Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2860
Revised: November 5, 2010
Accepted: November 12, 2010
Published online: June 21, 2011
AIM: To evaluate the impact of the ITGA2 gene polymorphism on gastric cancer risk.
METHODS: A hospital-based case-control study was conducted, including 307 gastric cancer patients and 307 age- and gender-matched control subjects. The genotypes were identified by polymerase chain reaction-restriction fragment length polymorphism assay.
RESULTS: The frequencies of the wild and variant genotypes in cases were significantly different from those of controls (P = 0.019). Compared with individuals with the wild genotype CC, subjects with the variant genotypes (CT + TT) had a significantly higher risk of gastric cancer (adjusted odds ratio = 1.57, 95% CI = 1.13-2.17, P = 0.007). In stratified analyses, the elevated gastric cancer risk was especially evident in older individuals aged > 58 years, nonsmokers and rural subjects. Further analyses revealed that the variant genotypes were associated with poor tumor differentiation and adjacent organ invasion in the sub-analysis of gastric cancer patients.
CONCLUSION: The ITGA2 gene C807T polymorphism may be associated with an increased risk of gastric cancer, differentiation and invasion of gastric cancer.
-
Citation: Chen J, Liu NN, Li JQ, Yang L, Zeng Y, Zhao XM, Xu LL, Luo X, Wang B, Wang XR. Association between
ITGA2 C807T polymorphism and gastric cancer risk. World J Gastroenterol 2011; 17(23): 2860-2866 - URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2860.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2860
Gastric cancer remains a major public health issue as the fourth most common cancer type and the second leading cause of cancer death worldwide[1,2]. Nearly half of the gastric cancer cases occur in China[3]. Although the cause of gastric cancer is largely unknown, it has been shown that diet, tobacco smoking, alcohol, gastroesophageal reflux and Helicobacter pylori (H. pylori) infection are associated with the risk of this cancer[4-7]. As genetic polymorphisms are responsible for the inter-individual variation and diversity, they have been recently considered as the main genetic elements involved in the development of common and complex diseases, including various cancers. Like many malignancies, it is believed that gastric cancer is the result of interactions between environmental factors and genetic factors[8]. Our previous epidemiological studies also provided the evidence that genetic polymorphisms were associated with the risk of gastric cancer[9-12].
Integrins are members of a family of cell-surface heterodimeric proteins that mediate cell-matrix and cell-cell interactions. The 18 α-subunits and 8 β-subunits form together at least 25 different integrins, each pair being specific for a unique set of ligands. It has been demonstrated that integrins may play a crucial role in carcinogenesis, tumor behavior and metastasis[13,14]. Several integrins such as α2β1, αIIbβ3 and αvβ3 are considered as key factors for cancer development and progression. Integrin α2β1, also known as platelet glycoprotein Ia-IIa, is expressed by epithelial cells, and its level of expression in tumor cells is associated with motility, invasiveness and cellular differentiation[15-17]. Several studies have shown that integrin α2β1 expression is closely associated with invasion and metastasis of gastric cancer[18-21].
The integrin, α2 gene (ITGA2) is located on chromosome 5q23-31. A silent change in the coding region at nucleotide 807 (TTT/TTC at codon Phe253) has been identified. The C807T single nucleotide polymorphism (NCBI SNP ID: rs1126643) of the ITGA2 gene was associated with the integrin α2β1 density. The genotype 807 TT was associated with a higher receptor density and the genotype 807 CC with a lower density, whereas heterozygous individuals expressed intermediate receptor levels[22,23].
Recent studies indicated that the ITGA2 gene C807T polymorphism was associated with various diseases, including stroke, retinal vein occlusion, acute coronary syndrome, colorectal cancer, and breast cancer[24-29]. To the best of our knowledge, there has been no study that assessed the association between the polymorphism and gastric cancer risk.
Given that the roles of ITGA2 in the progression of gastric cancer as well as the effect of the polymorphism in ITGA2 gene on the receptor function, it is plausible that the polymorphism may be associated with the risk of gastric cancer. To test the hypothesis, we performed a hospital-based case-control study in a Chinese population.
This hospital-based case-control study consisted of 307 consecutive inpatients with histologically confirmed gastric cancers without synchronous and/or metachronous secondary malignancy and a population-based and sex- and age-matched 307 cancer-free inpatients as controls. All subjects were recruited between March 2005 and November 2009 from the patients who were admitted to the First Affiliated Hospital of Nanjing Medical University. The most common causes for hospitalization in the control subjects were hernias, appendicitis, hydrocele, cholecystitis and cataract. All subjects were of unrelated Han nationality from Jiangsu Province or its surrounding regions. Information on age, gender, smoking status, residence (urban or rural), body weight and personal medical history was collected by questionnaire. Individuals who formerly or currently smoked ≥ 10 cigarettes per day for at least 2 years were defined as smokers. Depth of tumor invasion and local lymph node status were classified according to the TNM classification criteria of International Union Against Cancer[30]. Differentiation was graded according to World Health Organization classification. The study was approved by the Ethics Committee of Nanjing Medical University First Affiliated Hospital and informed consent was obtained from all the participating subjects.
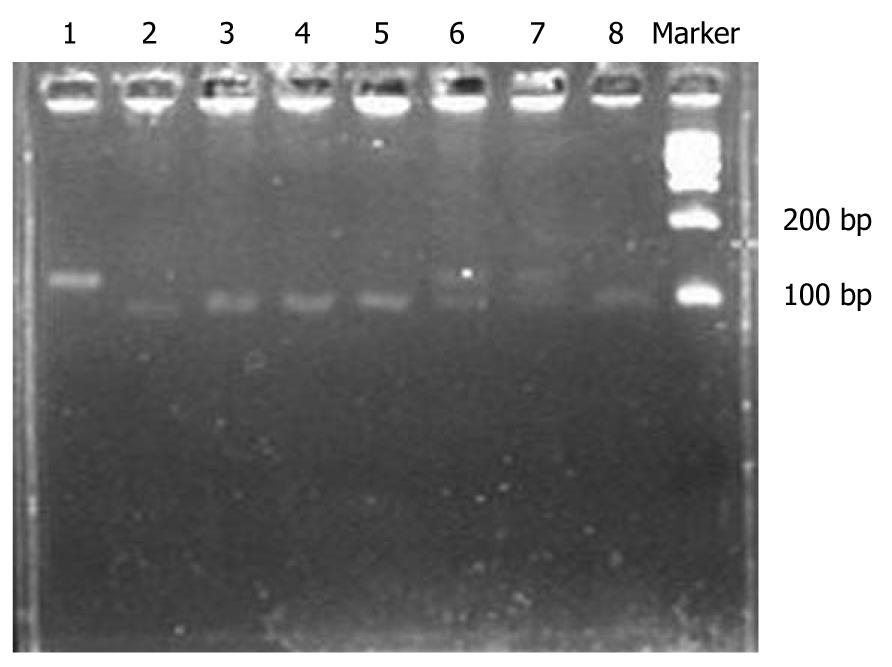
The protocol for genomic DNA extraction was described in our previous study[9]. The polymerase chain reaction (PCR)-restriction fragment length polymorphism assay was used to identify the ITGA2 C807T genotypes. The PCR was performed in a total volume of 20 μL reaction mixtures containing 2 μL 10 × PCR buffer (MBI Fermentas), 1.75 mmol/L MgCl2, 0.25 μmol/L each primer (forward 5'-GTGTTTAACTTGAACACATAT-3', reverse 5'-ACCTTGCATATTGAATTGCTT-3'), 0.15 mmol/L dNTP, 1 unit Taq polymerase (MBI fermentas) and 150 ng genomic DNA. The amplification protocol is as follows: primary denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, then a final elongation at 72°C for 5 min. The 115 bp PCR products including the polymorphic site were digested at 65°C for 12 h, using restriction enzyme Taq I (MBI Fermentas) and then separated on a 3% ethidium bromide-stained agarose gel. The wild-type homozygotes (CC) produced two bands at 92 and 23 bp, while the variant homozygotes (TT) produced one band at 115 bp, and the heterozygous (CT) produced three bands at 115, 92 and 23 bp (Figure 1). To control the quality of genotyping, all assays were conducted by two researchers separately in a blind fashion. In addition, a 10% masked samples were randomly selected and retested, and the reproducibility was 100%.
Statistical analyses were conducted using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and P < 0.05 was considered statistically significant. Quantitative variables departing from the normal distribution including age and weight were summarized as median and analyzed by Mann-Whitney rank sum test. Pearson’s χ2 test was used to compare the difference in the distribution of categorical variables and genotype frequencies between cases and controls. The Hardy-Weinberg equilibrium of the ITGA2 genotypes was estimated for cases and controls by a goodness-of-fit χ2 test. Odds ratio (OR) and 95% CI were calculated to evaluate the association between the polymorphism and the risk of gastric cancer. Carriers of the wild genotype CC were used as the reference. The crude OR was obtained using the Woolf approximation method and the adjusted OR was calculated by unconditional logistic regression method, with adjustment for age, sex, smoking status, residence, hypertension and diabetes.
A total of 614 subjects (307 cases and 307 controls) were analyzed. Baseline demographic characteristics of the study groups are shown in Table 1. The age distribution and proportion of males were quite similar due to the fact that we selected the age- and gender-matched subjects. The two groups were similar with respect to residence, history of hypertension and diabetes. Nevertheless, compared with controls, gastric cancer patients had a lower body-weight (P = 0.001) and more smokers were found among gastric cancer cases than among the controls (26.71% vs 17.26%, P = 0.005).
| Characteristics | Cases (n = 307) | Controls (n = 307) | P value |
| Gender (male) | 231 (75.2) | 231 (75.2) | 1.000 |
| Age1 (yr), (range) | 59 (50-68) | 58 (49-66) | 0.145 |
| Weight1 (kg), (range) | 62 (55-70) | 65 (57-72.75) | 0.001 |
| Hypertension | 65 (21.17) | 59 (19.22) | 0.546 |
| Diabetes | 17 (5.54) | 24 (7.82) | 0.262 |
| Smoking | 82 (26.71) | 53 (17.26) | 0.005 |
| Residence | |||
| Rural | 139 (45.28) | 139 (45.28) | 1.000 |
| Urban | 168 (54.72) | 168 (54.72) |
Table 2 shows the frequency distributions of the genotypes and their association with gastric cancer risk by unadjusted OR, adjusted OR and 95% CI. The genotype distributions in cases and controls were consistent with those from the Hardy-Weinberg equilibrium model (P = 0.988, P = 0.675, respectively). The frequencies of the ITGA2 genotype were significantly different between gastric cancer cases and controls (P = 0.019). Compared with the control group, T allele frequency was significantly higher in the case group (P = 0.024). With the wild genotype CC as reference, we found that the CT genotype was associated with an increased risk of gastric cancer (adjusted OR = 1.54, 95% CI = 1.10-2.18, P = 0.013). Individuals with the variant genotypes (CT + TT) had a 1.57-fold increased risk of developing gastric cancer (adjusted OR = 1.57, 95% CI = 1.13-2.17, P = 0.007).
| ITGA2 genotype | Cases1 | Controls1 | Crude OR (95% CI) | P value | Adjusted OR2 (95% CI) | P value |
| Overall | 307 | 307 | ||||
| CC | 141 (45.93) | 170 (55.37) | 1.00 | 1.00 | ||
| CT | 135 (43.97) | 113 (36.81) | 1.44 (1.03-2.01) | 0.033 | 1.54 (1.10-2.18) | 0.013 |
| TT | 31 (10.10) | 24 (7.82) | 1.56 (0.87-2.78) | 0.133 | 1.62 (0.90-2.91) | 0.112 |
| CT + TT | 166 (54.07) | 137 (44.63) | 1.46 (1.06-2.01) | 0.019 | 1.57 (1.13-2.17) | 0.007 |
| C allele | 417 (67.92) | 453 (73.78) | ||||
| T allele | 197 (32.08) | 161 (26.22) |
As shown in Table 3, stratified analyses were performed by the median age of controls (58 years), sex, smoking status, and residence. The elevated risk of gastric cancer associated with the variant genotypes was noteworthy in subjects aged > 58 years (adjusted OR = 1.88, 95% CI = 1.17-3.03, P = 0.010), but not in subjects aged ≤ 58 years. In non-smoking subjects, the variant genotypes were associated with a 51% increased risk of gastric cancer (adjusted OR = 1.51, 95% CI = 1.05-2.18, P = 0.028), whereas the correlation was not statistically significant in smoking subjects. When stratified by residence, the elevated risk was evident in rural subjects (adjusted OR = 2.35, 95% CI = 1.42-3.90, P = 0.001), but not in urban subjects. No statistically significant difference was observed in the association of the polymorphism and susceptibility to gastric cancer between males and females.
| Variable | (CT + TT)/CC | Crude OR (95% CI) | P value | Adjusted OR1 (95% CI) | P value | |
| Cases | Controls | |||||
| Age (yr), (median) | ||||||
| ≤ 58 | 81 (26.4)/68 (22.1) | 80 (26)/85 (27.7) | 1.27 (0.81-1.97) | 0.298 | 1.31 (0.83-2.06) | 0.247 |
| > 58 | 85 (27.7)/73 (23.8) | 57 (18.6)/85 (27.7) | 1.74 (1.10-2.75) | 0.018 | 1.88 (1.17-3.03) | 0.010 |
| Sex | ||||||
| Females | 45 (14.7)/31 (10.1) | 38 (12.4)/38 (12.4) | 1.45 (0.76-2.76) | 0.255 | 1.52 (0.73-2.93) | 0.206 |
| Males | 110 (35.8)/121 (39.4) | 99 (32.2)/132 (43) | 1.21 (0.85-1.73) | 0.304 | 1.29 (0.82-2.01) | 0.287 |
| Smoking status | ||||||
| Smokers | 40 (13)/42 (13.7) | 19 (6.2)/34 (11.1) | 1.70 (0.84-3.46) | 0.141 | 1.87 (0.89-3.94) | 0.100 |
| Non-smokers | 126 (41)/99 (32.3) | 118 (38.4)/136 (44.3) | 1.47 (1.02-2.10) | 0.037 | 1.51 (1.05-2.18) | 0.028 |
| Residence | ||||||
| Urban | 89 (29)/79 (25.7) | 84 (27.4)/84 (27.4) | 1.13 (0.73-1.73) | 0.585 | 1.17 (0.76-1.81) | 0.479 |
| Rural | 77 (25.1)/62 (20.2) | 53 (17.2)/86 (28) | 2.02 (1.25-3.25) | 0.004 | 2.35 (1.42-3.90) | 0.001 |
We also observed the correlations between the ITGA2 variant genotypes and clinicopathologic features of gastric cancer patients in this study (Table 4). A significantly increased risk was found in individuals with the variant genotypes in both poorly differentiated tumors (adjusted OR = 2.21, 95% CI = 1.12-4.38, P = 0.022) and adjacent invaded organs (adjusted OR = 2.12, 95% CI = 1.10-4.07, P = 0.024) of gastric cancer. However, no significant association was observed between the polymorphism and lymph node metastasis or tumor location.
| Variable | CT + TT | CC | Crude OR (95% CI) | P value | Adjusted OR2 (95% CI) | P value |
| Tumor differentiation | ||||||
| Well | 42 | 42 | 1 | 1 | ||
| Moderate | 65 | 69 | 0.94 (0.55-1.63) | 0.830 | 0.94 (0.54-1.63) | 0.828 |
| Poor | 55 | 27 | 2.04 (1.09-3.82) | 0.027 | 2.21 (1.12-4.38) | 0.022 |
| Depth of tumor infiltration | ||||||
| T1 | 24 | 30 | 1 | 1 | ||
| T2 | 21 | 17 | 1.54 (0.67-3.56) | 0.308 | 1.76 (0.73-4.25) | 0.208 |
| T3 | 34 | 40 | 1.06 (0.53-2.15) | 0.866 | 1.10 (0.52-2.32) | 0.797 |
| T4 | 83 | 51 | 2.03 (1.07-3.86) | 0.030 | 2.12 (1.10-4.07) | 0.024 |
| Lymph node metastasis | ||||||
| Negative | 59 | 51 | 1 | 1 | ||
| Positive | 103 | 87 | 1.02 (0.64-1.64) | 0.923 | 0.98 (0.61-1.58) | 0.942 |
| Localization | ||||||
| Cardia | 38 | 41 | 1 | 1 | ||
| Non-cardia | 128 | 100 | 1.38 (0.83-2.31) | 0.217 | 1.38 (0.81-2.35) | 0.231 |
In the present study, we investigated the role of ITGA2 gene C807T polymorphism in gastric cancer susceptibility in a Chinese population. We found that the polymorphism may be associated with an increased risk of gastric cancer, differentiation and invasion of gastric cancer.
It has been reported that integrin α2β1 is one of the key factors accelerating tumor progression and metastasis in various types of cancers[15-21,31]. Koike et al[20] found that the α2 integrin was expressed in the intestinal-type and diffuse-type gastric carcinoma cells, and invasion through basement membrane and type I collagen gel was inhibited by anti-α2 integrin monoclonal antibody, indicating that the α2 integrin plays an important role in invasion of gastric carcinoma cells. Another study conducted by Lee et al[32] elucidated the potential mechanisms underlying the spreading and invasiveness of gastric carcinoma cells, the integrin transduces signaling directly via engagements with extracellular matrix proteins, thereby leading to the regulation of downstream intracellular signaling molecules. It also functions in collaborative (indirect) signaling, in which integrins cosignal with other membrane receptor-mediated signal pathways, e.g. growth factor receptors, G-protein coupled receptors or the transforming growth factor β1 signaling pathway.
The ITGA2 gene C807T polymorphism is associated with integrin density, but the precise molecular mechanism remains unclear. It is a silent polymorphism in codon 253 (Phe253Phe) and does not cause an altered structure of the integrin molecule, but in linkage disequilibrium with a yet unknown functional polymorphism affecting ITGA2 expression. Another explanation could be a direct effect on the stability of the ITGA2 mRNA, which resulted in a change of the amount of integrin protein being expressed.
Limited studies have reported the association between the polymorphism in ITGA2 gene and cancer risks, although the results remain inconsistent[27-29]. Gerger et al[27] found that the ITGA2 gene C807T polymorphism was associated with reduced colorectal cancer risk (OR = 0.77, 95% CI = 0.64-0.94, P = 0.011). In their another case-control study, they found that carriers of the most common ITGA2 haplotype (807C_1648G) had a decreased risk for breast cancer (OR = 0.72, 95% CI = 0.53-0.98)[28]. Nevertheless, Ayala et al[29] reported that no association was observed between the ITGA2 gene C807T polymorphism and breast cancer risk.
Based on these studies, we conducted this hospital-based case-control study to investigate the association between the ITGA2 gene C807T polymorphism and the risk of gastric cancer in a Chinese population. The frequency of the variant T allele in our control group was 26.22%, which was similar to that in another study in a Chinese Taiwanese population (27.1%)[33] and HapMap database (26.7% for Han Chinese). Our results showed that the variant genotypes had a 57% increased risk of developing gastric cancer.
In the subgroup analyses, we found that the polymorphism was associated with the increased risk of gastric cancer in the subgroup of the subjects aged > 58 years, but not in the subjects aged ≤ 58 years. Milne et al[34] indicated that carcinogenesis is considered as accumulation of genetic events, and gastric cancer has a steep slope for age-specific increase in incidence. The increased risk observed in older subjects implies that the ITGA2 genotype effects tend to be age specific. The polymorphism may contribute to elevated integrin α2β1 levels beyond the age of 58, thus representing a significant risk factor in this age group. However, this is just a hypothesis to interpret the results of our study, and further research is warranted to clarify the mechanism underlying the interaction between the polymorphism and age.
Similarly, in statistical analyses stratified by smoking status, a significant association was observed in non-smokers, but not in smokers. Tobacco smoking has been undoubtedly accepted as a independent risk factor for gastric cancer[3,5,6]. The association between the polymorphism and gastric cancer risk could be masked by the overwhelming accumulated exposure to tobacco carcinogens in smokers so that the association is more evident in nonsmokers.
We also noted that increased risk of gastric cancer associated with the polymorphism was pronounced in rural subjects, but not in urban subjects. It has been suggested that the genetic differences have their strongest effects under conditions of low environmental pollution[9,35]. Our results plausibly agree with the hypothesis that the genetic effects might be more prominent in the better environments of rural areas[9]. However, this result may be found accidentally, further studies are needed to verify it.
In addition, in the stratified analyses by clinicopathological characteristics of gastric cancer, we observed a significant correlation of the variant genotypes with poorly differentiated tumors. Similarly, Langsenlehner et al[28] suggested that a histological grade of 3 or 4 was found more often in breast cancer subjects with TT genotype. The result is consistent with our findings. In contrast, Yasoshima et al[21] found no correlation between the expression of integrin α2β1 and histopathological features such as the histological grade, stromal type, and infiltrating growth pattern. We also observed the significant association of the variant genotypes with adjacent organ invasions. Several studies have suggested that integrin α2β1 was closely associated with invasion and metastasis in gastric cancer or tumor cells[18-21,31]. These studies might explain the result we observed. However, no correlation between the polymorphism and lymph node metastasis or location of gastric cancer was found in the stratified analyses. Because the number of cases in the subgroups was relatively small and clinicopathological variables were obtained at the time of diagnosis, our findings should be interpreted with caution before being confirmed in further studies. Thus, large-sized studies which prospectively follow up the clinical outcome, especially the survival rate, may be required to elucidate the association between the polymorphism and gastric cancer progression as well as prognosis.
Some limitations may exist in the present study. First, our study is a hospital-based case-control study, so we can not rule out the selection and recall bias. Nevertheless, the T allele frequency in control subjects is quite similar to that reported in HapMap database for Han Chinese in Beijing (0.262 in our study vs 0.267 in HapMap database) and the genotype distributions of cases and controls were in Hardy-Weinberg equilibrium. The second limitation is our relatively small sample size, with 307 cases and 307 controls. So gene-environment interactions may have been underpowered in stratified analyses. However, our preliminary data certainly provides some interesting information and valuable guidance for the future studies in this area. Finally, no enough information on H. pylori status was available in cases and controls, because of the ethical reasons.
In conclusion, the present study provides evidence that the ITGA2 gene C807T polymorphism is associated with an increased risk of gastric cancer in a Chinese population. The association is especially evident in older individuals, non-smokers and rural subjects, and the variant genotypes may also play a role in the differentiation and invasion of gastric cancer, indicating that the polymorphism may be a useful diagnostic marker for genetic susceptibility to gastric cancer. Further studies with larger samples and functional studies are needed to elucidate the role of genetic variations in ITGA2 and the pathogenesis of gastric cancer.
Integrin α2β1 has been considered as a key factor for cancer development and progression, especially in gastric cancer. Polymorphisms in ITGA2 gene is responsible for the expression of integrin α2β1. Recent studies indicated that the ITGA2 gene C807T polymorphism was associated with cancer risk.
Using polymerase chain reaction-restriction fragment length polymorphism method, this study explored the relationship between ITGA2 C807T polymorphism and gastric cancer risk.
The results suggest that the polymorphism is associated with the elevated risk of gastric cancer in a Chinese population, especially in older individuals aged > 58 years, nonsmokers, and rural subjects. Further analyses revealed that the polymorphism may play a role in differentiation and invasion of gastric cancer.
The results of this study could help further understand the genetic determinants of gastric cancer. The polymorphism may be a useful diagnostic marker for genetic susceptibility to gastric cancer.
Integrins are members of a family of cell-surface heterodimeric proteins that mediate cell-matrix and cell-cell interactions. Single nucleotide polymorphisms represent a natural genetic variability at a high density in the human genome, which are responsible for the inter-individual variation and diversity. They have been recently considered as the main genetic elements involved in the development of common and complex diseases, including various cancers.
The current study was designed, processed and concluded well, deserving publication.
Peer reviewer: Ki-Baik Hahm, MD, PhD, Professor, Gachon Graduate School of Medicine, Department of Gastroenterology, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon, 406-840, South Korea
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 2. | Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121-125. |
| 4. | Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132:3467S-3470S. |
| 5. | Galanis DJ, Lee J, Kolonel LN. The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer. 1997;79:1840-1841. |
| 6. | Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford JL. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277-1284. |
| 7. | Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, Shu XO, Jin F, Fraumeni JF, Gao YT. Body mass index and the risk of cancers of the gastric cardia and distal stomach in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 1997;6:481-485. |
| 8. | Wu MS, Chen CJ, Lin JT. Host-environment interactions: their impact on progression from gastric inflammation to carcinogenesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1878-1882. |
| 9. | Zhu H, Yang L, Zhou B, Yu R, Tang N, Wang B. Myeloperoxidase G-463A polymorphism and the risk of gastric cancer: a case-control study. Carcinogenesis. 2006;27:2491-2496. |
| 10. | Gu H, Yang L, Tang N, Zhou B, Zhu H, Sun Q, Cong R, Wang B. Association of endothelin-converting enzyme-1b C-338A polymorphism with gastric cancer risk: a case-control study. Eur J Cancer. 2008;44:1253-1258. |
| 11. | Yang L, Gu HJ, Zhu HJ, Sun QM, Cong RH, Zhou B, Tang NP, Wang B. Tissue inhibitor of metalloproteinase-2 G-418C polymorphism is associated with an increased risk of gastric cancer in a Chinese population. Eur J Surg Oncol. 2008;34:636-641. |
| 12. | Gu H, Yang L, Sun Q, Zhou B, Tang N, Cong R, Zeng Y, Wang B. Gly82Ser polymorphism of the receptor for advanced glycation end products is associated with an increased risk of gastric cancer in a Chinese population. Clin Cancer Res. 2008;14:3627-3632. |
| 13. | Parise LV, Lee J, Juliano RL. New aspects of integrin signaling in cancer. Semin Cancer Biol. 2000;10:407-414. |
| 14. | Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91-100. |
| 15. | Zutter MM, Santoro SA. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137:113-120. |
| 16. | Gui GP, Puddefoot JR, Vinson GP, Wells CA, Carpenter R. Altered cell-matrix contact: a prerequisite for breast cancer metastasis? Br J Cancer. 1997;75:623-633. |
| 17. | Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853-1858. |
| 18. | Matsuoka T, Yashiro M, Nishimura S, Inoue T, Fujihara T, Sawada T, Kato Y, Seki S, Hirakawa-Ys Chung K. Increased expression of alpha2beta1-integrin in the peritoneal dissemination of human gastric carcinoma. Int J Mol Med. 2000;5:21-25. |
| 19. | Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, Kuo ML. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem. 2007;282:34594-34604. |
| 20. | Koike N, Todoroki T, Komano H, Shimokama T, Ban S, Ohno T, Fukao K, Watanabe T. Invasive potentials of gastric carcinoma cell lines: role of alpha 2 and alpha 6 integrins in invasion. J Cancer Res Clin Oncol. 1997;123:310-316. |
| 21. | Ura H, Denno R, Hirata K, Yamaguchi K, Yasoshima T. Separate functions of alpha2beta1 and alpha3beta1 integrins in the metastatic process of human gastric carcinoma. Surg Today. 1998;28:1001-1006. |
| 22. | Kritzik M, Savage B, Nugent DJ, Santoso S, Ruggeri ZM, Kunicki TJ. Nucleotide polymorphisms in the alpha2 gene define multiple alleles that are associated with differences in platelet alpha2 beta1 density. Blood. 1998;92:2382-2388. |
| 23. | Corral J, González-Conejero R, Rivera J, Ortuño F, Aparicio P, Vicente V. Role of the 807 C/T polymorphism of the alpha2 gene in platelet GP Ia collagen receptor expression and function--effect in thromboembolic diseases. Thromb Haemost. 1999;81:951-956. |
| 24. | Carlsson LE, Santoso S, Spitzer C, Kessler C, Greinacher A. The alpha2 gene coding sequence T807/A873 of the platelet collagen receptor integrin alpha2beta1 might be a genetic risk factor for the development of stroke in younger patients. Blood. 1999;93:3583-3586. |
| 25. | Dodson PM, Haynes J, Starczynski J, Farmer J, Shigdar S, Fegan G, Johnson RJ, Fegan C. The platelet glycoprotein Ia/IIa gene polymorphism C807T/G873A: a novel risk factor for retinal vein occlusion. Eye (Lond). 2003;17:772-777. |
| 26. | Casorelli I, De Stefano V, Leone AM, Chiusolo P, Burzotta F, Paciaroni K, Rossi E, Andreotti F, Leone G, Maseri A. The C807T/G873A polymorphism in the platelet glycoprotein Ia gene and the risk of acute coronary syndrome in the Italian population. Br J Haematol. 2001;114:150-154. |
| 27. | Gerger A, Hofmann G, Langsenlehner U, Renner W, Weitzer W, Wehrschütz M, Wascher T, Samonigg H, Krippl P. Integrin alpha-2 and beta-3 gene polymorphisms and colorectal cancer risk. Int J Colorectal Dis. 2009;24:159-163. |
| 28. | Langsenlehner U, Renner W, Yazdani-Biuki B, Eder T, Wascher TC, Paulweber B, Clar H, Hofmann G, Samonigg H, Krippl P. Integrin alpha-2 and beta-3 gene polymorphisms and breast cancer risk. Breast Cancer Res Treat. 2006;97:67-72. |
| 29. | Ayala F, Corral J, González-Conejero R, Sánchez I, Moraleda JM, Vicente V. Genetic polymorphisms of platelet adhesive molecules: association with breast cancer risk and clinical presentation. Breast Cancer Res Treat. 2003;80:145-154. |
| 30. | Sobin LH, Wittekind CH, editors . TNM classification of malignant tumors. 5th ed. New York: Wiley & Sons Inc 1997; 59-62. |
| 31. | Chan BM, Matsuura N, Takada Y, Zetter BR, Hemler ME. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991;251:1600-1602. |
| 32. | Lee MS, Kim TY, Kim YB, Lee SY, Ko SG, Jong HS, Kim TY, Bang YJ, Lee JW. The signaling network of transforming growth factor beta1, protein kinase Cdelta, and integrin underlies the spreading and invasiveness of gastric carcinoma cells. Mol Cell Biol. 2005;25:6921-6936. |
| 33. | Chen CH, Lo YK, Ke D, Liu CK, Liou CW, Wu HL, Lai ML. Platelet glycoprotein Ia C807T, Ib C3550T, and IIIa Pl(A1/A2) polymorphisms and ischemic stroke in young Taiwanese. J Neurol Sci. 2004;227:1-5. |
| 34. | Milne AN, Carvalho R, Morsink FM, Musler AR, de Leng WW, Ristimäki A, Offerhaus GJ. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Mod Pathol. 2006;19:564-572. |
| 35. | Hung RJ, Boffetta P, Brennan P, Malaveille C, Gelatti U, Placidi D, Carta A, Hautefeuille A, Porru S. Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis. 2004;25:973-978. |