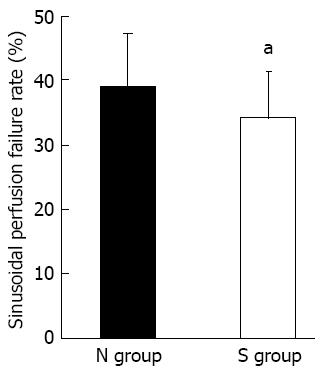
The following parameters were analyzed: (1) the number of adherent platelets, i.e., platelets firmly attached to the sinusoidal endothelium for longer than 20 s [the number of adherent platelets in the scanned acini was counted and results were expressed as the number of adherent platelets per field (1 field = approximately 0.2 mm2)]; (2) the number of adherent platelets adhering to KCs; (3) the number of KCs; and (4) the sinusoidal perfusion failure rate (%) as an index of microcirculatory disturbance, calculated as the ratio of non-perfused sinusoids among the sinusoids observed in one acinus after 120 min of reperfusion.
We immunohistochemically assessed the number of KCs in the acini. To compare the differences between the normal liver group and the mild steatotic liver group before ischemia and after reperfusion, liver tissues were obtained from each group both before ischemia and at the end of the surgical procedure, and from another animal before ischemia. The tissues were fixed in 10% formalin and embedded in paraffin and cut into 4 μm-thick sections. It was immersed in 0.03% hydrogen peroxidase to block endogenous peroxidase activity, and then blocked with 2% bovine serum albumin to reduce background staining. To specifically recognize KCs, mouse anti-rat ED2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States) was used as the primary antibody. The sections were incubated with primary diluted antibodies (1:50) at room temperature for 60 min. Primary antibody reactions were enhanced using horseradish peroxidase EnVision (Dako Japan, Tokyo, Japan). The immunoreaction was visualized with 0.05% 3,3-diaminobenzidine solution. After washing in distilled water, specimens were counterstained with hematoxylin then mounted. The number of ED2-positive cells per acinus was counted in five randomly chosen acini.
As a marker of liver deterioration, serum ALT levels were measured using a Drychem 7000V autoanalyzer (Fuji Film, Tokyo, Japan). Serum was stored at -80 °C until use for cytokine determination. Levels of interleukin (IL)-6 were measured using commercial enzyme-linked immunosorbent assay kits (R and D Systems, Minneapolis, MN, United States).
Liver tissue was obtained before ischemia from the S group to assess the degree of steatosis and after 120 min of reperfusion from each group to assess the histological changes due to IR. The samples were fixed with 10% formalin, and embedded in paraffin. Thin sections (4 μm) were prepared and stained with hematoxylin and eosin (HE). Tissue damage was evaluated in 5 randomly selected high-power fields (×200).
All data are expressed as mean ± SD. The Mann-Whitney U test and analysis of variance were used, followed by Scheffe’s test. P values < 0.05 were considered statistically significant.
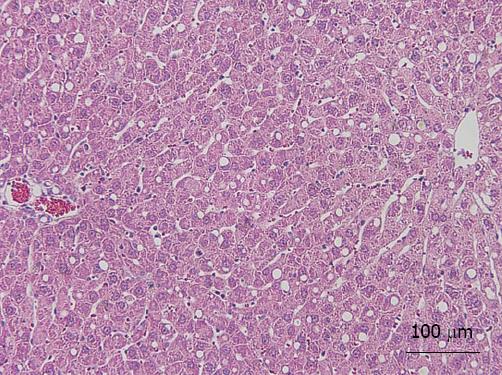
Mild steatosis of the liver was induced by feeding a CDD. The CDD-induced steatotic liver is an established experimental model, in which morphological and functional features are very similar to those of the clinical steatotic liver[14]. The rats fed on CDD for 2 wk developed mild steatotic livers. They were characterized by microvesicular lipid droplet filtration in 10% to 20% of hepatocytes (HE stain) (Figure 2). These findings were identified as a mild degree of steatosis of the liver. We preoperatively confirmed similar findings by liver biopsy in several animals.
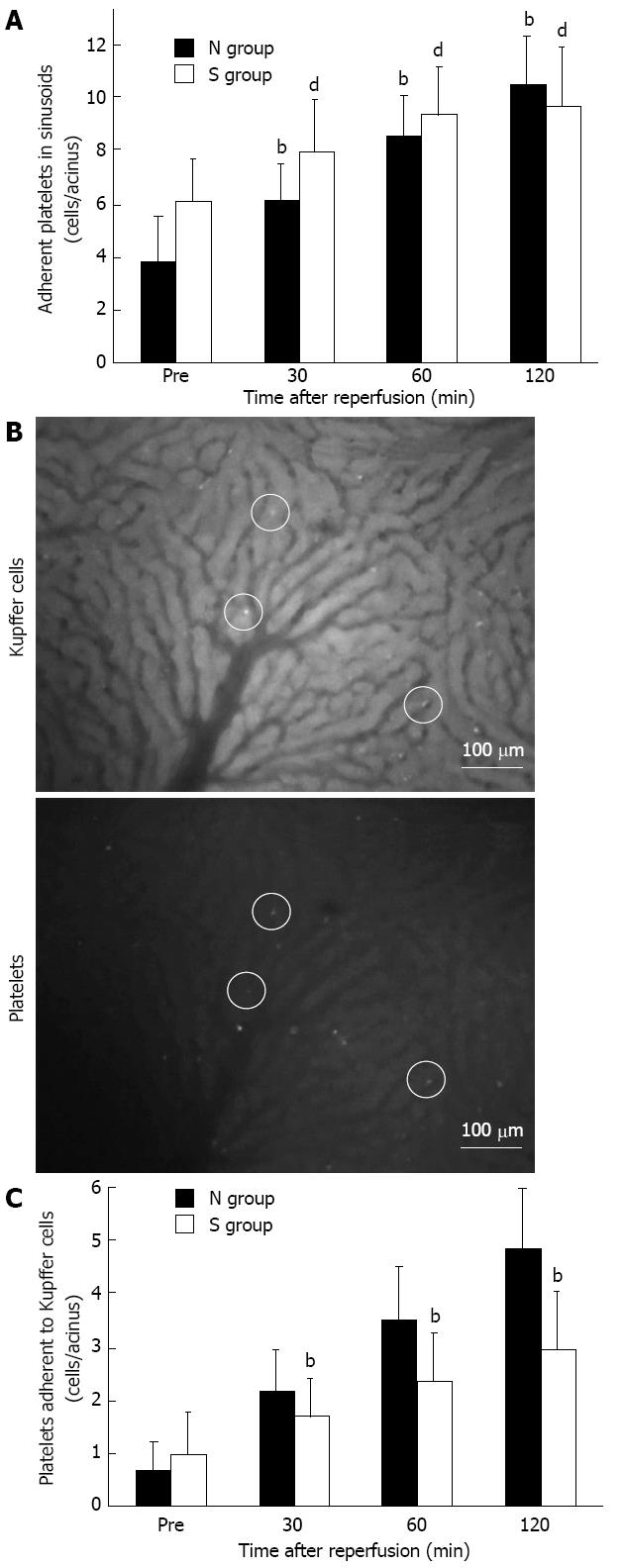
The number of adherent platelets in sinusoids increased along with the reperfusion time both in the N group and in the S group (Figure 3A). In the S group, the number of adherent platelets adhering to KCs was significantly less than in the N group at 30 min after reperfusion and concomitant with the reperfusion period (Figure 3B and C). In addition, there was no significant difference between the two groups in the number of blood platelets (data not shown).
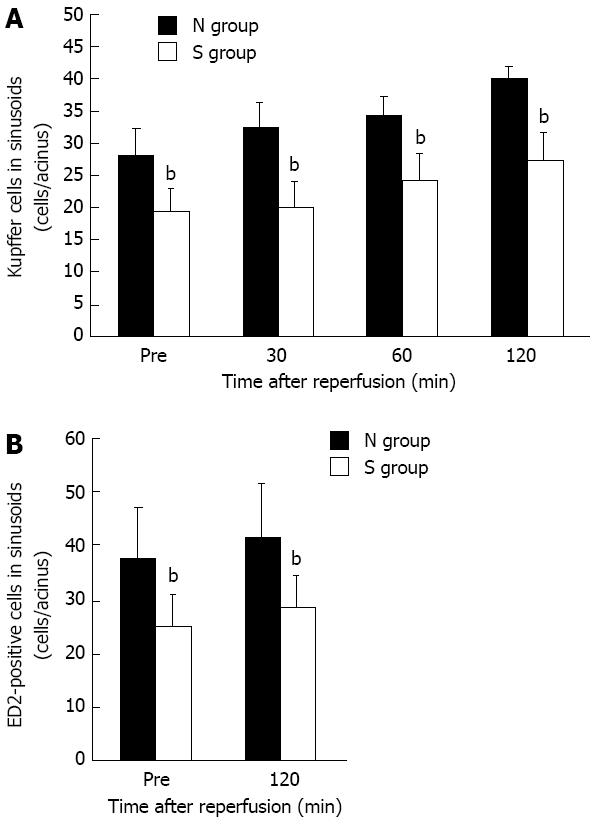
The mild steatotic change in the liver significantly decreased the number of KCs in sinusoids compared with the normal liver at any point in time before and after ischemia reperfusion with IVM study (Figure 4A). In the IVM observation, the counted KCs represented only KCs labeled by liposome entrapment methods. In addition, the number of KCs was verified immunohistochemically. In immunohistochemical staining, the numbers of ED2-positive cells were lower in the S group than in the N group already at the time before ischemia and after 120 min of reperfusion (Figure 4B).
Vollmar et al[15] reported that the sinusoidal perfusion rate was one of the indexes of reperfusion injury. After 120 min of reperfusion, the rate of sinusoidal perfusion failure was significantly higher in the N group than in the S group (Figure 5). The perfusion of sinusoids in which KCs and platelets adhered had a failure rate of about 50% in both groups (data not shown). In contrast, the perfusion failure rate of sinusoids in which KCs adhering to platelets was not observed remained at approximately 30% in both groups (data not shown). This indicated that the interaction between KCs and platelets was associated with sinusoidal perfusion failure.
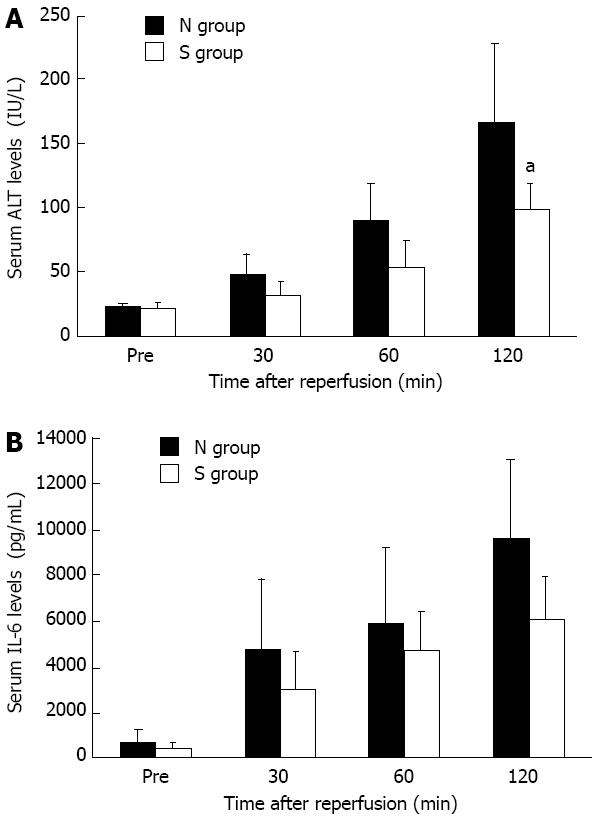
Serum ALT level as a measure of hepatic parenchymal impairment was significantly lower in the S group compared with the N group after 120 min of reperfusion (Figure 6A). The concentration of serum IL-6 had a tendency to be lower in the S group than in the N group, but there was no significant difference (Figure 6B).
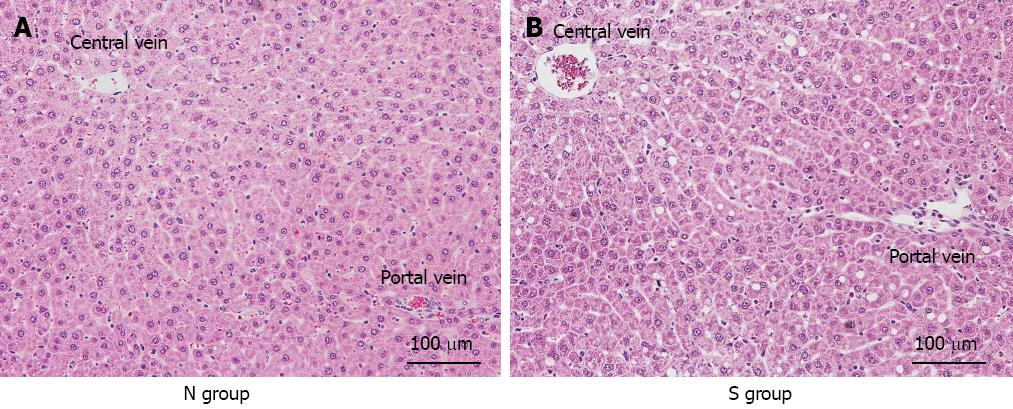
In the S group, histological damage to the liver, such as disturbance of the sinusoidal structure and sinusoidal narrowing, was slightly greater than in the N group. Necrotic changes were not observed in either group (Figure 7).
Steatosis of the liver is a common disorder with several different etiologies. This disorder is one of the most common obstacles in liver surgery, since steatotic livers are susceptible to some stressful loads, especially IR injury[1,16]. With the increase in steatotic liver patients in the world, the number of mild steatotic liver cases is also increasing[17]. A recent analysis of liver transplants, in which the graft liver suffered from cold ischemia, reported that the degree of liver graft steatosis is an important determinant of IR injury and correlated with the rate of postoperative complications and mortality[18,19]. In warm ischemia, however, the influence of the degree of hepatic steatosis on liver dysfunction is unclear. In this study, we demonstrated that IR injury in mild steatotic liver was attenuated compared with that in normal liver, and that it resulted from the reduction in the interaction between KCs and platelets due to the decreased number of KCs.
It was reported that there are two distinct periods of liver injury after warm IR[20,21]. The early period of IR injury, which occurs within 120 min after reperfusion, is characterized by KC-induced reactive oxygen species (ROS)[21,22]. KC production and the release of ROS result in acute hepatocellular injury. In addition, in response to the exposure to activated KCs, neutrophils accumulate in the post-ischemic liver. In the late period of IR injury, inflammatory responses from accumulating neutrophils induce hepatocyte injury, which appears more than 6 h after reperfusion[22,23]. Elevated liver enzymes and apoptosis of hepatocytes and sinusoidal endothelial cells (SECs) can already be observed in the early period[10,24]. A reduction in the early period of IR injury, for instance KC depletion, also leads to inhibition of injury in the late period[25]. Recently, some studies have focused on the function of platelets in hepatic IR injury[26,27]. Sindram et al[24] reported that platelets caused SEC apoptosis and significantly contributed to IR injury. Khandoga et al[28] have shown that warm hepatic IR induced rolling and adhesion of platelets to SECs as well as accumulation of platelets in sinusoids. We previously reported that platelet-SEC interactions occur earlier than leukocyte responses after reperfusion, and that adhesion of platelets requires the presence of activated KCs[11]. In addition, based on our study using the IVM system and electron microscopy, we also reported that platelet-KC interaction as well as platelet-SEC interaction contributes to early-period hepatic IR injury[10,24,28]. Most of the events that determine the extent of IR injury, such as KC activation, platelet adhesion to KCs or SECs, and neutrophil accumulation, occur in the early period of IR injury. Therefore, we focused on observation until 120 min after reperfusion. In this study, we demonstrated that the adherence of platelets to KCs decreased and reperfusion injury was reduced in the mild steatotic liver. The number of adherent platelets in sinusoids increased along with the reperfusion time both in the normal liver group and in the mild steatotic liver group. This suggests that the adherent ability of platelets was not reduced in the mild steatotic liver group. Therefore, we considered that the reduction in KC-platelet interaction was a result of the decreased number of KCs in the mild steatotic liver.
KCs are more likely to be activated in the steatotic liver[5,29]. In addition, hepatic IR activates KCs[30]. However, it is unknown whether KC activation after IR increases in steatotic liver more than in normal liver. After IR, KCs secrete pro-inflammatory cytokines including IL-6[31,32]. Moreover, as described above, activated KCs cause adhesion of platelets to KCs, and lead to later leukocyte accumulation[11]. We consider that serum IL-6 levels reflect the degree of the interaction between KCs and platelets according to the activity of KCs. In the present study, elevation of serum IL-6 levels after IR was less in mild steatotic liver than in normal liver. Our results indicated that in the mild steatotic liver, IL-6 secretion was suppressed because of the decreased number of KCs, even if KCs were activated after IR.
In our present study, we demonstrated that there were fewer KCs in the sinusoids in our mild steatotic liver than in the normal liver. The results were confirmed by both the IVM study and immunohistochemical examination. Several studies reported on the change in the number of KCs in steatotic liver models. Shono et al[33] reported that the number of KCs in a particular subgroup could change, for example, the proportion of CD68 positive KCs decreased in their steatotic model induced by a high-fat diet and a high-cholesterol diet. Veteläinen et al[34] investigated the difference between a methionine-choline deficient diet (MCD) model and a CDD model in their effect on the number of KCs, and reported that the number of KCs increased in the MCD model, but did not change in the CDD model. The degree of steatosis was moderate in their CDD model, and severe in their MCD model. These investigators indicated that the difference in the method of inducing steatosis of the liver resulted in a change in the number of KCs in the sinusoids. In addition, Guo et al[35] reported that the number of KCs was reduced in their steatotic liver model induced by palmitoleate, a monounsaturated fatty acid. Thus, differences in nutrient factors may influence the number of KCs in the steatotic liver. We supposed that a change in nutrient conditions induced by CDD might lead to a decrease in KCs as well as mild steatosis of the liver. The relationship between KCs and steatotic liver has not been well established yet. Further research using various steatotic liver models and various degrees of steatosis will be necessary to elucidate the impact of steatotic liver on KCs.
Steatotic liver patients in the clinical setting of hepatic surgery have tended to increase[17]. IR injury is closely involved with complications in the steatotic liver after hepatic resection[36]. It is known that the steatotic liver is a risk factor for postoperative complications[9,36]. Some investigators reported that patients with a steatotic liver who received a major hepatectomy were more likely to suffer from infective, wound-related, hepatobiliary and gastrointestinal postoperative complications[37]. The decreased tolerance of steatotic liver to IR injury is a result of impaired microcirculation due to hepatocytes with fat deposition[38]. Between the mild and severe steatotic liver, there are differences in microcirculatory disturbances due to differences in the degree of fat deposition. A recent meta-analysis revealed a significant association between the degree of steatosis and increased risk of postoperative complications and mortality[9]. Several investigators reported that postoperative complications, especially infectious complications, and mortality increased in patients with severe steatotic liver compared with those with mild steatotic liver[9,37]. It will be necessary to evaluate IR injury in the moderate to severe steatotic liver in a similar experimental model in the future.
On the other hand, there is a report that postoperative liver failure was slight in the mild steatotic liver, and so mild steatotic liver can be an indication for hepatic surgery[39]. Moreover, mild to moderate seteatotic livers have been accepted as a marginal graft in transplantation[18,19]. Our study suggested that IR injury does not depend on the degree of fat deposition. In addition, our results provide some evidence that postoperative outcomes after liver resection or transplantation are not aggravated in mild steatotic liver.
In conclusion, in mild steatosis liver induced by CDD, hepatic IR injury was attenuated compared with the normal liver. The small number of KCs in the sinusoids decreased the number of KCs adhering to platelets, and resulted in decreased interaction between KCs and platelets.
The steatotic liver is well known to be sensitive to ischemia-reperfusion (IR) injury. Increased microcirculatory deterioration is suggested as a reason for this increased liver damage in the steatotic liver. However, it is unclear whether IR injury increases in the mild steatotic liver. Previously, authors reported that the interaction between Kupffer cells (KCs) and platelets played a key role in hepatic IR injury. This study investigated the effect of mild steatotic liver on IR injury, focusing on Kupffer cells and platelets.
KC activation plays a pivotal role in hepatic IR injury. Upon activation by hepatic ischemia, KCs release reactive oxygen species and inflammatory mediators, such as cytokines and chemokines. In addition, KC activation leads to platelet and neutrophil accumulation in hepatic sinusoids. On the other hand, KCs are more likely to be activated in the steatotic liver.
This is the first study focusing on KCs and platelets in IR injury in the mild steatotic liver. The authors demonstrated that hepatic IR injury in the mild steatotic liver was attenuated compared with normal liver. The small number of KCs in the sinusoids decreased the number of KCs adhering to platelets, and resulted in decreased interaction between KCs and platelets.
The authors suggest that IR injury in the steatotic liver does not depend on the degree of fat deposition. The results of this study provide some evidence that the postoperative outcomes after liver resection or transplantation are not aggravated in the mild steatotic liver.
Intravital fluorescence microscopy (IVM) of the rat liver is a high-resolution real-time technique that allows authors visualize hepatic sinusoidal perfusion. The availability of an enormous number of different fluorescent markers for ex vivo and in vivo staining has extended the possibilities of IVM from purely morphological analysis to the study of complex physiological and pathological events.
The manuscript demonstrated a decrease in IR injury in mild steatotic liver in Wistar rats by feeding with a choline-deficient diet compared to normal rats. In addition, the results also showed that the attenuation of IR injury was associated with a decrease in KC number and platelet-KC adhesion in acini. The study is well designed and the results are interesting and conceivable.
P- Reviewers Acquaviva R, Han JY S- Editor Wen LL L- Editor Cant MR E- Editor Li JY
| 1. | Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625-1631. [PubMed] |
| 2. | Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken). 2008;291:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Federico A, D’Aiuto E, Borriello F, Barra G, Gravina AG, Romano M, De Palma R. Fat: a matter of disturbance for the immune system. World J Gastroenterol. 2010;16:4762-4772. [PubMed] |
| 4. | Koti RS, Yang W, Glantzounis G, Quaglia A, Davidson BR, Seifalian AM. Effect of ischaemic preconditioning on hepatic oxygenation, microcirculation and function in a rat model of moderate hepatic steatosis. Clin Sci (Lond). 2005;108:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076-1082. [PubMed] |
| 6. | Omagari K, Morikawa S, Nagaoka S, Sadakane Y, Sato M, Hamasaki M, Kato S, Masuda J, Osabe M, Kadota T. Predictive factors for the development or regression of Fatty liver in Japanese adults. J Clin Biochem Nutr. 2009;45:56-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, Kafetzis I, Castaing D, Bismuth H. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538-1540. [PubMed] |
| 8. | Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54-61. [PubMed] |
| 9. | de Meijer VE, Kalish BT, Puder M, Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Tamura T, Kondo T, Pak S, Nakano Y, Murata S, Fukunaga K, Ohkohchi N. Interaction between Kupffer cells and platelets in the early period of hepatic ischemia-reperfusion injury--an in vivo study. J Surg Res. 2012;178:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, Myronovych A, Tadano S, Hisakura K, Ikeda O. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Massberg S, Eisenmenger S, Enders G, Krombach F, Messmer K. Quantitative analysis of small intestinal microcirculation in the mouse. Res Exp Med (Berl). 1998;198:23-35. [PubMed] |
| 13. | Watanabe R, Munemasa T, Matsumura M, Fujimaki M. Fluorescent liposomes for intravital staining of Kupffer cells to aid in vivo microscopy in rats. Methods Find Exp Clin Pharmacol. 2007;29:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hayashi M, Tokunaga Y, Fujita T, Tanaka K, Yamaoka Y, Ozawa K. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282-287. [PubMed] |
| 15. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 16. | Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48 Suppl 1:S104-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 18. | Perez-Daga JA, Santoyo J, Suárez MA, Fernández-Aguilar JA, Ramírez C, Rodríguez-Cañete A, Aranda JM, Sánchez-Pérez B, Montiel C, Palomo D. Influence of degree of hepatic steatosis on graft function and postoperative complications of liver transplantation. Transplant Proc. 2006;38:2468-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Briceño J, Padillo J, Rufián S, Solórzano G, Pera C. Assignment of steatotic livers by the Mayo model for end-stage liver disease. Transpl Int. 2005;18:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 21. | Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115-136. [PubMed] |
| 22. | Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 616] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 24. | Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology. 2000;118:183-191. [PubMed] |
| 25. | Giakoustidis DE, Iliadis S, Tsantilas D, Papageorgiou G, Kontos N, Kostopoulou E, Botsoglou NA, Gerasimidis T, Dimitriadou A. Blockade of Kupffer cells by gadolinium chloride reduces lipid peroxidation and protects liver from ischemia/reperfusion injury. Hepatogastroenterology. 2003;50:1587-1592. [PubMed] |
| 26. | Cywes R, Packham MA, Tietze L, Sanabria JR, Harvey PR, Phillips MJ, Strasberg SM. Role of platelets in hepatic allograft preservation injury in the rat. Hepatology. 1993;18:635-647. [PubMed] |
| 27. | Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Khandoga A, Biberthaler P, Messmer K, Krombach F. Platelet-endothelial cell interactions during hepatic ischemia-reperfusion in vivo: a systematic analysis. Microvasc Res. 2003;65:71-77. [PubMed] |
| 29. | Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, Zorn M, Büchler MW, Schemmer P. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011;18:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 31. | Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol. 2010;16:1285-1292. [PubMed] |
| 32. | Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Shono S, Habu Y, Nakashima M, Sato A, Nakashima H, Miyazaki H, Kinoshita M, Tsumatori G, Shinomiya N, Seki S. The immunologic outcome of enhanced function of mouse liver lymphocytes and Kupffer cells by high-fat and high-cholesterol diet. Shock. 2011;36:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 2012;7:e39286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [PubMed] |
| 38. | El-Badry AM, Moritz W, Contaldo C, Tian Y, Graf R, Clavien PA. Prevention of reperfusion injury and microcirculatory failure in macrosteatotic mouse liver by omega-3 fatty acids. Hepatology. 2007;45:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Cho JY, Suh KS, Kwon CH, Yi NJ, Lee KU. Mild hepatic steatosis is not a major risk factor for hepatectomy and regenerative power is not impaired. Surgery. 2006;139:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |