Published online Feb 28, 2010. doi: 10.3748/wjg.v16.i8.973
Revised: November 25, 2009
Accepted: December 2, 2009
Published online: February 28, 2010
AIM: To search for further immunodominant peptides of the pyruvate dehydrogenase complex E2-component (PDC-E2) recognized by antimitochondrial antibodies (AMA) in primary biliary cirrhosis (PBC).
METHODS: Sera from 95 patients with PBC were tested by enzyme-linked immunosorbent assay against 33 synthetic overlapping peptides (25 amino acids; aa) covering the entire length of the E2-subunit of PDC-E2. Furthermore, the inner lipoyl peptide 167-184 was used in an unlipoylated and a lipoylated form as well as coupled to ovalbumin. Sera from 11 AMA negative/ANA positive PBC patients, 63 patients with other liver disorders and 22 healthy blood donors served as controls.
RESULTS: Of the 95 PBC-sera, 74% reacted with the peptide 475-499 and 58% with the peptide 407-431 located within the catalytic domain of PDC-E2. Patients with other disorders or healthy controls were positive in only up to 18%. Antibodies to the unlipoylated and lipoylated peptide 167-184 within the inner lipoyl domain were found in only 5% and 11% of the PBC sera, respectively; using ovalbumin-coupled peptides, the incidence increased up to 57% (unlipoylated form).
CONCLUSION: Peptides within the catalytic site of PDC-E2 rather than the previously reported lipoyl binding peptide 167-184 may represent major immunodominant epitopes recognized by AMA in PBC.
- Citation: Braun S, Berg C, Buck S, Gregor M, Klein R. Catalytic domain of PDC-E2 contains epitopes recognized by antimitochondrial antibodies in primary biliary cirrhosis. World J Gastroenterol 2010; 16(8): 973-981
- URL: https://www.wjgnet.com/1007-9327/full/v16/i8/973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i8.973
Antimitochondrial antibodies (AMA) are one of the most important criteria for the diagnosis of primary biliary cirrhosis (PBC), a chronic cholestatic liver disease of unknown etiology, which affects mainly middle-aged women and leads to a destruction of small bile ducts. Their target antigen named M2[1,2] is attached to the inner mitochondrial membrane[3] and consists of five components[4], which have been identified in the following years on molecular bases as subunits of the 2-oxo acid dehydrogenase complex of the inner mitochondrial membrane: the pyruvate dehydrogenase complex (PDC), the 2-oxoglutarate dehydrogenase complex and the branched-chain 2-oxo acid dehydrogenase complexes[5-7]. Each complex comprises multiple copies of three component enzymes termed E1 (a thiamine pyrophosphate-dependent decarboxylase), E2 (a coenzyme A-dependent decarboxylase), and E3 (a dihydrolipoyl dehydrogenase). The major M2-antigen which is recognized by nearly 90% of PBC sera is the E2 component of PDC.
The immunodominant epitope within PDC-E2 has been mapped to the region associated with the inner lipoyl domain wherein a lysine residue binds the lipoic cofactor for the enzyme with a minimum of 75 residues necessary for characteristic antibody recognition[6,8,9] suggesting that a conformational autoepitope may be primarily recognized[9]. A minor epitope is associated with the outer lipoyl domain[6,10]. Hitherto, epitope mapping with PBC sera has linked AMA-reactivity to the highly conserved amino acids surrounding the lipoyl-lysine K173, particularly linear peptides AEIETDKATIGFEVQEEG (corresponding to aa 167-184 within the human PDC-E2 but primarily labeled aa 81-100[6] according to its location within a subclone pRMIT-603 used to identify the immunodominant epitope) or LLAEIETDKATIGF (165-178)[11]. It was shown that these short peptides absorbed most reactivity with PBC sera by enzyme-linked immunosorbent assay (ELISA), albeit only at a serum dilution of 1:80 000[6]. Binding of lipoic acid cofactor to K173 has been discussed to be necessary for antibody reaction[10,12].
Until now, the large fragment of the catalytic domain of PDC-E2 (aa 331-560) has been regarded as ‘immunologically silent’ due to negative results in several studies[6,9,12-14]. In the present study we used peptides spanning the whole PDC-E2 enzyme, and it will be shown that sera from PBC patients recognize epitopes within the catalytic domain in an even higher incidence than those in the inner lipoyl domain.
Sera from 95 patients with clinically well defined PBC were analyzed. All patients had been seen by one of the authors (Berg C), and all were AMA/anti-M2-anti-PDC-E2 positive. Detailed clinical, biochemical and histological data are given in Table 1.
| Parameters | |
| Females:males | 88:7 |
| Age (yr) | |
| mean ± SD | 50 ± 9.8 |
| Range | 21-70 |
| Biochemical parameters1 | |
| AP (U/L) (normal < 120) | 453 ± 451 |
| GT (U/L) (normal < 50) | 154 ± 143 |
| ASAT (U/L) (normal < 35) | 39.4 ± 42.6 |
| ALAT (U/L) (normal < 35) | 51 ± 49.3 |
| Cholinesterase (kU/L) (normal > 5) | 4.7 ± 1.7 |
| Bilirubin (mg%) (normal < 1.5) | 1.62 ± 0.8 |
| Cholesterol (mg/dL) (normal < 200) | 251 ± 69 |
| Eosinophils (%) (normal < 4) | 3.61 ± 2.36 |
| IgG globulins (mg%) (normal < 1800) | 1679 ± 855 |
| IgA globulins (mg%) (normal < 400) | 269 ± 158 |
| IgM globulins (mg%) (normal < 280) | 416 ± 255 |
| Histological data2 | |
| PBC stage I/II (n) | 45 |
| PBC stage III/IV (n) | 20 |
| Serological data | |
| Antimitochondrial antibodies (number positive) | |
| AMA (IFT) (titer > 1:160) | 95 |
| Anti-M2 (ELISA) | |
| Total | 95 |
| IgG type | 87 |
| IgM type | 72 |
| Anti-PDC-E2 (ELISA) | |
| Total | 95 |
| Antinuclear antibodies (number positive) (titer > 1:160) | |
| Nuclear dots (sp 100) | 16 |
| Nuclear membrane (gp 210) | 10 |
| Centromeres | 8 |
Liver biopsy had been performed in 65 of the patients and was interpreted by Dr. Bianchi L, Professor, University of Basel, Switzerland. All patients had been followed for more than 5 years (range: 5-28 years). At time of first serum analysis, 65 had not yet received any therapy, 30 were already treated with ursodeoxycholic acid (at least 6 mo, doses 1000-1500 mg/d).
Furthermore, sera from 11 patients with clinically and histologically proven PBC being AMA negative but ANA positive were investigated. Five had antibodies to nuclear dots (sp 100), three to nuclear membranes (gp 210) and two to centromeres.
The investigations have been approved by the local Ethics committee.
As controls we used sera from 36 patients with untreated autoimmune hepatitis, 27 patients with alcoholic liver disease, and 32 patients with collagen disorders. All patients have been seen and followed either by Berg C or Klein R Diagnosis was based on clinical, biochemical and serological parameters.
Sera from 22 healthy blood donors (kindly provided by Dr. Wernet D, Professor, Institute for Transfusionmedicine, University of Tubingen, Germany) were used as controls, all being negative for AMA/anti-M2 as shown by immunofluorescence test (IFT), ELISA and Western blotting. All sera were stored at -20°C.
The M2-antigen was released from beef heart submitochondrial particles by chloroform treatment as described[2]. Commercial PDC (from porcine heart) was obtained from Sigma-Aldrich (St. Louis, USA).
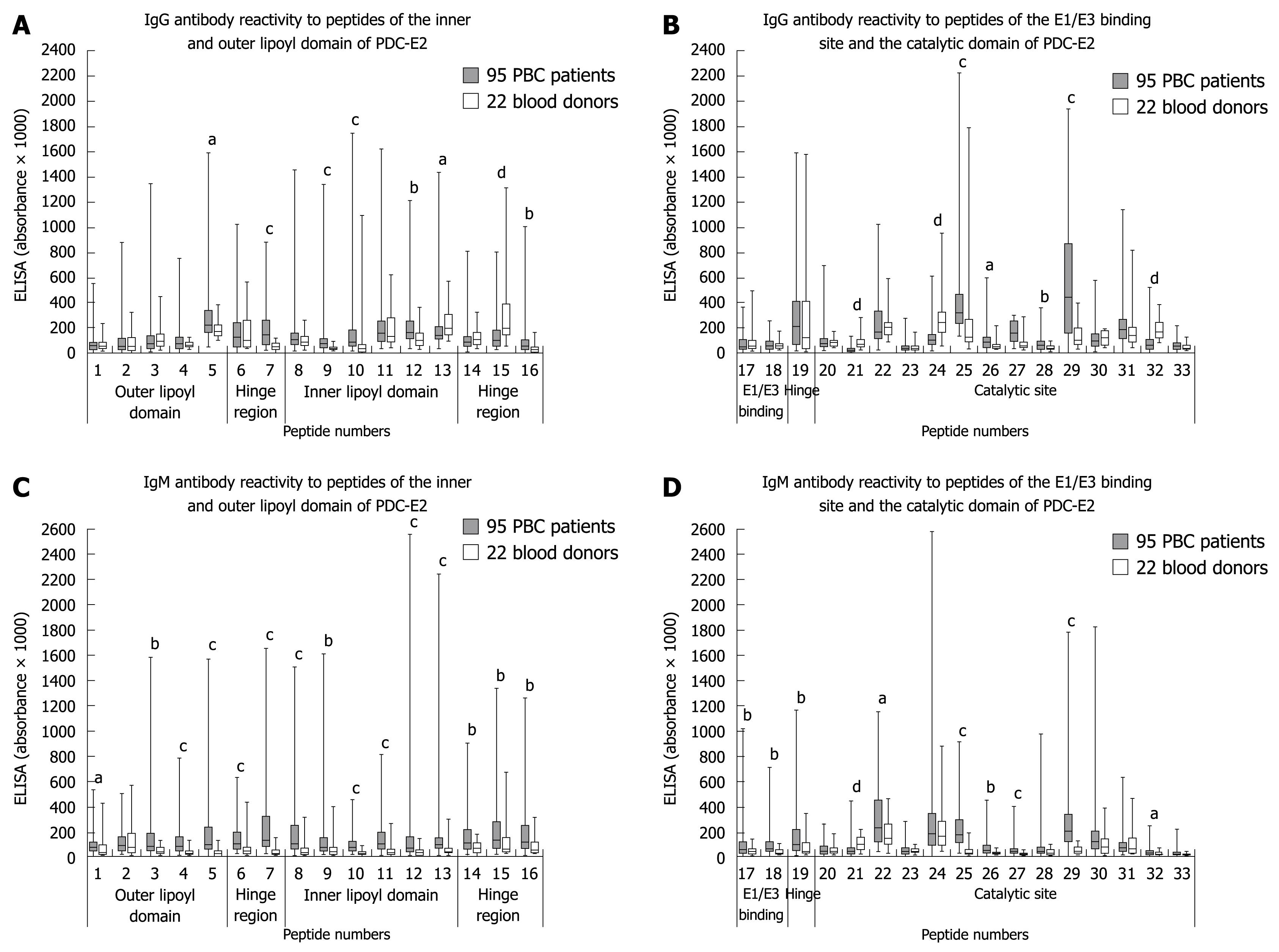
Thirty-three synthetic overlapping peptides covering the entire length of PDC-E2 were obtained from Biotrend (Cologne, Germany). Peptides were 25 amino acids (aa) long with 8 overlapping aa (Figure 1).
| Peptide No. | Amino acid | PBC patients | Healthy individuals (n = 22) | ||
| Anti-M2+ (n = 95) | AMA-/ANA+ (n = 11) | ||||
| Outer lipoyl domain (aa 1-80) | 1 | -2-23 | 4 (4) | 0 | 1 (5) |
| 2 | 16-40 | 6 (6) | 0 | 2 (9) | |
| 3 | 33-57 | 10 (11) | 0 | 2 (9) | |
| 4 | 50-74 | 15 (16) | 0 | 0 | |
| 5 | 67-91 | 26 (27) | 0 | 2 (9) | |
| Hinge | 6 | 84-108 | 17 (18) | 0 | 3 (14) |
| 7 | 101-125 | 42 (44)1 | 0 | 0 | |
| Inner lipoyl domain (aa 133-217) | 8 | 118-142 | 14 (15) | 0 | 1 (5) |
| 9 | 135-159 | 32 (34) | 0 | 0 | |
| 10 | 152-176 | 28 (29) | 0 | 2 (9) | |
| 11 | 169-193 | 11 (12)2 | 0 | 2 (9) | |
| 12 | 186-210 | 16 (17) | 0 | 1 (5) | |
| 13 | 203-227 | 6 (6) | 0 | 3 (14) | |
| Hinge | 14 | 220-244 | 4 (4) | 0 | 2 (9) |
| 15 | 237-261 | 3 (3) | 0 | 4 (18) | |
| 16 | 254-278 | 20 (21) | 0 | 1 (5) | |
| E1/E3-binding site (aa 272-303) | 17 | 271-295 | 3 (3) | 0 | 1 (5) |
| 18 | 288-312 | 7 (7) | 0 | 1 (5) | |
| Hinge | 19 | 305-329 | 19 (20) | 0 | 3 (14) |
| Catalytic site (aa 331-561) | 20 | 322-346 | 12 (13) | 2 (18) | 1 (5) |
| 21 | 339-363 | 0 | 0 | 1 (5) | |
| 22 | 356-380 | 19 (20) | 0 | 1 (5) | |
| 23 | 373-397 | 2 (2) | 1 (9) | 1 (5) | |
| 24 | 390-414 | 1 (1) | 1 (9) | 2 (9) | |
| 25 | 407-431 | 53 (56)3 | 0 | 4 (18) | |
| 26 | 424-448 | 10 (11) | 0 | 1 (5) | |
| 27 | 441-465 | 5 (5) | 0 | 2 (9) | |
| 28 | 458-482 | 18 (19) | 0 | 0 | |
| 29 | 475-499 | 55 (58)4 | 0 | 1 (5) | |
| 30 | 492-516 | 9 (9) | 0 | 0 | |
| 31 | 509-533 | 17 (18) | 0 | 2 (9) | |
| 32 | 526-550 | 2 (2) | 0 | 1 (5) | |
| 33 | 543-561 | 11 (12) | 0 | 1 (5) | |
Peptide 11 contained the immunodominant decameric epitope consisting of aa IETDKATIGF as reported by Van de Water et al[6], which had been extended to a 15 amino acid peptide by Amano et al[15] (IETDKATIGFEVQEE). All peptides were high-performance liquid chromatography (HPLC)-purified (more than 90% purity). An irrelevant peptide was used as background control. The peptides were reconstituted at 5 mg/mL DMSO and stored at -20°C.
The immunodominant peptide 167-184 (AEIETDKATIGFEVQEEG) was synthesized (Biotrend, Cologne, Germany) in an unlipoylated form and a form in which lipoic acid was coupled to lysine at position 173 (167-184-LA)[12]. Purity and conjugation was proven by HPLC (elution time 984.5 m/z vs 1076.7 m/z; purity > 95%). Since these peptides may be recognized only when attached to a carrier[12], both forms of peptides were also coupled to ovalbumin (Biotrend, Cologne, Germany). For this purpose, at the C-terminal end a cysteine had to be added (AEIETDKATIGFEVQEEGC-OVA) which had to be substituted by a lysine for coupling the LA-conjugated [AEIETDK(alpha-lipoic)ATIGFEVQEEGK-OVA]peptide. The presence of the hydrophobic lipoic acid moiety covalently bound to the peptide was again confirmed by the significant differences in HPLC-elution profiles when peptides without or with LA were analyzed (elution time 1.067.8 m/z vs 1.163.3 m/z; purity > 93%).
In the IFT cryostat sections from rat liver, kidney, heart, stomach and human thyroid were used to detect AMA and other autoantibodies[16].
The ELISA for the detection of anti-M2/PDC-E2 antibodies was performed as described[17].
Antibody reactivity with PDC-E2 peptides was determined using microtiter plates (Maxisorp, Nunc, Denmark) coated with 100 μL of each peptide at a concentration of 25 μg/mL in coating buffer (hydrogen bicarbonate buffer, 0.2 mol/L, pH 9.6) overnight at 4°C. After extensive washing and blocking with phosphate buffered saline (PBS) (60 mmol/L, pH 7.4) containing 1% bovine serum albumin (BSA) for 60 min they were incubated with 100 μL of patients’ sera at a dilution of 1:500 for 90 min at room temperature. After washing with PBS containing 0.2% Triton X 100 and 0.5% BSA, the wells were incubated with peroxidase-conjugated monovalent anti-human IgG- and IgM-antibodies from goat (Dianova, Hamburg, Germany; dilution 1:3000) for 60 min at room temperature, washed as above, and AMA reactivity detected using orthophenylendiamine as substrate. Antibody reactivity was given as absorbance × 1000.
Optimal peptide concentrations (25 μg/mL) and serum dilutions (1:500) had been determined by serial dilutions prior to the study.
Normal ranges for antibody reactivities with all antigens/peptides were determined by analysis of 22 healthy donors. Mean value of their absorbance plus double the standard deviation was defined as cut off value.
For the comparison of antibody reactivity in different groups of patients, SPSS version 15.0 was used applying the non parametric Mann-Whitney test. For analysis of paired data the Wilcoxon signed rank test was used. Differences with P <0.05 were considered statistically significant.
Sera from 95 patients with PBC all being anti-M2/PDC-E2 positive in the ELISA using the purified M2-antigen and the commercially available PDC were tested against 33 peptides (25 mers) spanning the whole sequence of PDC-E2.
Surprisingly, the reactivity of the PBC sera with the peptides 3, 10 and 11 containing the immunodominant lipoyl binding epitopes in the outer (aa 41-53) and inner lipoyl domain (aa 167-183)[6,10] was rather low although significantly higher than that of healthy controls (Figure 2A and C). Only 11% had IgG-antibodies to peptide 3, 29% to peptide 10, and 12% to peptide 11; IgM-antibodies were observed in 28%, 37%, and 39%, respectively. In contrast, up to 74% of the PBC sera reacted with the two peptides 25 and 29 within the catalytic domain and the peptide 7 (aa 101-125) in the first hinge region (Tables 2 and 3), and IgG and IgM reactivity towards these three peptides was significantly higher in PBC patients than in controls (Figure 2).
| Peptide No. | Amino acid | PBC patients | Healthy individuals (n = 22) | ||
| Anti-M2+ (n = 95) | AMA-/ANA+ (n = 11) | ||||
| Outer lipoyl domain (aa 1-80) | 1 | -2-23 | 7 (7) | 0 | 1 (5) |
| 2 | 16-40 | 10 (11) | 0 | 2 (9) | |
| 3 | 33-57 | 27 (28) | 0 | 0 | |
| 4 | 50-74 | 33 (35) | 1 (9) | 1 (5) | |
| 5 | 67-91 | 42 (44) | 0 | 1 (5) | |
| Hinge | 6 | 84-108 | 31 (33) | 0 | 1 (5) |
| 7 | 101-125 | 52 (55)1 | 1 (9) | 2 (9) | |
| Inner lipoyl domain (aa 133-217) | 8 | 118-142 | 34 (36) | 0 | 1 (5) |
| 9 | 135-159 | 35 (37) | 0 | 2 (9) | |
| 10 | 152-176 | 35 (37) | 0 | 1 (5) | |
| 11 | 169-193 | 37 (39)2 | 0 | 2 (9) | |
| 12 | 186-210 | 27 (28) | 0 | 1 (5) | |
| 13 | 203-227 | 23 (24) | 0 | 2 (9) | |
| Hinge | 14 | 220-244 | 26 (27) | 0 | 0 |
| 15 | 237-261 | 43 (45) | 0 | 3 (14) | |
| 16 | 254-278 | 33 (35) | 0 | 1 (5) | |
| E1/E3-binding site (aa 272-303) | 17 | 271-295 | 15 (16) | 0 | 0 |
| 18 | 288-312 | 24 (25) | 0 | 1 (5) | |
| Hinge | 19 | 305-329 | 36 (38) | 0 | 3 (14) |
| Catalytic site (aa 331-561) | 20 | 322-346 | 1 (1) | 0 | 0 |
| 21 | 339-363 | 4 (4) | 0 | 0 | |
| 22 | 356-380 | 24 (25) | 0 | 1 (5) | |
| 23 | 373-397 | 10 (11) | 0 | 0 | |
| 24 | 390-414 | 23 (24) | 1 (9) | 3 (14) | |
| 25 | 407-431 | 55 (58)3 | 0 | 1 (5) | |
| 26 | 424-448 | 21 (22) | 0 | 0 | |
| 27 | 441-465 | 16 (17) | 0 | 0 | |
| 28 | 458-482 | 15 (16) | 0 | 0 | |
| 29 | 475-499 | 70 (74)4 | 0 | 1 (5) | |
| 30 | 492-516 | 14 (15) | 0 | 2 (9) | |
| 31 | 509-533 | 6 (6) | 0 | 2 (9) | |
| 32 | 526-550 | 4 (4) | 0 | 0 | |
| 33 | 543-561 | 9 (9) | 0 | 1 (5) | |
The peptides 15, 21 and 24 were the only ones whose reactivity was lower with PBC sera as compared to control sera (Figure 2A, B and D).
Most PBC sera recognized several peptides in parallel. IgM-antibodies generally reacted with a higher diversity of peptides than IgG-antibodies (IgG: mean + SD: 5.3 ± 7.7 peptides, median: 3 peptides, range: 0-30 peptides; IgM: mean ± SD: 9 ± 7.1 peptides, median: 7 peptides, range: 0-28 peptides). Two PBC sera had neither IgG- nor IgM-antibodies to any of the 33 peptides although both sera showed high antibody reactivities towards PDC and M2 in the ELISA.
Sera from patients with AMA negative/ANA positive PBC hardly reacted with any of the peptides (Tables 2 and 3).
Incidence and reactivity of antibodies to the different peptides in sera from patients with autoimmune hepatitis, alcoholic liver disease and collagen disorders resembled that in healthy controls (data not shown).
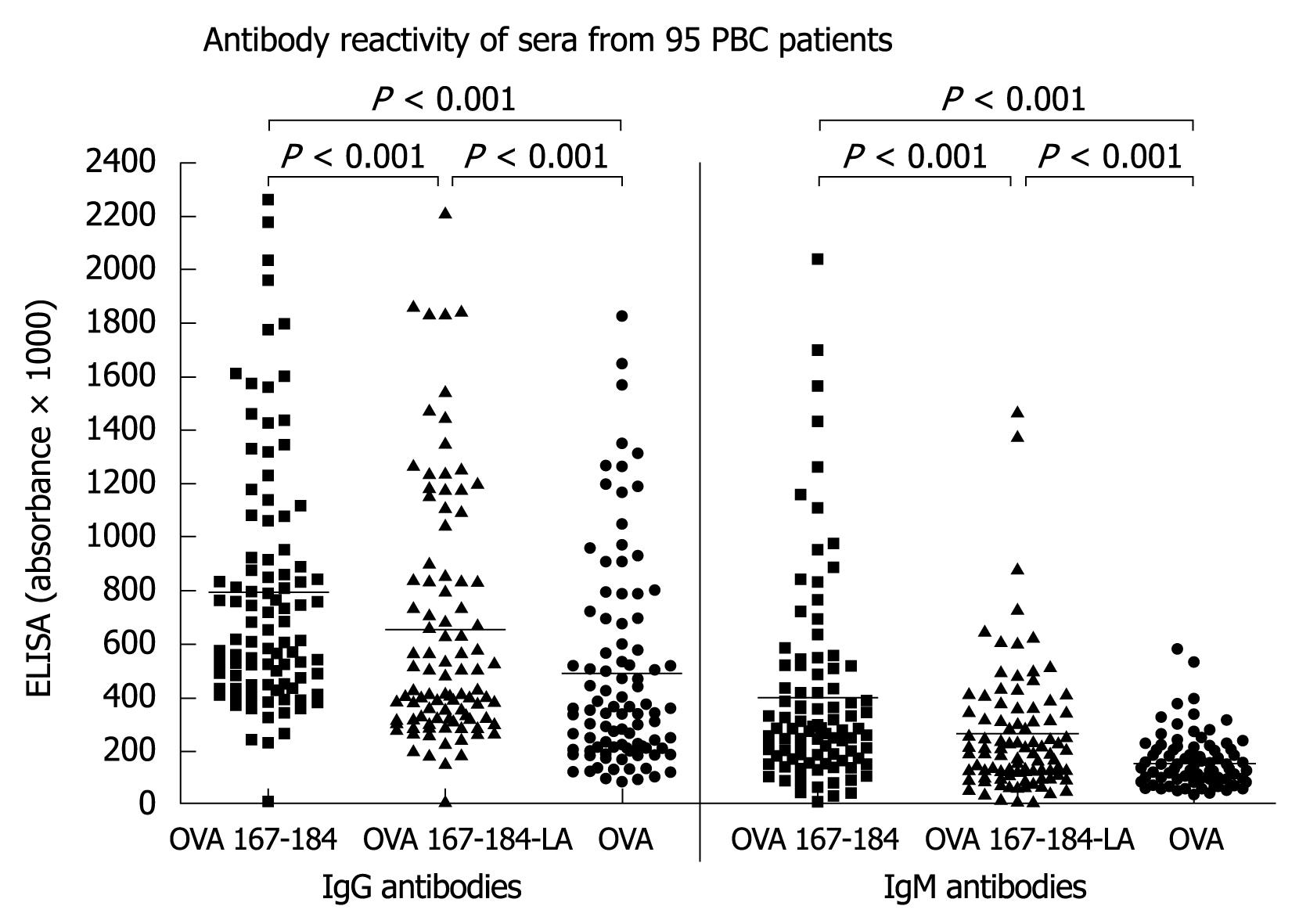
In view of the fact that the 25 mers peptide 10 (aa 152-176) and 11 (aa 169-193) used in the epitope mapping above did not completely respond to the published sequence 167-184 (AEIETDKATIGFEVQEEG), we also synthesized the latter peptide in an unlipoylated form and a form containing lipoic acid (LA) at aa K173 (peptide 167-184-LA) and applied both forms in the ELISA. In accordance to a previous study[12], also with these peptides reactivity of the 95 PBC sera was low (Table 4).
| Peptides of the inner lipoyl domain | IgG-antibodies | IgM-antibodies | ||||
| Positive n (%) | mean absorbance × 1000 ± SD (median) | Cut off value | Positive n (%) | mean absorbance × 1000 ± SD (median) | Cut off value | |
| 152-176 (present study peptide 10) | 28 (29) | 153 ± 243 (87) | 150 | 35 (37) | 95 ± 77 (73) | 80 |
| 169-193 (present study peptide 11) | 11 (12) | 210 ± 219 (157) | 300 | 37 (39) | 143 ± 120 (101) | 120 |
| 167-184 | 0 | 113 ± 63 (93) | 100 | 5 (5) | 53 ± 79 (20) | 100 |
| 167-184-LA | 3 (3) | 135 ± 71 (111) | 100 | 10 (11) | 78 ± 107 (40) | 100 |
| OVA-167-1841 | 42 (44) | 316 ± 260 (271) | 100 | 54 (57) | 254 ± 318 (117) | 100 |
| OVA-167-184-LA1 | 21 (22) | 175 ± 280 (148) | 100 | 29 (31) | 113 ± 217 (52) | 100 |
In order to find out whether the antibodies may recognize a conformational epitope the peptides were coupled to ovalbumin (OVA-peptide 167-184 and OVA-peptide 167-184-LA)[12]. With these conjugated peptides reactivity was stronger, but most sera showed a high reactivity already with OVA alone (Figure 3). IgG- and IgM-reactivity towards the unlipoylated OVA 167-184 was significantly higher than that towards the lipoylated form (OVA 167-184-LA), reactivity towards both forms of peptides was significantly higher than that against OVA alone.
After subtracting the reactivities with OVA from that with the OVA-coupled peptides, 42 (44%) of the 95 PBC sera had IgG-antibodies to the unlipoylated (OVA 167-184) and 21 (22%) to the lipoylated peptide (OVA 167-184-LA) (Table 4). IgM antibodies were found in 57% and 31%, respectively.
In order to see whether UDCA-treatment may influence the reactivity with distinct peptides we compared the reaction of 65 patients who were without any therapy at the time of serological analysis with that of 30 patients who received UDCA for at least 6 mo. However, no significant differences in antibody titers were observed for any of these antigens/peptides between the two groups (data not shown), and also mean number of peptides recognized by each patient did not differ [IgG: untreated group: mean ± SD 5.7 ± 6 peptides, median 4 peptides, treated group: 4.5 ± 5.1 peptides, median 2 peptides (P = 0.11); IgM: untreated group: 9.2 ± 7 peptides, median 7 peptides, treated group: 8.5 ± 7 peptides, median 6 peptides (P = 0.65)].
The inner lipoyl domain of PDC-E2 and especially the peptide aa167-184 has been previously considered the prominent immunodominant epitope recognized by sera from PBC-patients, although there is no general consensus[6,10,11,15,18-20]. In these analyses sera from PBC patients were incubated with the peptide, and it was shown that it absorbed the anti-M2 activity, albeit only at high serum dilutions. In other studies fusion proteins were used containing the peptide and tested by ELISA against PBC sera revealing positive results. However, using this peptide in the ELISA, no significant reactivity with PBC sera was observed[5,12]. There was evidence that antigenicity could be improved after coupling the peptide to ovalbumin and/or lipoylation of K173[12], but this is also still controversially discussed. Accordingly, the uncertainty on reactivity with the linear epitope and an absolute requirement for lipoylation of PDC-E2 for antibody reactivity strongly suggests that rather a conformational epitope within the inner lipoyl domain may represent the immunodominant epitope as outlined by several authors[9,11].
In the present study analyzing a large group of 95 patients with clinically, serologically and histologically defined PBC we could largely confirm these data. Thus, testing the sera by ELISA against 25 mer peptides spanning the whole sequence of PDC-E2, we found only a weak antibody reactivity to peptide 10 (aa 152-176) or peptide 11 (aa 169-193) containing the lipoyl binding K173 and a low incidence of positive results. Since these peptides derived from the peptide scan did not completely correspond to the published peptide 167-184, we also synthesized this epitope but again did not obtain significant reactions. In accordance with a previous report, antigenicity could be increased by coupling with OVA[12] while lipoylation had no additional effect. However, already antibody reactivity to OVA itself was rather high indicating that this protein is not suitable for coupling autoantigens to detect conformational autoantibodies in sera from patients with autoimmune disorders. The wide distribution of antibodies to OVA in the general population is a well known phenomenon[21].
In contrast, we found strongly reactive linear epitopes in the first hinge region (peptide 7, aa 101-125) and in the catalytic domain of PDC-E2 - until now regarded as ‘immunologically silent’[9,14]. Thus, up to 55% of the 95 PBC sera had antibodies of the IgG- or IgM-type to peptide 7 and up to 74% to the peptides aa 407-431 (peptide 25) or aa 475-499 (peptide 29) in the catalytic domain. The specificity of this reaction was underlined by the finding that sera from patients with AMA negative PBC or other disorders did not react with these peptides. Although there have been several studies analyzing the catalytic domain they failed to detect relevant antibody reactivities[6,9,12], but most of them used larger peptide sequences indicating that - in contrast to antibodies to the inner lipoyl domain - antibodies to the catalytic domain may be rather directed against linear and not against conformational peptides.
The reaction of PBC sera with the catalytic domain is of interest since it has been reported by several authors that anti-PDC-E2 antibodies inhibit the PDC-E2 enzyme activity, which has been attributed until now to their binding to the inner lipoyl domain[22-25]. However, since the catalytic domain contains the active site an enzyme inhibition by interference with this region seems even more likely. Thus, the catalytic centre is formed by a long channel across the interface between the catalytic domains of two neighbored E2-subunits. The opening of the channel pointing towards the outer face of the molecule forms the lipoamide binding site, whereas the opposite entrance corresponds to the coenzyme A (CoA) binding site. In the middle of the catalytic centre there is a pair formed by H534 and Ser480 from neighboring E2-subunits (nomenclature according to human PDC) which separates these two substrate binding sites and is directly involved in the transacetylase reaction[26-32]. Our observation in the present study that highest antibody reactivity in PBC sera was obtained with peptide 29 (aa 475-499) which contains Ser480, might, therefore, also explain the inhibitory potency of PBC sera on PDC-E2-enzyme activity. The inner catalytic domain as a whole shares less than 35% sequence identity in prokaryote and human PDC. In contrast, the structures at the active sites are highly conserved in pro- and eukaryotes. The reactivity of autoantibodies with evolutionary highly conserved enzymes and their interaction with enzyme function in vitro is a quite frequently observed phenomenon in autoimmune disorders in general[33,34].
The etiopathogenetic role of anti-M2/PDC-E2 antibodies for PBC is still a matter of debate. Coupling of the inner lipoyl domain with 2-octynoic acid instead of lipoic acid has been discussed to increase antigenicity of the enzyme PDC-E2, and common environmental, cosmetic and food additives containing this 2-octynoic acid have, therefore, be postulated to play a role in their induction by formation of an altered PDC-E2 in the sense of a neoantigen[15,35,36]. Our observation that anti-PDC-E2 antibodies react preferentially with the active site of the catalytic domain of PDC-E2 is strongly suggestive for a further mechanisms including molecular mimicry or defects in apoptosis[5,37,38]. Interestingly, similarity alignment (http://www.expasy.org) revealed that a sequence consisting of the five aa TFTIS within peptide 29 containing the active site S480 shares an identity of 80% with a five-aa-peptide within an envelope protein of human β-retrovirus previously cloned from patients with PBC[39]. The relevance of this observation is, of course, still rather speculative.
Another peptide strongly recognized by PBC sera (up to 58%) was the peptide 25. However, 18% of healthy controls also reacted with this peptide indicating that probably natural autoantibodies towards this region exist. Also the fact that antibody reactivity to some peptides within the catalytic domain did not differ significantly between healthy individuals and PBC-patients (i.e. peptide 22, 30, 31) or were even lower in the PBC sera than in sera of the controls (peptides 15, 21 and 24) may point towards the existence of natural autoantibodies to several PDC-E2 epitopes as also outlined by others[5,13]. It is well known that natural autoantibodies, which play an important role in the ‘first line defense’ react preferentially with archaic enzymes, and this would fit with our observation. Interestingly, we also observed antibodies to the PDC-E2 complex in sera from healthy family members of PBC-patients, but these seem to recognize other epitopes than the patients’ sera (manuscript in preparation).
In general, most sera recognized several epitopes on the entire PDC-E2 molecule, and diversity of IgM antibodies was even higher than that of IgG antibodies. Using overlapping octameric peptides representing the inner lipoyl domain of PDC-E2 similar observations were made by Mackay et al[5], and they also noted that normal human sera reacted with multiple peptides, although levels of reactivity were generally lower than those observed using PBC sera[5,40].
Two of the 95 PBC sera did not react with any of the 33 peptides although they were strongly anti-M2/PDC-E2 positive in ELISA and Western blotting again underlining the importance of conformational epitopes. Our preliminary findings that anti-M2/PDC-activity could not be significantly absorbed with any of the linear 167-184 peptides or with peptide 25 or 29 (data not shown), fit to this concept. Based upon molecular modeling of antibodies reacting with antigens, over 90% of B-cell epitopes are thought to be conformational[41,42]. Attempts to map B-cell epitopes have, therefore, met with mixed success depending largely on the system under investigation. Since there are so many different types of autoepitopes, linear, conformational, cryptic, etc.[43], no universally applicable method is available that allows for the identification of all autoepitopes. Performing those analyses one has, therefore, to be aware of the techniques’ advantages and disadvantages. Peptide scan as used in the present study does not allow determining conformational epitopes but it has the advantage that even cryptic epitopes can be detected. This method has been proven useful in the identification of immunodominant epitopes of several autoantigens for instance in collagen disorders[43]; and considering the concept of “epitope-spreading” it is not unlikely that the multiple immunoreactivity towards several linear and conformational epitopes in an autoimmune disease starts with cross-reactivity to a single (linear) peptide which may lead us to the initiating agent.
In conclusion, we have firmly documented that PBC sera react preferentially with peptides of the catalytic domain of PDC-E2. However, it still has to be proven whether this catalytic domain is also targeted by T-cells. Furthermore, the data again underline the importance of conformational epitopes for AMA-reactivity in PBC.
Antimitochondrial antibodies (AMA) reacting with the 2-oxo acid dehydrogenase complex of the inner mitochondrial membrane are highly specific for the serological diagnosis of primary biliary cirrhosis (PBC), a chronic cholestatic liver disorder of unknown etiology.
The M2-antigen consists of five components which have been identified as subunits of the 2-oxo acid dehydrogenase complex: the pyruvate dehydrogenase complex (PDC), the 2-oxoglutarate dehydrogenase complex and the branched-chain 2-oxo acid dehydrogenase complexes. Each complex comprises multiple copies of three component enzymes termed E1, E2, and E3. The major M2-antigen which is recognized by nearly 90% of PBC sera is the E2 component of PDC.
Within PDC-E2 an immunodominant epitope has been identified which is associated with the inner lipoyl domain (aa 167-184) wherein a lysine residue (K173) binds the lipoic cofactor for the enzyme. Binding of lipoic acid cofactor to K173 has been discussed to be necessary for antibody reaction. Replacement of this lipoic acid by 2-octynoic acid seemed even to enhance antibody binding. It was, therefore, speculated that xenobiotics may lead to an accumulation of 2-octynoic acid hereby preventing binding of lipoic acid to the PDC-E2 and generation of neoantigen with the consequence of generation of autoreactive B- and T-cells. The present study contradicts this concept to some extend indicating that epitopes within the catalytic site of PDC-E2 are even stronger reactive with AMA.
The identification of immunodominant epitopes recognized by autoreactive T- or B-cells may improve our understanding with respect to their generation (for instance as consequence of cross reactivity with infectious agents or induction by neoantigens) and their possible pathogenetic role in PBC.
The 2-oxoacid dehydrogenase complex is a multi enzyme complex located at the inner membrane of mitochondria which catalyzes the oxidative decarboxylation of 2-oxo acids to the corresponding acyl-coenzyme A. It is not yet known why this non-organ specific protein complex becomes an autoantigen recognized by autoantibodies in a disease affecting mainly bile ducts such as PBC.
The authors analyse immunodominant targets of PDC-E2 in sera of about 100 patients with PBC. Interestingly, they show that the catalytic site of PDC-E2 contains several immunodominant epitopes. The group has an outstanding expertise in the field of clinical immunology. The data presented in the manuscript add to the understanding of immunological events in PBC.
Peer reviewers: Henning Schulze-Bergkamen, MD, Henning Schulze-Bergkamen, First Medical Department, University of Mainz, Langenbeckstr, 1, 55101 Mainz, Germany; Sun-Lung Tsai, MD, PhD, Professor, Director, Hepatogastroenterology Section, Department of Internal Medicine and Liver Research Unit, Department of Medical Research, Chi Mei Medical Center, 901 Chung Hwa Road, Young-Kang City, Tainan County 710, Taiwan, China
S- Editor Wang JL L- Editor O’Neill M E- Editor Zheng XM
| 1. | Berg PA, Klein R, Lindenborn-Fotinos J, Klöppel W. ATPase-associated antigen (M2): marker antigen for serological diagnosis of primary biliary cirrhosis. Lancet. 1982;2:1423-1426. |
| 2. | Lindenborn-Fotinos J, Sayers TJ, Berg PA. Mitochondrial antibodies in primary biliary cirrhosis. VI. Association of the complement fixing antigen with a component of the mitochondrial F1-ATPase complex. Clin Exp Immunol. 1982;50:267-274. |
| 3. | Berg PA, Muscatello U, Horne RW, Roitt IM, Doniach D. Mitochondrial antibodies in primary biliary cirrhosis. II. The complement fixing antigen as a component of mitochondrial inner membranes. Br J Exp Pathol. 1969;50:200-208. |
| 4. | Lindenborn-Fotinos J, Baum H, Berg PA. Mitochondrial antibodies in primary biliary cirrhosis: species and nonspecies specific determinants of M2 antigen. Hepatology. 1985;5:763-769. |
| 5. | Mackay IR, Whittingham S, Fida S, Myers M, Ikuno N, Gershwin ME, Rowley MJ. The peculiar autoimmunity of primary biliary cirrhosis. Immunol Rev. 2000;174:226-237. |
| 6. | Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791-1799. |
| 8. | Fussey SP, Bassendine MF, James OF, Yeaman SJ. Characterisation of the reactivity of autoantibodies in primary biliary cirrhosis. FEBS Lett. 1989;246:49-53. |
| 9. | Surh CD, Coppel R, Gershwin ME. Structural requirement for autoreactivity on human pyruvate dehydrogenase-E2, the major autoantigen of primary biliary cirrhosis. Implication for a conformational autoepitope. J Immunol. 1990;144:3367-3374. |
| 10. | Fussey SP, Ali ST, Guest JR, James OF, Bassendine MF, Yeaman SJ. Reactivity of primary biliary cirrhosis sera with Escherichia coli dihydrolipoamide acetyltransferase (E2p): characterization of the main immunogenic region. Proc Natl Acad Sci USA. 1990;87:3987-3991. |
| 11. | Koike K, Ishibashi H, Koike M. Immunoreactivity of porcine heart dihydrolipoamide acetyl- and succinyl-transferases (PDC-E2, OGDC-E2) with primary biliary cirrhosis sera: characterization of the autoantigenic region and effects of enzymatic delipoylation and relipoylation. Hepatology. 1998;27:1467-1474. |
| 12. | Tuaillon N, Andre C, Briand JP, Penner E, Muller S. A lipoyl synthetic octadecapeptide of dihydrolipoamide acetyltransferase specifically recognized by anti-M2 autoantibodies in primary biliary cirrhosis. J Immunol. 1992;148:445-450. |
| 13. | Chen QY, Mackay IR, Fida S, Myers MA, Rowley MJ. Natural and disease associated autoantibodies to the autoantigen, dihydrolipoamide acetyltransferase, recognise different epitopes. J Autoimmun. 1998;11:151-161. |
| 14. | Rowley MJ, Scealy M, Whisstock JC, Jois JA, Wijeyewickrema LC, Mackay IR. Prediction of the immunodominant epitope of the pyruvate dehydrogenase complex E2 in primary biliary cirrhosis using phage display. J Immunol. 2000;164:3413-3419. |
| 15. | Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874-5883. |
| 16. | Berg PA, Doniach D, Roitt IM. Mitochondrial antibodies in primary biliary cirrhosis. I. Localization of the antigen to mitochondrial membranes. J Exp Med. 1967;126:277-290. |
| 17. | Klein R, Pointner H, Zilly W, Glässner-Bittner B, Breuer N, Garbe W, Fintelmann V, Kalk JF, Müting D, Fischer R. Antimitochondrial antibody profiles in primary biliary cirrhosis distinguish at early stages between a benign and a progressive course: a prospective study on 200 patients followed for 10 years. Liver. 1997;17:119-128. |
| 18. | Bellucci R, Oertelt S, Gallagher M, Li S, Zorn E, Weller E, Porcheray F, Alyea EP, Soiffer RJ, Munshi NC. Differential epitope mapping of antibodies to PDC-E2 in patients with hematologic malignancies after allogeneic hematopoietic stem cell transplantation and primary biliary cirrhosis. Blood. 2007;109:2001-2007. |
| 19. | Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210-225. |
| 20. | Yeaman SJ, Fussey SP, Danner DJ, James OF, Mutimer DJ, Bassendine MF. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988;1:1067-1070. |
| 21. | Kilshaw PJ, McEwan FJ, Baker KC, Cant AJ. Studies on the specificity of antibodies to ovalbumin in normal human serum: technical considerations in the use of ELISA methods. Clin Exp Immunol. 1986;66:481-489. |
| 22. | Jois J, Omagari K, Rowley MJ, Anderson J, Mackay IR. Enzyme inhibitory antibody to pyruvate dehydrogenase: diagnostic utility in primary biliary cirrhosis. Ann Clin Biochem. 2000;37:67-73. |
| 23. | Robertson CA, Coppel RL, Prindiville T, Fregeau D, Kaplan M, Dickson ER, Gershwin ME. The relative affinity of recombinant dihydrolipoamide transacetylase for autoantibodies in primary biliary cirrhosis. Hepatology. 1990;11:717-722. |
| 24. | Teoh KL, Mackay IR, Rowley MJ, Fussey SP. Enzyme inhibitory autoantibodies to pyruvate dehydrogenase complex in primary biliary cirrhosis differ for mammalian, yeast and bacterial enzymes: implications for molecular mimicry. Hepatology. 1994;19:1029-1033. |
| 25. | Van de Water J, Fregeau D, Davis P, Ansari A, Danner D, Leung P, Coppel R, Gershwin ME. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988;141:2321-2324. |
| 26. | Mattevi A, Obmolova G, Kalk KH, Westphal AH, de Kok A, Hol WG. Refined crystal structure of the catalytic domain of dihydrolipoyl transacetylase (E2p) from Azotobacter vinelandii at 2.6 A resolution. J Mol Biol. 1993;230:1183-1199. |
| 27. | Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217-222. |
| 28. | Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265:8971-8974. |
| 29. | Smolle M, Prior AE, Brown AE, Cooper A, Byron O, Lindsay JG. A new level of architectural complexity in the human pyruvate dehydrogenase complex. J Biol Chem. 2006;281:19772-19780. |
| 30. | Yu X, Hiromasa Y, Tsen H, Stoops JK, Roche TE, Zhou ZH. Structures of the human pyruvate dehydrogenase complex cores: a highly conserved catalytic center with flexible N-terminal domains. Structure. 2008;16:104-114. |
| 31. | Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272:19746-19751. |
| 32. | Mattevi A, Obmolova G, Schulze E, Kalk KH, Westphal AH, de Kok A, Hol WG. Atomic structure of the cubic core of the pyruvate dehydrogenase multienzyme complex. Science. 1992;255:1544-1550. |
| 33. | Bogdanos DP, Dalekos GN. Enzymes as target antigens of liver-specific autoimmunity: the case of cytochromes P450s. Curr Med Chem. 2008;15:2285-2292. |
| 34. | Kiechle FL, Quattrociocchi-Longe TM, Brinton DA, Gordon SC, Sykes E, Elkhalifa MY. Autoantibodies to specific enzymes: a review. Ann Clin Lab Sci. 1996;26:195-207. |
| 35. | Bruggraber SF, Leung PS, Amano K, Quan C, Kurth MJ, Nantz MH, Benson GD, Van de Water J, Luketic V, Roche TE. Autoreactivity to lipoate and a conjugated form of lipoate in primary biliary cirrhosis. Gastroenterology. 2003;125:1705-1713. |
| 36. | Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, Colvin ME, Lam KS, Coppel RL, Ansari A. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956-2963. |
| 37. | Berg PA, Klein R. Mitochondrial antigen/antibody systems in primary biliary cirrhosis: revisited. Liver. 1995;15:281-292. |
| 38. | Jones DE. Pathogenesis of primary biliary cirrhosis. Clin Liver Dis. 2008;12:305-321; viii. |
| 39. | Xu L, Sakalian M, Shen Z, Loss G, Neuberger J, Mason A. Cloning the human betaretrovirus proviral genome from patients with primary biliary cirrhosis. Hepatology. 2004;39:151-156. |
| 40. | Rowley MJ, Maeda T, Mackay IR, Loveland BE, McMullen GL, Tribbick G, Bernard CC. Differing epitope selection of experimentally-induced and natural antibodies to a disease-specific autoantigen, the E2 subunit of pyruvate dehydrogenase complex (PDC-E2). Int Immunol. 1992;4:1245-1253. |
| 41. | Blundell TL, Sibanda BL, Sternberg MJ, Thornton JM. Knowledge-based prediction of protein structures and the design of novel molecules. Nature. 1987;326:347-352. |
| 42. | Horsfall AC, Hay FC, Soltys AJ, Jones MG. Epitope mapping. Immunol Today. 1991;12:211-213. |
| 43. | Mahler M, Blüthner M, Pollard KM. Advances in B-cell epitope analysis of autoantigens in connective tissue diseases. Clin Immunol. 2003;107:65-79. |