Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6237
Revised: August 7, 2013
Accepted: August 20, 2013
Published online: October 7, 2013
Processing time: 126 Days and 13.3 Hours
AIM: To investigate the effects of nilotinib in a rat model of trinitrobenzene sulfonic acid (TNBS)-induced colitis.
METHODS: Twenty-one Wistar albino female rats obtained from Dokuz Eylul University Department of Laboratory Animal Science were categorized into a control (n = 7), TNBS (n = 7) and nilotinib group (n = 7). Saline was administered orally for 14 d to the control and the TNBS group. The TNBS group received rectal TNBS on the first day while saline was administered to the control group. The nilotinib group received 20 mg/kg nilotinib for 14 d in 2 divided doses, starting the same day as TNBS administration. For 14 d, the rats were fed a standard diet, and their weights were recorded daily. After sacrifice, colon tissue samples from each group were scored for macroscopic and microscopic pathology. Apoptotic indices were determined by the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling method. Platelet-derived growth factor receptor (PDGFR) alpha and beta levels were assessed through immunohistochemistry staining scores and compared among the groups. Tissue and serum tumor necrosis factor (TNF) alpha levels were determined by enzyme-linked immunosorbent assay.
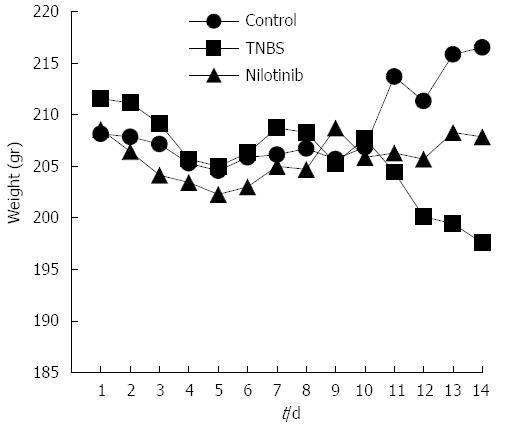
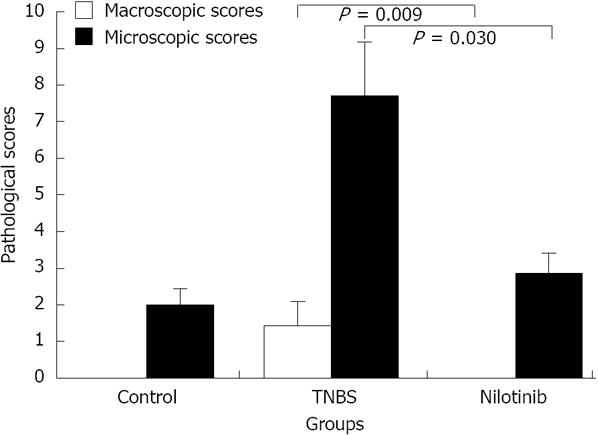
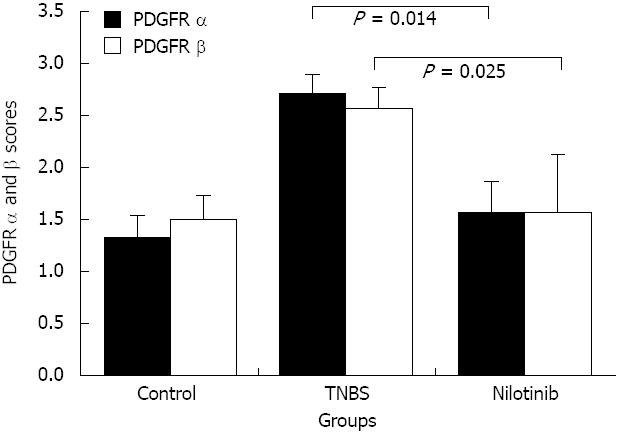
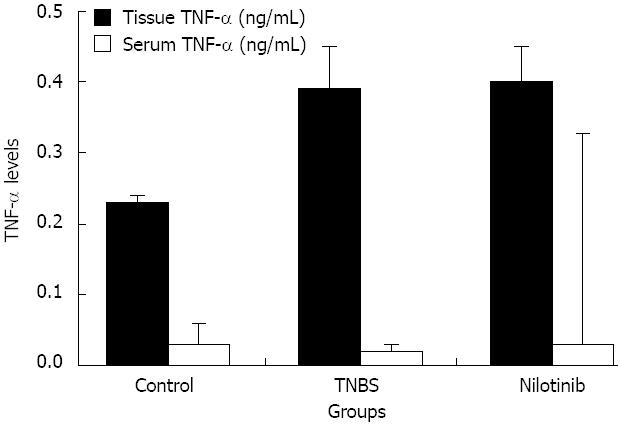
RESULTS: Between days 1 and 14, the nilotinib group rats lost significantly less weight than the TNBS group rats (-0.7 g vs -14.0 g, P = 0.047). The difference in weight between the control and nilotinib groups was also statistically significant (+8.3 g vs -0.7 g, P = 0.031). From day 7 to day 14, the weight differences of the control group vs the TNBS group, the TNBS group vs the nilotinib group, and the control group vs the nilotinib group were all statistically significant (+8.0 g vs -11.1 g, P = 0.007; -11.1 g vs +2.9 g, P = 0.015; +8.0 g vs +2.9 g, P = 0.042, respectively). Macroscopic and microscopic scores were significantly lower in the nilotinib group than in the TNBS group (0.00 ± 0.00 vs 1.43 ± 0.65, P = 0.009; 2.86 ± 0.55 vs 7.71 ± 1.48, P = 0.030, respectively). However, these scores were similar between the nilotinib and control groups. While no significant difference for the nilotinib vs control groups could be determined for PDGFR alpha and beta scores, PDGFR alpha and beta scores were lower in the nilotinib group than in the TNBS group. Furthermore, the TNF alpha levels in the serum, tissue and apoptosis scores were similar between the nilotinib and TNBS groups.
CONCLUSION: Nilotinib prevents weight loss, facilitates mucosal healing by improving the pathological scores without introducing variation into the apoptotic scores or TNF alpha levels.
Core tip: Unresponsiveness to medical treatment in refractory inflammatory bowel disease (IBD) still poses a therapeutic challenge. To detect an alternative treatment option, we selected nilotinib based on the fact that tyrosine kinases inhibitors affect several key components in the pathogenesis of IBD, including tumor necrosis factor (TNF) alpha, platelet-derived growth factor receptor (PDGFR), and apoptosis. In a trinitrobenzene sulfonic acid-induced colitis rat model, we concluded that nilotinib has a significant effect on weight loss and on macroscopic and microscopic pathological scores, leading to significant mucosal healing. Although nilotinib caused a decrease in the PDGFR alpha and PDGFR beta levels, it did not have a significant effect on the apoptotic scores or TNF alpha levels.
- Citation: Ataca P, Soyturk M, Karaman M, Unlu M, Sagol O, Dervis Hakim G, Yilmaz O. Nilotinib-mediated mucosal healing in a rat model of colitis. World J Gastroenterol 2013; 19(37): 6237-6244
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6237
Chronic intestinal inflammation is characterized by the pathological responses of the adaptive and innate immune systems. These responses are central to the pathological mechanisms that lead to inflammatory bowel disease (IBD)[1]. Genetic and environmental factors, infectious agents, the structure of the enteric flora, and immune system dysfunctions are key elements in the pathogenesis of IBD, and thus, these are targets for many drugs developed to treat IBD[2,3]. However, unresponsiveness to medical treatment in IBD still poses a therapeutic challenge. Previous studies examining the therapeutic effectiveness of selecting drugs in patients with ulcerative colitis (UC) reported the rates of remission to be 47%-81% with rectal 5-aminosalicylic acid (5-ASA), 9%-30% with oral 5-ASA, and 42%-82% with thiopurines[4-6].
Monoclonal tumor necrosis factor (TNF) alpha inhibitors are currently the treatment of choice, especially in severe and resistant cases of IBD. However, decreased responses or resistance to the TNF alpha inhibitor infliximab have been reported. Previous studies have reported an average clinical remission rate at week 8 of 33% (range, 27.5%-38.8%) with the use of infliximab in IBD patients[7]. Clinical remission was maintained in 33% (range, 25.6%-36.9%) of patients treated with infliximab at week 30[7]. In a randomized, placebo-controlled 52-wk study examining the effectiveness of adalimumab, another anti-TNF agent, the IBD remission rate was significantly higher than the placebo, regardless of treatment with steroids (13.3% and 5.7%, respectively; P = 0.035)[8].
Mucosal healing has emerged as a key therapeutic objective in the treatment of IBD and is able to predict sustained clinical remission and resection-free survival in patients. Mucosal healing is achieved in approximately 30% of IBD patients receiving corticosteroid therapy and in as many as 60% of IBD patients receiving anti-TNF therapies[9-11]. Approximately 20% of IBD patients, however, do not respond to anti-TNF therapy and require surgical intervention[12]. These findings emphasize the importance of discovering new medical treatment options for IBD because the currently available treatments are insufficient for a substantial number of patients.
Tyrosine kinases (TKs) are enzymes that play a role in normal cell function, metabolism, growth, differentiation, and apoptosis. TK inhibitors are drugs that block the action of these enzymes. Although they are typically used as anticancer drugs, they have recently been considered for use in noncancer proliferative diseases and for inflammatory conditions. Imatinib, the best-known member of this class of drugs, is specific for TK receptor sites and suppresses the Abelson proto-oncogene (ABL), the c-kit proto-oncogene, platelet-derived growth factor receptor (PDGFR), macrophage colony-stimulating factor receptor, TNF alpha, and inducible nitric oxide synthase[13]. Nilotinib is a more potent inhibitor of TKs than imatinib. In studies involving patients with lung fibrosis, nilotinib has been shown to reduce interleukin (IL)-6, IL-1 beta, TNF alpha, tumor growth factor beta 1, and PDGFR beta levels more significantly than imatinib and had a potent antifibrotic effect[14].
In the literature, there are a few reports suggesting that TK inhibitors may be effective in IBD. In a case report by Magro et al[15], a patient diagnosed with Crohn’s disease (CD) and chronic myeloid leukemia (CML) remained in remission for 3 years on imatinib therapy alone, without the use of mesalamine or steroids. Cuzzocrea et al[16] demonstrated that the development of colitis in dinitrobenzene sulfonic acid (DNBS)-induced colitis animal models was reduced by the TK inhibitor tyrphostin AG126.
The present study was planned based on the demonstrated success of nilotinib in previous studies and on the fact that TK inhibitors affect several key components in the pathogenesis of IBD, including TNF alpha, PDGFR, and nitric oxide (NO) synthesis. For this purpose, we evaluated the efficacy of nilotinib on weight, macroscopic and microscopic pathological scores, TNF alpha levels, PDGFR levels, and the apoptotic index in a rat model of trinitrobenzene sulfonic acid (TNBS)-induced colitis. This study is the first to evaluate the efficacy of nilotinib in a rat colitis model.
Approval was obtained from the animal ethics council of Dokuz Eylul University Medical Faculty (DEUTF). The DEUTF Hospital Experimental Research Laboratory provided 21 female Wistar albino rats weighing 200-250 g (mean weight: 209.43 ± 8.92 g) for use in this study.
The rats were maintained in a room at a temperature of 23 ± 2 °C under a 12-h light/dark cycle at the DEUTF Experimental Animal Laboratory. Before and during the study, they were fed a standard diet, and their weights were monitored daily. The animals were also allowed water ad libitum.
The rats were divided into 3 groups, each consisting of 7 rats: the control group, TNBS group and nilotinib group. After 24 h of fasting, 0.25 mL of the physiological serum was intracolonically administered to the control group rats through a cannula inserted 8 cm proximal to the anus, using a rectally inserted flexible polypropylene catheter. To induce colitis, the rats in the other 2 groups received an intracolonic solution treated with 0.5 mL of 100 mg/mL TNBS (Sigma, Germany) dissolved in 30% ethanol and administered through a cannula. Before catheter insertion, short-term sedation was provided through ether anesthesia. Neither group of rats treated with TNBS encounter any instance of perforation or death due to colonic ulceration. The TNBS and control groups received a saline placebo for 14 d through an orogastric tube. Nilotinib 20 mg/kg/d (Novartis Pharma AG, Basel, Switzerland) was administered in 2 divided doses to the nilotinib group for 14 d through an orogastric tube, beginning on the same day as TNBS administration.
Blood and tissue samples for pathological examination were obtained from all of the rats under ether anesthesia at the end of the 14-d period. All of the animals were then sacrificed by decapitation. The abdominal cavities were opened by a midline incision, and the entire length of the large intestines was dissected from the distal ileum to the rectum. After washing with saline, the large intestinal tissues were fixed with buffered formalin.
A pathologist blinded to the group identity of the intestinal samples performed pathological evaluations of all of the tissue samples. Each intestinal column was opened longitudinally, according to the method reported by Vilaseca et al[17], and macroscopic scoring was performed. Tissue sections of the gross ulcerative lesions and surrounding normal mucosa were then stained with hematoxylin-eosin (HE). The pathologist then performed microscopic scoring according to the method reported by Dieleman et al[18].
The pathologist stained all tissue samples using the TUNEL method. Mucosal crypts and apoptotic cells were counted along the surface epithelium under a microscope (Olympus DX51) at a magnification of × 400. Using the TUNEL technique, all of the cut sections were preserved with lysine for 3 nights at 37 °C and then for 1 night at 60 °C in an incubator. Thereafter, deparaffinization was performed with 3 cycles of xylene. The tissue sections were then rehydrated by flushing with a series of alcohol solutions of decreasing degrees (absolute, 96%, 80%, and 70%) and then stored in distilled water for 5 min. Proteinase K (Proteinase K, Invitrogen, Carlsbad, CA, United States) was applied for 10 min at room temperature. The sections were then washed twice with phosphate-buffered solution (PBS) for a period of 2 min each. After drying the cross-sections, 3% H2O2 (Merck, Germany) was applied for 5 min, and the sections were then washed with PBS twice for 5 min each. The cross-sectional slices were then dried, and an equilibration buffer (ApopTag Plus peroxidase kit, Millipore, Billerica, MA, United States) was applied for 10 min at room temperature. A total of 55 μL of the enzyme terminal deoxynucleotidyl transferase was then applied to each cross-section. The cross-sections were closed with a coverslip (ApopTag Plus peroxidase kit, Millipore, Billerica, MA, United States) and incubated for 1 h at 37 °C. Stop/wash buffer (ApopTag Plus peroxidase kit, Millipore, Billerica, MA, United States) was then applied to the sections removed from the incubator for 10 min at room temperature. The sections were then washed with PBS at room temperature 3 times for 1 min each, dried, and incubated with anti-streptavidin-peroxidase (ApopTag Plus peroxidase kit, Millipore, Billerica, MA, United States) at room temperature for 30 min. The sections were then washed with PBS 4 times for 2 min to determine the visibility of the TUNEL reaction before being stained with diaminobenzidine (DAB) (DAB-PLUS kit; Invitrogen, Carlsbad, CA, United States). After washing with distilled water, ground staining was performed using methyl green. After three changes of the searing process with xylene for 20 min, it was closed with Entella.
The tissue samples obtained from the ileum were introduced into 2 mL microcentrifuge tubes and stored at -80°C until the day of the study. These tissues were then removed and warmed to 4 °C. Next, 60-80 mg pieces were obtained from these samples and placed into a tube containing 5-mm-diameter stainless steel beads and a phosphate buffer with a 1:7 ratio (pH 7.2). Microcentrifuge tubes were introduced into a pre-chilled TissueLyser LT device and replaced into a TissueLyser (Qiagen-Germany) tissue homogenization device. Next, an enzyme-linked immunosorbent assay (ELISA) was performed on tissue supernatants, and serum was obtained via centrifugation for the identification of TNF alpha in accordance with the manufacturer’s recommendations (Invitrogen Rat TNF-alpha, Carlsbad, CA, United States). Finally, the ELISA plates were spectrophotometrically evaluated at 450 nm (Biotech Synergy HT; Winooski, VT, United States).
PDGFR alpha and beta levels were assessed through staining scores and compared among the groups by immunohistochemistry. For immunohistochemical staining, 2-3 micron sections were stored overnight in an incubator at 40 °C. The following day, the sections were washed with xylene, a descending alcohol series, and distilled water for 20 min. They were then boiled for 20 min in EDTA solution at pH 8. Next, they were stored in DakoFlex peroxidase solution for 5 min and washed again with Tris-buffered saline. A primary antibody was then applied. PDGFR alpha in a 1:100 dilution (NOVUS Biologicals, NBP1-19 423, Littleton, CO, United States) and PDGFR beta in a 1:50 dilution (NOVUS Biologicals, NBP1-19 473; Littleton, CO, United States) were stored for 30 min, washed with Tris buffer, stored in DakoFlex HRP solution for 20 min, washed with Tris buffer again, and stored in DakoFlex DAB for 7 min. The samples were again washed with Tris-buffered saline, kept under tap water for 5 min, stained with Mayer’s hematoxylin solution for 10 min, washed with tap water for 1 min, rinsed in an alcohol series, and cleaned with xylene for 5-10 min.
PDGFR alpha and beta positivity was determined according to a devised scoring system. According to this system, a score of 1 was assigned if PDGFR alpha and beta positivity was confirmed in inflammatory cells and in the cells of the lamina propria, stroma, and submucosal endothelium. A score of 2 was assigned if increased expression of PDGFR alpha and beta was confirmed in the lamina propria and submucosa. A score of 3 was assigned if PDGFR alpha and beta positivity was confirmed with widespread staining in the ulcerated areas or in the inflammatory cells, fibroblasts, endothelial cells, submucosa, and mucosa of the surrounding tissue.
All statistical procedures were performed using SPSS software (version 15.0). The Kruskal-Wallis test was used for multigroup comparisons, and the Mann-Whitney U test was used to compare the means of 2 groups. A P value less than 0.05 was considered statistically significant.
Bloody diarrhea was observed on day 1 of rectal TNBS administration in all 14 of the rats in the 2 experimental groups; no bloody diarrhea was observed in the control group. In the TNBS and nilotinib groups, the diarrhea was semi-solid on day 5. In the nilotinib group, normal stools were observed after day 7. During rectal saline administration under ether anesthesia in the control group, respiratory arrest developed in 1 rat, which remained stable after CPR. However, the animal’s general condition deteriorated over the next few d, and the animal died on day 6 of the experiment. An autopsy was not performed on this rat.
On the first experimental day, the average weights were similar among all of the study groups (P > 0.05), and the average weights were examined daily (Figure 1). The average weight of the control group increased to 8.3 g at the end of 14 d. The TNBS group, however, lost an average of 14 g throughout the study, and the nilotinib group lost an average of 0.7 g. There was a significant difference among the groups with regard to the average weight change throughout the study (P = 0.006). The difference in weight between the control and nilotinib groups was statistically significant (+8.3 and -0.7 g, respectively, P = 0.031). The TNBS group lost significantly more weight than the nilotinib group (-14.0 and -0.7 g, respectively; P = 0.047) and the control group (-14.0 and +8.3 g, respectively, P = 0.008).
Between day 7 and day 14, the weights of the control group increased by an average of 8 g; those of the nilotinib group increased by an average of 2.9 g; and those of the TNBS group decreased by an average of 11.1 g. Comparing the average increase in weights over this time period among all 3 of the groups, there was a significant difference observed (P = 0.004). From day 7 to day 14, the weight differences of the control rats vs the TNBS rats, the TNBS rats vs the nilotinib rats, and the control rats vs the nilotinib rats were statistically significant (+8.0 and -11.1 g, P = 0.007; -11.1 and +2.9 g, P = 0.015; +8.0 and +2.9 g, P = 0.042, respectively).
The mean macroscopic pathological scores of the control and nilotinib groups were 0, while the macroscopic pathological score in the TNBS group was 1.43 ± 0.65. When the distribution of macroscopic scores based on rats was examined, all scores from the control and nilotinib group rats were “0”, which is noteworthy. The control and nilotinib groups were similar in terms of macroscopic scores (P > 0.05). Macroscopic scores were significantly lower in the control and nilotinib groups than in the TNBS group (0.00 ± 0.00 and 1.43 ± 0.65, P = 0.014; 0.00 and 1.43 ± 0.65, P = 0.009, respectively) (Figure 2).
The mean microscopic scores in the control, TNBS, and nilotinib groups were 2.0 ± 0.45, 7.71 ± 1.48, and 2.86 ± 0.55, respectively. The mean microscopic scores were significantly lower in the control and nilotinib groups than in the TNBS group (2.0 ± 0.45 and 7.71 ± 1.48, P = 0.034; 2.86 ± 0.55 and 7.71 ± 1.48, P = 0.030, respectively). The control and nilotinib groups were similar in terms of the mean microscopic scores (P > 0.05) (Figure 2).
With regard to the PDGFR alpha and beta scoring system, the PDGFR alpha scores in the control, TNBS, and nilotinib groups were 1.33 ± 0.21, 2.71 ± 0.18, and 1.57 ± 0.30, respectively. There was a significant difference among the groups (P = 0.007). The PDGFR alpha scores were significantly lower in the control and nilotinib groups than in the TNBS group (1.33 ± 0.21 and 2.71 ± 0.18, P = 0.004; 1.57 ± 0.30 and 2.71 ± 0.18, P = 0.014, respectively). The control and nilotinib groups were similar in terms of the PDGFR alpha scores (P > 0.05) (Figure 3).
The mean PDGFR beta scores in the control, TNBS, and nilotinib groups were 1.50 ± 0.22, 2.57 ± 0.20, and 1.57 ± 0.30, respectively. There was a statistically significant difference among all of the groups in terms of the mean PDGFR beta scores (P = 0.020). The PDGFR beta scores were significantly lower in the control and nilotinib groups than in the TNBS group (1.50 ± 0.22 and 2.57 ± 0.20, P = 0.011; 1.57 ± 0.30 and 2.57 ± 0.20, P = 0.025, respectively). The PDGFR beta scores of the control and nilotinib groups were similar (P > 0.05) (Figure 3).
The mean serum TNF alpha levels in the control, TNBS, and nilotinib groups were 0.03 ± 0.03, 0.02 ± 0.01, and 0.03 ± 0.01 pg/mL, respectively. There was no statistically significant difference observed among the groups in terms of the mean serum TNF alpha levels (P > 0.05) (Figure 4). The average tissue TNF alpha levels in the control, TNBS, and nilotinib groups were 0.23 ± 0.01, 0.39 ± 0.06, and 0.40 ± 0.05 ng/mL, respectively. There was a significant difference observed among the groups (P = 0.002). TNF alpha levels were significantly lower in the control group than in the TNBS or nilotinib groups (0.23 ± 0.01 and 0.39 ± 0.06 ng/mL, P = 0.002; 0.23 ± 0.01 and 0.40 ± 0.05 ng/mL, P = 0.003, respectively). However, there was no statistically significant difference between the TNBS and nilotinib groups in terms of the mean tissue TNF alpha levels (P > 0.05) (Figure 4).
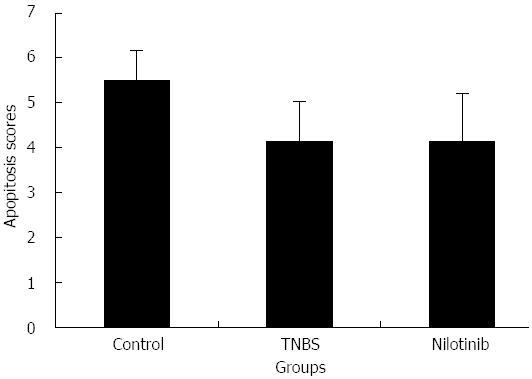
The mean number of apoptotic cells detected by the TUNEL method in the control, TNBS, and nilotinib groups was 5.50 ± 0.67, 4.14 ± 0.88, and 4.14 ± 1.06, respectively. The difference among the groups was not statistically significant (P > 0.05) (Figure 5).
IBDs, such as CD and UC, are chronic recurrent intestinal inflammatory conditions. Genetic, environmental, microbial, and immune factors play a role in the etiopathogenesis of IBDs. Despite the development of biological therapies and advancements in genetic technology, treatment options remain limited for refractory cases.
Mucosal healing has emerged as a key treatment goal for IBD and allows the prediction of sustained clinical remission and resection-free survival in affected patients. Mucosal healing can be achieved in approximately 30% of patients receiving corticosteroid therapy and in 60% of patients receiving anti-TNF agents[9-12]. Approximately 20% of IBD patients do not respond to anti-TNF therapy and require surgical intervention[12]. Thus, the currently available medical treatment options are ineffective in a substantial group of patients with IBD.
Nilotinib, which was used in this study, is a strong TK inhibitor that was initially approved for use in patients with imatinib-intolerant and imatinib-resistant Philadelphia chromosome-positive chronic or accelerated phase-CML and has since been approved as a frontline therapy in chronic phase CML[19]. Nilotinib is more potent than imatinib, which inhibits the autophosphorylation of various kinases, such as BCR-ABL, PDGFR, and c-KIT[19]. Nilotinib is generally well tolerated. Due to the lack of Src family kinase inhibition, myelosuppression is an infrequent adverse event that occurs less frequently with nilotinib than with other TK inhibitors[19]. The most common manageable adverse events are rash, pruritus, fatigue and headache. Neutropenia, anemia, thrombocytopenia, elevations of liver enzymes, cardiac toxicity, namely QT prolongation, fluid retention, edema, and weight gain are among the less common side effects[19]. TK inhibitors affect several key components in the pathogenesis of IBD, including TNF alpha, PDGFR, and NO synthesis. In this study, we evaluated the efficacy of nilotinib on weight, macroscopic and microscopic pathological scores, TNF alpha and PDGFR levels, and the apoptotic index in rat models with TNBS-induced colitis. There are no previous reports in the literature evaluating the efficacy of nilotinib in either a rat model of colitis or in human colitis.
In the present study, the weights of the control and experimental rats were monitored daily. At the end of 14 d, rats in the nilotinib group had lost significantly less weight than rats in the TNBS group (P = 0.047). These results are similar to those obtained in the study by Cuzzocrea et al[16], in which weight loss was significantly reduced by 7 d of treatment with the TK inhibitor tyrphostin AG126 in a DNBS-induced colitis animal model. The TK inhibitor used in the study by Cuzzocrea et al[16] is different from that used in our study. However, our study indicates that nilotinib does have a positive effect on weight in animal models with colitis.
The first therapeutic target in drug studies for the treatment of IBD was the regression of disease-related symptoms. The most important reason for this was that the agents used in the treatment of IBD were not disease-modifying drugs. In more recent studies, however, the primary endpoint in evaluating the therapeutic efficacy of drugs used to treat IBD has been “mucosal healing”[20]. With mucosal healing as the therapeutic target, continuous clinical remission and survival without surgery can be achieved[21]. The mucosal healing rates of anti-TNF agents have been reported at approximately 60% in the active ulcerative colitis trials (ACT)-1 and ACT-2 studies[7,10]. In the present study, the effects of nilotinib on mucosal healing and pathological macroscopic and microscopic scores yielded quite remarkable results. The macroscopic and microscopic pathological scores of intestinal tissue from the nilotinib group were similar to those of the control group (P > 0.05) but significantly lower than those of the TNBS group (P = 0.009; P = 0.030, respectively). In our study, the similar microscopic and macroscopic scores of the nilotinib and control groups constituted the most important evidence of the mucosal healing effect of nilotinib. Indeed, in the study conducted by Cuzzocrea et al[16], the rats treated with the TK inhibitor showed significant histological improvements after treatment compared with the control rats. This result parallels the results of our study. Although there are no human studies investigating the use of TK inhibitors in patients with IBD, the case report by Magro et al[15] representing a case of long-standing remission from Crohn’s disease under imatinib therapy supports these results. However, a number of caveats should be noted regarding the present study. This is the first study of nilotinib in a rat colitis model. The current study was unable to compare the efficacy of nilotinib with that of other IBD agents or to assess the adverse events of nilotinib. Generally, as found in previous studies on CML, nilotinib has been a well-tolerated agent with manageable adverse effects. The findings of this study have a number of important implications for future practice. Further experimental investigations could provide more definitive evidence for human studies.
In our study, to determine the effectiveness of nilotinib on colitis, the PDGFR alpha, PDGFR beta, TNF alpha, and apoptosis levels were compared among the groups. Similar to the results observed in the macroscopic and microscopic pathological scores, the PDGFR alpha and beta scores were significantly lower in the nilotinib group than in the TNBS group (P = 0.014, P = 0.025) but were similar to the control group. There are no other studies investigating the effects of TK inhibitors on the levels of PDGFR alpha and beta in a colitis animal model. Histologically, in IBD, the intestinal microvasculature shifts into a tight angiogenic structure characterized by the increased secretion of angiogenic integrins and mediators into the inflamed mucosa[22]. PDGF alpha and beta are 2 of the angiogenic mediators whose levels increase in IBD[23]. Increased PDGF alpha and beta activity can be found in the fibrotic areas adjacent to the active ulcer areas in IBD. Kumagai et al[24] detected the increased expression of PDGF and PDGFR in the areas with active fibrosis in IBD, and they considered that this contributes to the development of IBD. The macrovascular results of this process were demonstrated as endothelial dysfunction in study of Principi et al[25] due to decreased brachial artery flow-mediated vasodilatation in patients with IBD. The results of our study suggest that nilotinib enacts its effect on mucosal healing in colitis by blocking PDGFR alpha and beta.
TNF alpha is a protein that plays a role in cell proliferation, differentiation, and cell survival. It is responsible for the expression of adhesion molecules, fibroblast proliferation, the release of procoagulant substances, the initiation of cytotoxic apoptosis, and the acute phase response[26]. It has a clearly defined role in the pathogenesis of IBD, and anti-TNF agents are currently being used in the successful treatment of IBD[10]. In IBD, induced apoptosis can be triggered by TNF alpha, which causes much larger leaks in the intestinal barrier[27]. Previous studies have demonstrated that TNF alpha and IL-1 beta, both proinflammatory cytokines synthesized in the colon, are reduced with TK inhibition[16,28]. In our study, the apoptotic indices and serum and tissue levels of TNF alpha were evaluated. The serum and tissue levels of TNF alpha and the apoptotic index in the nilotinib group were found to be similar to those in the TNBS group. Previously, it has been shown that TNF alpha levels on day 7 are significantly higher in acute models of colitis established through a single dose application of TNBS, compared to the model of chronic colitis using weekly TNBS administrations[29,30]. That the serum and tissue TNF alpha levels were similar in the nilotinib and TNBS groups in our study might be explained by the length of the experiment (14 d), during which a TNF alpha peak could not be obtained. Additionally, the apoptosis indices were similar between both groups in our study. D’Argenio et al[31] demonstrated the apoptotic cells and expressions of apoptotic proteins in TNBS-induced colitis over 4 wk. According to the results of this study, the apoptotic cell count was detected to be significantly decreased after first week by the TUNEL method[31]. The similar apoptotic scores detected in our study might be because the apoptotic cell peak could not be obtained after 14 d. Furthermore, the similar results of the TNF alpha levels and apoptosis scores in our study might also suggest that nilotinib has no significant effect on TNF alpha levels and apoptosis.
In conclusion, nilotinib has a significant effect on weight loss, as well as on the macroscopic and microscopic pathological scores in rats with TNBS-induced colitis, leading to significant mucosal healing. Although nilotinib caused a decrease in PDGFR alpha and PDGFR beta levels, it did not have a significant effect on apoptotic scores or TNF alpha levels.
Genetic and environmental factors, infectious agents, the structure of enteric flora, and immune system dysfunction are key elements in the pathogenesis of inflammatory bowel disease (IBD); thus, these are the targets for many drugs developed to treat IBD. Unresponsiveness to medical treatments in refractory IBD still poses a therapeutic challenge. Tyrosine kinases (TKs) are enzymes that play a role in normal cell function, metabolism, growth, differentiation, and apoptosis. To establish an alternative treatment option, they selected nilotinib based on the fact that TK inhibitors affect several key components in the pathogenesis of IBD, including tumor necrosis factor (TNF) alpha, platelet-derived growth factor receptor (PDGFR), and apoptosis.
Nilotinib is a TK inhibitor that is typically used as an anticancer drug. Recently, it has been considered for use in noncancerous proliferative diseases and for inflammatory conditions. Authors concluded that nilotinib has a significant effect on weight loss and macroscopic and microscopic pathological scores while leading to significant mucosal healing. Although nilotinib caused a decrease in the PDGFR alpha and PDGFR beta levels, it did not have a significant effect on apoptotic scores or TNF alpha levels.
Genetic, environmental, microbial, and immune factors play a role in the etiopathogenesis of IBDs. The currently available medical treatment options are ineffective in a substantial group of patients with IBD. Nilotinib, as used in this study, is a strong TK inhibitor. TK inhibitors affect several key components in the pathogenesis of IBD, including TNF alpha, PDGFR, and nitric oxide synthesis. Before this study, there were no reports in the literature evaluating the efficacy of nilotinib in a rat colitis model. One previous study showed that the use of a different TK inhibitor could successfully treat rat colitis. There are no human studies investigating the use of TK inhibitors in patients with IBD.
The results of this study suggest that nilotinib has a significant effect on weight loss, as well as on the macroscopic and microscopic pathological scores of rats with Trinitrobenzene sulfonic acid (TNBS)-induced colitis. Additionally, this treatment leads to significant mucosal healing and caused a decrease in PDGFR alpha and PDGFR beta levels, although it did not have a significant effect on apoptotic scores or TNF alpha levels. These results suggest that nilotinib may be effective in patients with IBD. The findings of this study have a number of important implications for the future practice. Therefore, further studies are needed to draw firm conclusions.
IBD are chronic inflammatory disorders of the gastrointestinal tract that have characteristic clinical, pathological, endoscopic and radiological features. TKs are enzymes that play a role in normal cell function, metabolism, growth, differentiation, and apoptosis. TK inhibitors are drugs that block the action of these enzymes. TNBS-induced colitis is a well-established animal model of mucosal inflammation that has been used for over 2 decades in the study of IBD pathogenesis, as well as in preclinical studies.
This is an excellent basic research animal study using a well-known model of TNBS-induced colitis in rats. The authors explored the ability of a tyrosine kinase inhibitor, nilotinib, to treat various clinical (weight determination), laboratory (TNF levels, apoptotic index) and pathological parameters (macroscopic and microscopic pathologic scores, PDGFR levels) and to quantify results. They were basically able to demonstrate mucosal healing effects, clinical improvements and decreased PDGFR alpha and beta levels; however, significant drops in the TNF peaks and apoptotic indices were not clearly shown.
P- Reviewers Actis GC, Ciccone MMM, Kopljar M S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77-97. [PubMed] |
| 2. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1505] [Article Influence: 83.6] [Reference Citation Analysis (2)] |
| 3. | Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;11:CD004118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Sutherland L, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2003;CD000543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Gisbert JP, Linares PM, McNicholl AG, Maté J, Gomollón F. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2880] [Article Influence: 144.0] [Reference Citation Analysis (2)] |
| 8. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 9. | TRUELOVE SC, WITTS LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954;2:375-378. [PubMed] |
| 10. | Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut. 2007;56:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, Bala M, Johanns J, Olson A, Hanauer SB. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, Wiesmann M, Woodman R, Gallagher N. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18:6977-6986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Rhee CK, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim YK, Kim KH, Kim TJ, Kim JW. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration. 2011;82:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Magro F, Costa C. Long-standing remission of Crohn’s disease under imatinib therapy in a patient with Crohn’s disease. Inflamm Bowel Dis. 2006;12:1087-1089. [PubMed] |
| 16. | Cuzzocrea S, McDonald MC, Mazzon E, Mota-Filipe H, Lepore V, Ciccolo A, Terranova ML, Britti D, Caputi AP, Thiemermann C. The tyrosine kinase inhibitor tyrphostin AG 126 reduced the development of colitis in the rat. Lab Invest. 2000;80:1439-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Vilaseca J, Salas A, Guarner F, Rodríguez R, Martínez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990;31:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 923] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 19. | Deremer DL, Katsanevas K, Ustun C. Critical appraisal of nilotinib in frontline treatment of chronic myeloid leukemia. Cancer Manag Res. 2011;3:65-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Chevaux JB, Vavricka SR, Rogler G, Lakatos PL, Schoepfer A, Peyrin-Biroulet L. Mucosal healing with anti-TNF antibodies. Digestion. 2012;86 Suppl 1:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 661] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 22. | Danese S, Sans M, Spencer DM, Beck I, Doñate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011;17:578-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (6)] |
| 24. | Kumagai S, Ohtani H, Nagai T, Funa K, Hiwatashi NO, Shimosegawa H. Platelet-derived growth factor and its receptors are expressed in areas of both active inflammation and active fibrosis in inflammatory bowel disease. Tohoku J Exp Med. 2001;195:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P, Bucci A, Zito A, Caputo P, Albano F. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848-5861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 194] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 27. | Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | De Backer O, Lefebvre RA. Is there a role for imatinib in inflammatory bowel disease? Inflamm Bowel Dis. 2008;14:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Fitzpatrick LR, Meirelles K, Small JS, Puleo FJ, Koltun WA, Cooney RN. A new model of chronic hapten-induced colitis in young rats. J Pediatr Gastroenterol Nutr. 2010;50:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Zhu MY, Lu YM, Ou YX, Zhang HZ, Chen WX. Dynamic progress of 2,4,6-trinitrobenzene sulfonic acid induced chronic colitis and fibrosis in rat model. J Dig Dis. 2012;13:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | D’Argenio G, Farrace MG, Cosenza V, De Ritis F, Della Valle N, Manguso F, Piacentini M. Expression of apoptosis-related proteins in rat with induced colitis. Int J Colorectal Dis. 2004;19:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |