Published online May 7, 2013. doi: 10.3748/wjg.v19.i17.2638
Revised: January 31, 2013
Accepted: February 8, 2013
Published online: May 7, 2013
Processing time: 257 Days and 1 Hours
AIM: To investigate the expression of interleukin (IL)-22 and its related proteins in biopsy specimens from patients with ulcerative colitis (UC) and UC-related carcinogenesis.
METHODS: Biopsy specimens were obtained from patients with inactive (n = 10), mild-to-moderately active (n = 30), severely active (n = 34), initial (n = 30), and chronic UC (n = 44), as well as UC patients with dysplasia (n = 10). Specimens from patients without colonic abnormalities (n = 20) served as controls. Chronic colitis in experimental mice was induced by 2.5% dextran sodium sulfate. The expression levels of IL-22, IL-23, IL-22R1 and phosphorylated STAT3 (p-STAT3) were determined by immunohistochemistry. Bcl-2, cyclin D1 and survivin expression was detected by Western blotting.
RESULTS: Patients with active UC had significantly more IL-22, IL-23, IL-22R1 and p-STAT3-positive cells than the patients with inactive UC and normal controls. Furthermore, IL-22 and related proteins were closely related to the severity of the colitis. The expression of IL-22 and IL-22R1 in the tissue of initial UC was stronger than in that of chronic UC, whereas the expression of p-STAT3 was significantly increased in chronic UC tissues. In dysplasia tissues, the expression level of IL-22 and related proteins was higher compared with controls. Mouse colitis model showed that expression of IL-22, IL-22R1 and IL-23 was increased with time, p-STAT3 and the downstream gene were also remarkably upregulated.
CONCLUSION: IL-22/STAT3 signaling pathway may be related to UC and UC-induced carcinogenesis and IL-22 can be used as a biomarker in judging the severity of UC.
Core tip: This study investigates the expression of interleukin (IL)-22, IL-22R1, IL-23, and STAT3 in ulcerative colitis (UC) and UC-related carcinogenesis (UC-CRC) tissues from human and mouse. The results showed that IL-22 and related proteins were closely related to the severity of colitis, and the expression level of IL-22 and related proteins was higher in dysplasia tissues. IL-22/STAT3 signaling pathway was related to UC and UC-CRC. IL-22 can be used as a biomarker for determining the severity of UC and as an interesting therapeutic target in active UC and UC-CRC.
- Citation: Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY, Sun C, Shi RH. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol 2013; 19(17): 2638-2649
- URL: https://www.wjgnet.com/1007-9327/full/v19/i17/2638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i17.2638
Ulcerative colitis (UC) is a subtype of chronic inflammatory bowel disease (IBD) of the large intestine. The disease is characterized by a dysregulated mucosal immune response. This aberrant immune response leads to the secretion of harmful cytokines that destroy the gastrointestinal tract epithelium, thereby causing further inflammation.
Several inflammatory cytokines have been associated with IBD, including the interleukins IL-1 and IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ. Among the inflammatory cytokines implicated in IBD pathogenesis, much interest has been focused on the recently-identified cytokine IL-22. IL-22 is a member of the IL-10 subfamily; its production is highly dependent on IL-23 in T-helper 17 (Th17)[1-3], Th1[4,5], NK-22[6,7], and CD11c+[8] cells. IL-22 is expressed by the novel Th22 cell lineages[9] and innate lymphoid cells (ILCs)[10]. IL-22-producing ILCs in humans are responsive to IL-23 signaling, as potentially important mediators of IBDs[10]. Th22 and Th17 cells may be implicated in the pathogenesis of several chronic inflammatory and autoimmune diseases such as IBD, psoriasis, ankylosing spondylitis, and rheumatoid arthritis[11-16].
IL-22 signaling is established when the cytokine binds to a heterodimeric receptor complex of IL-22R1 and IL-10R2. Given that IL-10R2 is a ubiquitous protein, cellular IL-22 responsiveness is mainly determined by IL-22R1 expression. IL-22R1 is specifically expressed in nonleukocytic cells such as those of the pancreas, skin, kidney, liver, and colon. IL-22R1 expression is detectable in epithelial cells of these organs, but not in their immune cells[17-20]. Therefore, IL-22 is unique among the cytokines because it cannot mediate autocrine or paracrine functions among leukocytes. Instead, IL-22 transmits information between leukocytes and the nonleukocytic cell compartment.
The STAT3 pathway for transcription activation appears to be a major mode of IL-22 signal transduction. Activated STAT3 is translocated from the cytoplasm to the nucleus, where it regulates genes involved in cell apoptosis, proliferation, migration, and survival. STAT3 has important functions in several autoimmune diseases. IL-22 mediates IL-23-induced acanthosis and dermal inflammation in psoriasis and IBD through the activation of STAT3[21]. When activated by IL-22, STAT3 can aggravate colitis by promoting the expression of inflammatory factors such as IL-8, IFN-γ, and the matrix metalloproteinases (MMPs)[22,23]. Previous studies investigated the role of IL-22 in UC and UC-related carcinogenesis (UC-CRC)[24-26]. These studies revealed that the IL-22/STAT3 pathway is involved in UC pathophysiology and carcinogenesis through the activity of inducible nitric oxide synthase (iNOS), DMBT1, and REGα[24-26]. However, the IL-22-induced phosphorylated STAT3 (p-STAT3) was considered a defense mechanism because it enhanced mucus production and goblet cell replacement in mouse models for acute colitis and wound healing[27-29]. Thus, the role of the IL-22/STAT3 signaling pathway in UC remains unclear. The current study investigates the significance of IL-22 and the IL-22/STAT3 signaling pathway in UC and UC-CRC, as well as its value as a therapeutic target for both diseases.
Colon biopsy via endoscopy was performed on 74 patients with UC, including 31 females and 43 males (median age, 45.9 years; range, 17-87 years). The controls included 6 females and 14 males (mean age, 45.6 years; range, 33-55 years). All samples were obtained at the First Affiliated Hospital of Nanjing Medical University from 2009 to 2011.
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Written informed consent was obtained from each patient. The diagnosis of UC was based on clinical, endoscopic, and histological findings. The patient characteristics and histological data are summarized in Table 1.
| Normal control n = 20 | Histological activity of UC | Types of UC | UC with dysplasia n = 10 | ||||
| Inactive n = 10 | Mild-moderate n = 30 | Severe n = 34 | Initial n = 30 | Chronic n = 44 | |||
| Gender (n) | |||||||
| Male | 14 | 5 | 16 | 22 | 16 | 27 | 6 |
| Female | 6 | 5 | 14 | 12 | 14 | 17 | 4 |
| Mean age3, yr | 40.6 ± 12.5 | 41.2 ± 13.3 | 44.7 ± 13.2 | 43.2 ± 17.1 | 42.8 ± 17.3 | 46.5 ± 13.6 | 44.7 ± 16.6 |
| Mean duration of disease3, yr | - | 6.14 ± 4.2 | 4.3 ± 2.3 | 3.7 ± 3.1 | 0.8 ± 0.6 | 5.1 ± 0.61 | 10.5 ± 1.42 |
| Extent of disease (n) | |||||||
| Extensive colitis | - | 0 | 8 | 19 | 8 | 19 | 3 |
| Left-side colitis | - | 4 | 13 | 9 | 13 | 13 | 4 |
| Proctitis | - | 6 | 9 | 6 | 9 | 12 | 3 |
| Treatment (n) | |||||||
| Aminosalicylates | - | 8 | 25 | 20 | 16 | 35 | 5 |
| Corticosteroids | - | 4 | 5 | 18 | 4 | 15 | 3 |
| Immunosuppressive agent | - | 0 | 0 | 1 | 0 | 1 | 0 |
| Biological agent | - | 0 | 0 | 0 | 0 | 0 | 0 |
| None | - | 3 | 2 | 0 | 10 | 5 | 2 |
The mouse experiments were conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the United States National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University. Male ICR mice were purchased and maintained at the Centre of Animal Facility, Nanjing Medical University. The mice were sacrificed by cervical dislocation. All efforts were made to minimize suffering. Chronic colitis was induced by administering 2.5% dextran sodium sulfate (DSS; ICN Biomedicals Inc., Irvine, CA, United States) to the drinking water in three seven-day cycles, which had a five-day recovery period after each cycle. During the recovery period, mice drank only normal water. The age-matched control mice received only normal drinking water throughout the entire study.
All the tissues were fixed overnight in 4% paraformaldehyde at 4 °C, processed, and cut into 5 μm-thick sections. The sections were then deparaffinized in xylene and rehydrated. Endogenous peroxidase activity was blocked by 3% H2O2 for 10 min at room temperature. Antigen retrieval was performed by 15 min of boiling in the preheated buffer (10 mmol/L of sodium citrate, pH = 6.0) at 200 W in a microwave. The slides were transferred to a humidor and blocked by incubating in 5% normal goat serum at room temperature for 1 h. The polyclonal rabbit antibodies used in this study included anti-p-STAT3 tyrosine 727, anti-IL-22, anti-IL-22R1, and anti-IL-23 (ab30647, ab18499, ab5984 and ab115759, respectively); these antibodies were diluted in 5% normal goat serum at ratios of 1:200, 1:200, 1:200 and 1:400, respectively. The sections were incubated in the respective antibodies overnight at 4 °C. The slides were subsequently incubated in the secondary antibody goat anti-rabbit IgG-biotin (B8895; Sigma-Aldrich, St. Louis, MO, United States) at room temperature for 40 min. The sections were incubated in the ABC-peroxidase solution (UltrasensitiveTM S-P kit, kit 9719; Maixin-Bio, China) for 30 min at room temperature, followed by counterstaining with hematoxylin. The immunohistochemistry (IHC) results were analyzed using Image Pro-Plus.
Proteins were extracted from the mouse tissues and quantified using a commercial protein assay (Bio-Rad Laboratories, CA, United States). The protein samples (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Immunoblot analysis was conducted using antibodies against total STAT3, p-STAT3 (S727), Bcl-2, cyclin D1, and survivin (Abcam Inc, MA, United States). The results were visualized using the chemiluminescent Pierce ECL Substrate Western blotting detection system (Thermo Scientific, IL, United States) and exposure to autoradiography film (Kodak XAR film).
The results were expressed as the mean ± SD. The two groups were compared using the Student’s t test or the Mann-Whitney U test, as appropriate. All statistical analyses were performed using the SPSS statistical software (version 13.0). P < 0.05 was considered to be statistically significant.
As shown in Table 1, we collected biopsy specimens from 20 healthy controls, 10 patients with inactive UC, 64 patients with active UC (including 30 patients with mild-to-moderate active UC and 34 patients with severely active UC), 30 patients with initial UC attacks, 44 patients with chronic UC, and 10 UC patients with dysplasia. The duration of disease was significantly longer in the groups with chronic UC and UC with dysplasia than in the other groups (P < 0.05). No other significant differences were observed among the other groups, regardless of the treatment used.
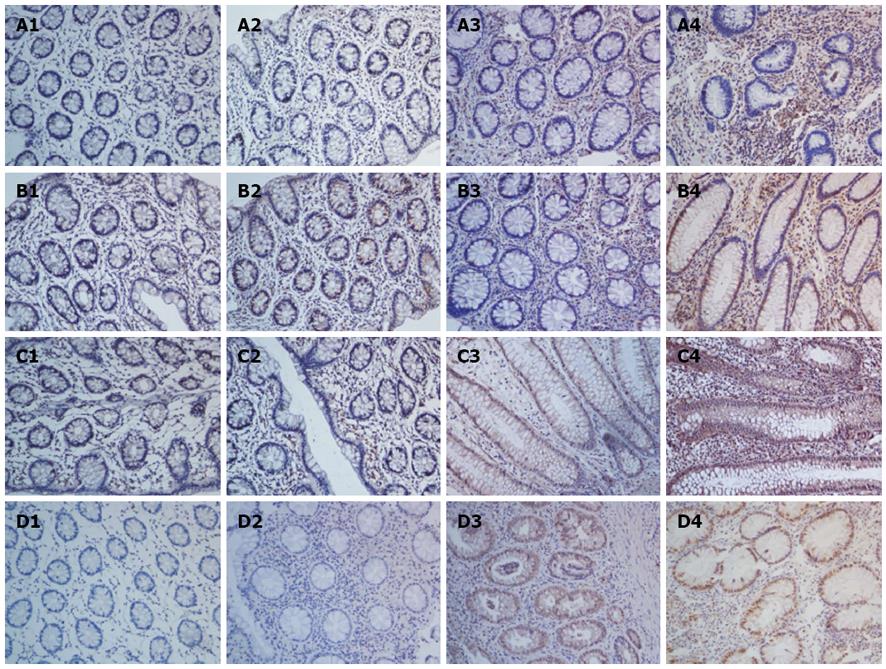
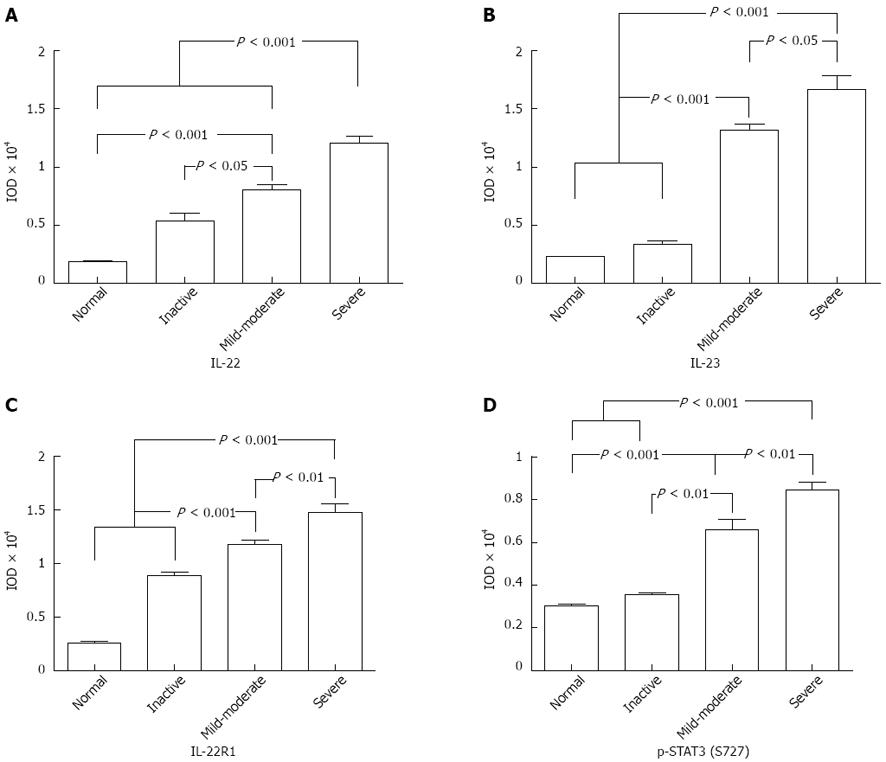
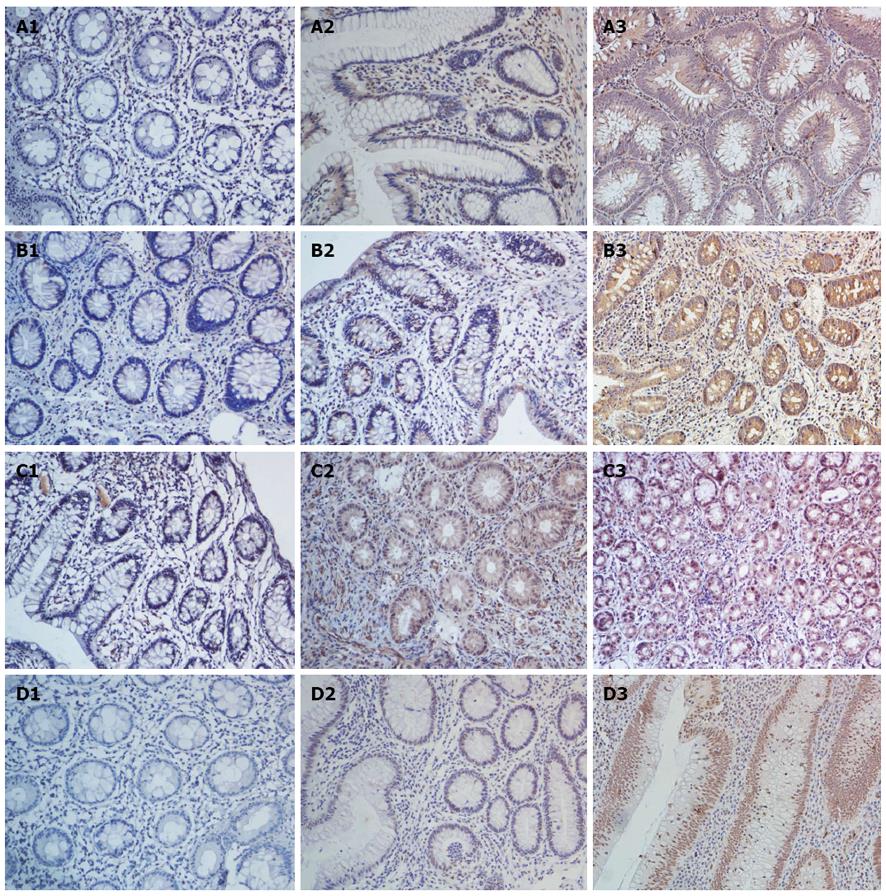
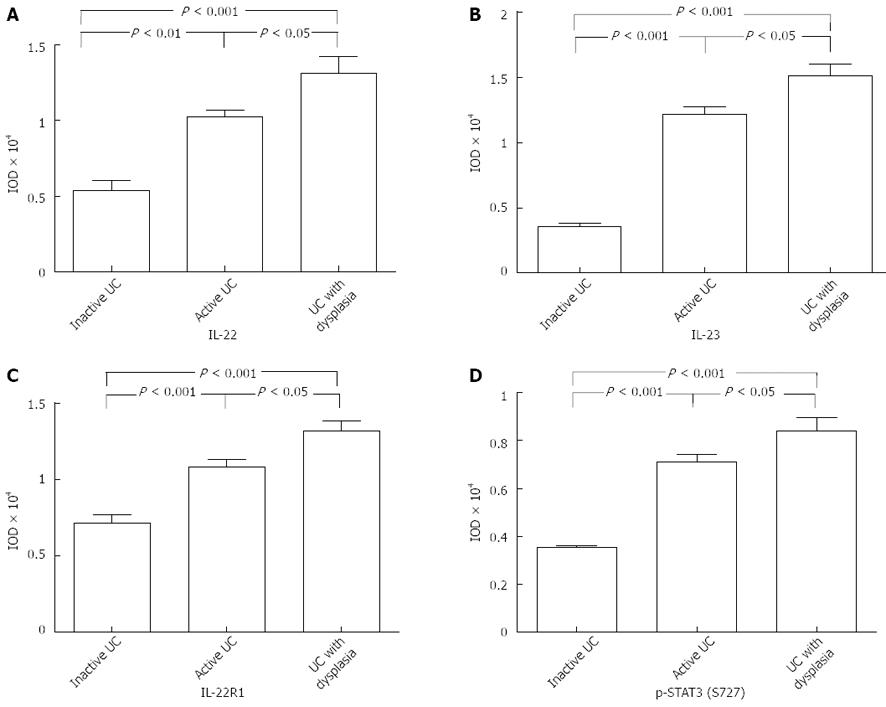
Immunohistochemical analysis showed that the IL-22 protein was mainly expressed in inflammatory cells of the colonic lamina propria, but not in the normal controls (Figure 1A). Tissues with mild-moderate and severe UC had significantly higher expression levels than those with inactive UC and normal colon tissues (mild-moderate vs normal, P < 0.001; mild-moderate vs inactive, P = 0.02, P < 0.05; severe vs normal, P < 0.001; severe vs inactive, P < 0.001; Figure 2A). Moreover, significantly more IL-22-positive cells were present in tissues of severe UC than in those of mild-moderate UC (severe vs mild-moderate, P < 0.001; Figure 2A). The results indicate that the expression of IL-22 was related to UC severity.
Previous studies have demonstrated that IL-22 production is highly dependent on IL-23 in Th17, NK-22, and lymphoid tissue-inducer cells[1-3,6,7]. Thus, we analyzed IL-23 expression in UC tissues using IHC. The results indicated that IL-23 was significantly highly upregulated in active UC, as compared with inactive UC and the controls (mild-moderate vs normal, P < 0.001; mild-moderate vs inactive, P < 0.001; severe vs normal, P < 0.001; severe vs inactive, P < 0.001; Figure 2B). Similarly, the IL-23 expression was stronger with severe UC than with mild-moderate UC (severe vs mild-moderate, P = 0.01, P < 0.05; Figure 2B). The positive region of IL-23 in UC was mostly confined to the intestinal epithelial cells (IECs) and inflammatory cells of the colonic lamina propria (Figure 1B).
We identified IL-22R1, another key molecule that is necessary for signal transmission. IL-22R1 was mainly localized in the IECs (Figure 1C). IL-22R1 was overexpressed in active UC (mild-moderate vs normal, P < 0.001; mild-moderate vs inactive, P = 0.0002, P < 0.001; severe vs normal, P < 0.001; severe vs inactive, P = 0.0002, P < 0.001; severe vs mild-moderate, P = 0.007, P < 0.01; Figure 2C).
Based on the downstream effects of IL-22, the activation of STAT3 was determined by staining with p-STAT3 at the S727 residue. IHC analysis showed that in the colon tissues resected from patients with UC, the p-STAT3 (S727) protein was mainly expressed in the nucleus of epithelial cells (Figure 1D). Its expression was significantly upregulated in the active UC tissues, particularly in severe UC (severe vs normal, P < 0.001; severe vs inactive, P < 0.001; severe vs mild-moderate, P = 0.0045, P <0.01; mild-moderate vs normal, P < 0.001; mild-moderate vs inactive, P = 0.0017, P < 0.01; Figure 2D).
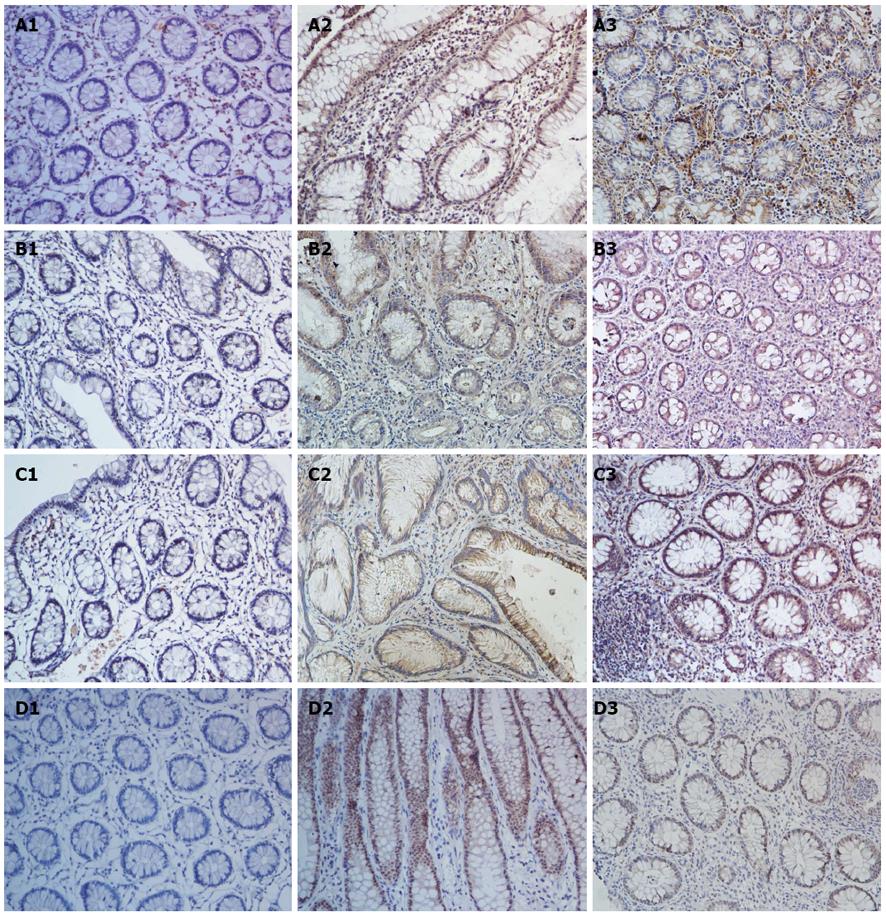
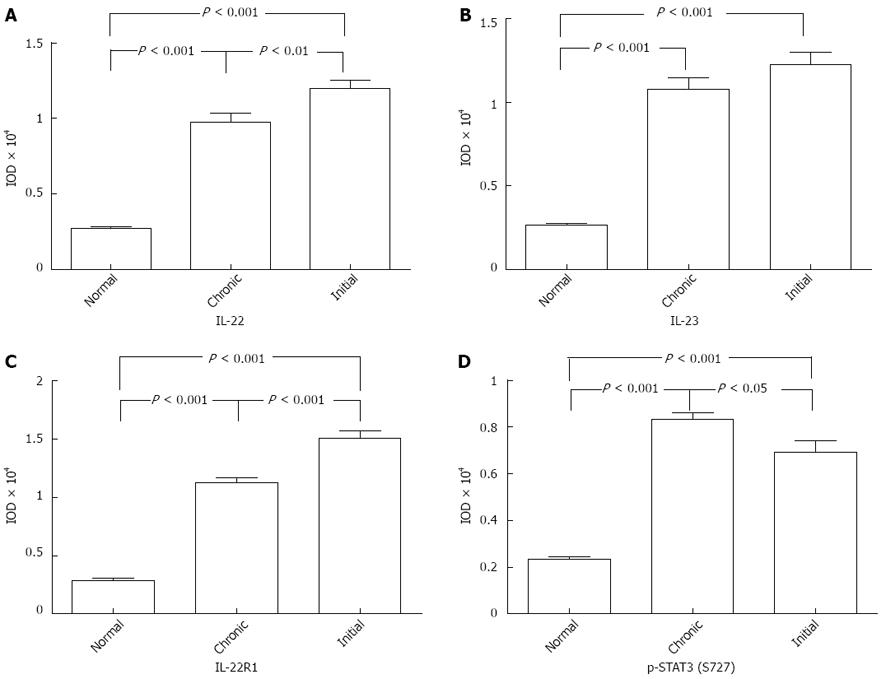
According to the clinical diagnostic standards, we classified UC into two types: initial and chronic UC. IHC indicated that IL-22 was significantly upregulated in the initial UC tissues than in the chronic UC tissues (initial vs chronic, P = 0.0077, P < 0.01; Figures 3A and 4A).
Similar to IL-22, IL-22R1 was more strongly expressed in the tissues of initial UC than in those of chronic UC (initial vs chronic, P < 0.001; Figures 3C and 4C). By contrast, the number of p-STAT3-positive cells was significantly higher in chronic UC tissues than in initial UC (chronic vs initial, P = 0.03, P < 0.05; Figures 3D and 4D). However, no significant differences were detected in terms of the IL-23 expression in these two groups (Figures 3B and 4B).
A positive correlation exists between the IL-22-positive cells and the severity of colitis in patients with UC. We investigated IL-22 expression in biopsy specimens from patients with UC-CRC (dysplasia) and analyzed the IL-22 levels in UC with dysplasia (Figure 5A). Significantly more IL-22-positive cells were observed in the dysplasia group than in the inflammatory group (P = 0.02; Figure 6A).
Given that IL-22 was highly upregulated in UC tissue with dysplasia, we studied the expression of the receptor IL-22R1, its upstream IL-23, and its downstream p-STAT3 (S727) in UC tissues with dysplasia, as compared with active and inactive UC tissues. The expression levels of IL-22R1, IL-23, and p-STAT3 were significantly higher in UC tissues with dysplasia than in the control group (IL-22R1: dysplasia vs active, P = 0.02; IL-23: dysplasia vs active, P = 0.01; p-STAT3: dysplasia vs active, P = 0.02; Figure 6B-D). The increased expression was strictly found at the dysplastic tissues of the patients (Figure 5B-D).
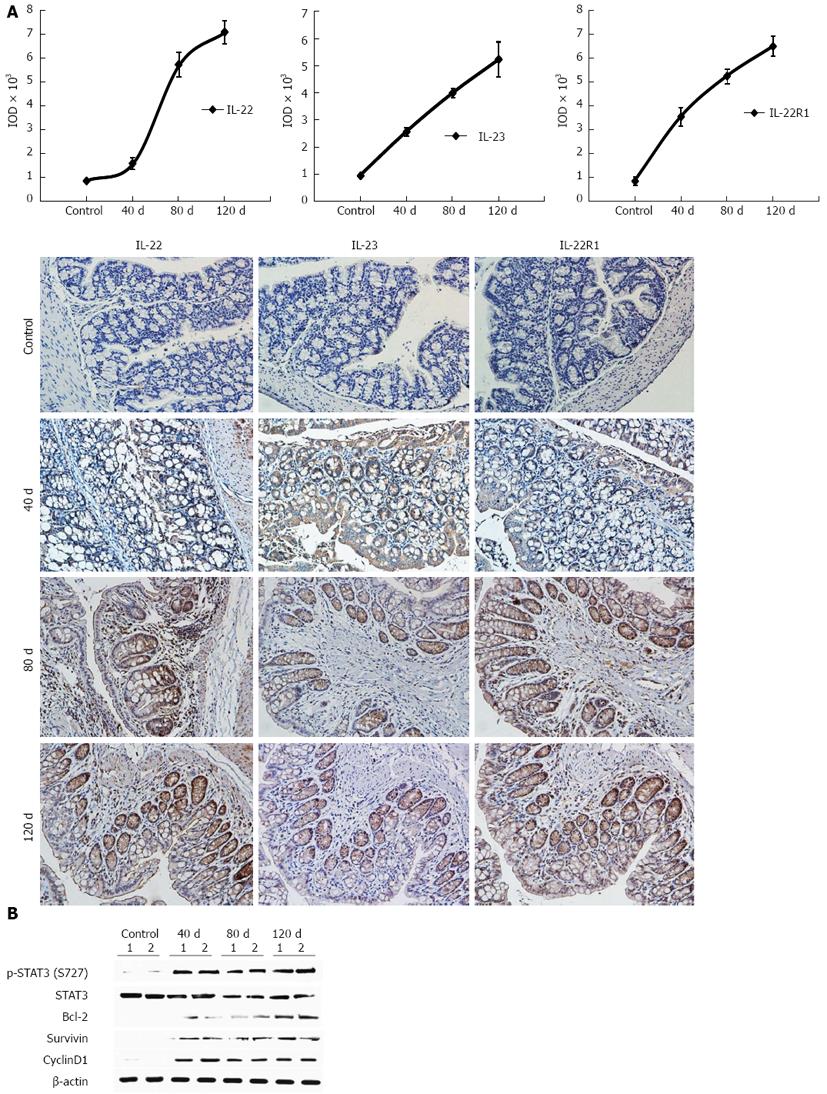
We induced experimental colitis by treating mice with DSS to study the role of IL-22 and its related proteins in the disease. Dynamic IL-22, IL-23, and IL-22R1 expression levels were investigated by IHC. p-STAT3 activity and its downstream gene expression were confirmed by Western blotting analysis at different time points (at days 40, 80, and 120). The expression levels of IL-22, IL-22R1, and IL-23 were increased with time (Figure 7A). STAT3 activation and the activity of its downstream cell proliferation-related genes, such as Bcl-2, cyclin D1, and survivin, were also investigated. All these genes had sustained expression over time (Figure 7B).
IBDs such as Crohn’s disease and ulcerative colitis are chronic inflammatory disorders of the gastrointestinal tract. Although their etiology is not completely understood, initiation and aggravation of the inflammatory process seem to be related to a massive local mucosal immune response. IL-22 belongs to the IL-10 family of cytokines; it has recently been shown to be preferentially expressed by Th17 and Th22 cells. These cells have been identified in the pathogenesis of certain chronic inflammatory diseases, including colitis, psoriasis, and rheumatoid arthritis. IL-22 targets innate immune pathways because of the restricted expression of IL-22 receptors on nonleukocytic cells, such as epithelial cells, keratinocytes, and hepatocytes; however, it does not recognize T- or B-cells[17-20]. Studies using genetically-engineered mice have demonstrated that epithelial STAT3 activation in DSS-induced colitis is dependent on IL-22, rather than on IL-6. Both IL-22 and STAT3 activation in epithelial cells is important for wound-healing, as demonstrated by in vivo experiments[27]. Sugimoto et al[28] found that the IL-22/STAT3 pathway contributes to the rapid amelioration of local intestinal inflammation by enhancing the production of membrane-bound mucins (Muc1, -3, -10, and -13) in a mouse model of acute colitis. However, IL-22 is also considered an inflammatory driver in IBD by acting on human colonic subepithelial myofibroblasts to stimulate secretion of proinflammatory cytokines such as MMPs, IL-1, IL-8, and INF-γ[30]. Highly elevated serum levels of IL-22 were correlated with disease severity in patients with Crohn’s disease (CD)[17]. Colitis mouse models have indicated that highly elevated IL-22 expression may directly or indirectly induce inflammation[31]. Moreover, the IL-22/STAT3 signaling pathway is important in inflammation and carcinogenesis during UC via its upregulation of iNOS and MMP production, respectively[23,24]. Here, we demonstrate that IL-22 contributes to the inflammatory severity of UC and UC-CRC by activating STAT3 in IECs.
The UC microenvironment is composed of IECs, macrophages, immunocytes, and so on. The interactions among these cells involve their secreted cytokines and consist of a positive feedback loop with persistent activation of the STAT3-enabling progression of UC. Similar to IL-23 and IL-22R1, an IL-22 positive feedback loop in the UC microenvironment was demonstrated in this study. Our results indicated the IL-22 overexpression in the inflammatory cells of the colonic lamina propria in UC tissues. Moreover, the sustained activation of STAT3 signaling in IECs was verified. Simultaneously, IL-22R1 expression was enhanced in IECs, thereby ensuring the transmission of the IL-22 signal.
The STAT3-regulated proinflammatory cytokine IL-23[32] was likewise overexpressed in human UC. IL-23 activates innate immune cells to secrete pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, as well as maintains the expansion of Th17 cells that express IL-22[33].
IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression[17]. We demonstrated that IL-22 is more highly expressed in active UC than in inactive UC and the normal control. Thus, the increased IL-22 signaling in active IBD supports the potential of an IL-22 signaling blockade as a therapeutic strategy for IBD. Furthermore, IL-23, IL-22, STAT3, and IL-22R1 are closely related to the colitis severity. Thus, positive feedback loops can further exacerbate inflammation. If left unchecked, these pathways may lead to the chronic immune pathology that is characteristic to IBD. Therefore, IL-22 could be used as a biomarker for determining UC severity.
STAT3 is a transcription factor that is activated by the binding of several cytokines, hormones, and growth factors to their respective receptors, including IL-22, IL-6, IL-23, and IL1-β[34]. We propose that STAT3 signaling is disrupted in chronic inflammation and carcinogenesis. The expression levels of total STAT3 and p-STAT3 in patients with UC were persistently elevated, with a positive correlation to the degree of inflammation[22]. Moreover, STAT3 is constitutively activated in a variety of human cancers, including colorectal cancer. This transcription factor is crucial in cancer cells because it regulates the transcription of genes involved in cell survival, apoptosis, and other cellular processes. Morikawa et al[34] found that p-STAT3 is significantly associated with poor prognosis in a data set of 724 colorectal cancers. Furthermore, STAT3 signaling has been reported to induce cancer-promoting inflammation and to inhibit antitumor immunity[35,36]. IL-6, a main activator of STAT3, has been proven to be important for promoting UC and UC-CRC[37]. IL-22, another inflammatory factor that predominantly activates STAT3, has been verified in the chronic hepatitis and hepatocellular carcinoma (HCC) microenvironment; it induces tumor growth, inhibits apoptosis, and promotes metastasis via STAT3 activation[38]. Consistent with other studies on chronic hepatitis and HCC, our results demonstrated that the expression levels of IL-23, IL-22, IL-22R1, and STAT3 are consistently highly expressed in human UC tissues. IL-22 and p-STAT3, in particular, are more constitutively upregulated in the chronic colitis than in the controls. During the chronic phase of the DSS-induced mice colitis model, IL-22, IL-22R1, and IL-23 were highly expressed over time. Likewise, p-STAT3 and its downstream Bcl-2, cyclin D1, and survivin genes were remarkably upregulated. Furthermore, the expression of IL-22, p-STAT3, IL-23, and IL-22R1 were significantly elevated in human UC tissues with dysplasia, as compared with inactive and active UC tissues. Our results showed that the expression levels of IL-22 and IL-22R1 were more highly elevated in the acute colitis phase, which is in accordance with earlier studies[27,28]. We propose that IL-22 may ameliorate intestinal inflammation by enhancing mucus production and goblet cell replacement in the early phase of inflammatory response. However, the persistent expression of IL-22 during the chronic phase of UC can strongly activate STAT3 phosphorylation in IECs, which is associated with the progression of human UC and UC-CRC[37] by upregulating genes for cell proliferation, anti-apoptosis, and survival.
In conclusion, our study provides clinical evidence that the IL-22/STAT3 signaling pathway is related to UC and UC-CRC. Moreover, IL-22 can be used as a biomarker for determining the severity of UC and as an interesting therapeutic target in active UC and UC-CRC.
It has been previously reported that interleukin (IL)-22, one of the cytokines secreted by Th17 cells, promotes a protective and inflammatory effect in inflammatory bowel disease (IBD) through STAT3 signaling activation.
The IL-22/STAT3 signaling pathway plays an important role in several autoimmune diseases, such as psoriasis, IBD, and so on. When activated by IL-22, STAT3 can aggravate colitis by promoting the expression of inflammatory factors such as IL-8, interferon-γ, and matrix metalloproteinases. However, some studies have found that the IL-22 induced phosphorylation of STAT3 is a defense mechanism that enhances mucus production and goblet cell replacement in mouse models of acute colitis and wound-healing. Thus, the role of the IL-22/STAT3 signaling pathway in ulcerative colitis (UC) remains unclear.
IL-22 may ameliorate intestinal inflammation by enhancing mucus production and goblet cell replacement during the early phase of the inflammatory response. However, the persistent expression of IL-22 in chronic phase of UC can strongly activate the phosphorylation of STAT3 in intestinal epithelial cells. p-STAT3 is associated with the progression of human UC and UC-related carcinogenesis (UC-CRC) because it upregulates the genes for cell proliferation, anti-apoptosis, and survival.
The authors report about the expression of IL-22, IL-22R1, IL-23, and STAT3 in biopsies of human UC and UC-related carcinogenesis. The study is well performed; it gives an overview on the expression of the before-mentioned factors in active and chronic UC and correlates IL-22 expression with disease severity.
P- Reviewer Guo JM S- Editor Zhai HH L- Editor Ma JY E- Editor Zhang DN
| 1. | Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1338] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 2. | Spolski R, Leonard WJ. Cytokine mediators of Th17 function. Eur J Immunol. 2009;39:658-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol. 2008;38:3265-3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397-5402. [PubMed] |
| 6. | Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1050] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 7. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 8. | Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1549] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 9. | Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573-3585. [PubMed] |
| 10. | Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 11. | Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 12. | Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, Hu NW, Ma DX, Li ZF, Yang Q. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577-2587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 14. | Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652-5661. [PubMed] |
| 15. | Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 17. | Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827-G838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 503] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1118] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 21. | Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 22. | Li F, Zou Y, Li X. Up-regulation of signal transducer and activator of transcription-3 is associated with aggravation of ulcerative colitis. Surgeon. 2010;8:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schütz A, Kovacic B, Friedrich K. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;9:279-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Radzikowski A, Banaszkiewicz A, Łazowska-Przeorek I, Grzybowska-Chlebowczyk U, Woś H, Pytrus T, Iwańczak B, Kowalska-Duplaga K, Fyderek K, Gawrońska A. Immunogenecity of hepatitis A vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Ziesché E, Bachmann M, Kleinert H, Pfeilschifter J, Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006-16015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 791] [Cited by in RCA: 858] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 28. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 566] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 29. | Yamamoto-Furusho JK, Miranda-Pérez E, Fonseca-Camarillo G, Sánchez-Muñoz F, Dominguez-Lopez A, Barreto-Zuñiga R. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, Haruna K, Kume S, Takeya M, Ogawa M. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696-705. [PubMed] |
| 31. | te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045-4051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 37. | Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227-235. [PubMed] |
| 38. | Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |