Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3371
Revised: April 10, 2010
Accepted: April 17, 2010
Published online: July 21, 2010
AIM: To investigate the effect of extracted soybean saponins on the growth of human colon cancer cells.
METHODS: WiDr human colon cancer cells were treated with 150, 300, 600 or 1200 ppm of soy saponin to determine the effect on cell growth, cell morphology, alkaline phosphatase (AP) and protein kinase C (PKC) activities, and P53 protein, c-Fos and c-Jun gene expression.
RESULTS: Soy saponin decreased the number of viable cells in a dose-dependent manner and suppressed 12-O-tetradecanol-phorbol-13-acetate-stimulated PKC activity (P < 0.05). Cells treated with saponins developed cytoplasmic vesicles and the cell membrane became rougher and more irregular in a dose-dependent manner, and eventually disassembled. At 600 and 1200 ppm, the activity of AP was increased (P < 0.05). However, the apoptosis markers such as c-Jun and c-Fos were not significantly affected by saponin.
CONCLUSION: Soy saponin may be effective in preventing colon cancer by affecting cell morphology, cell proliferation enzymes, and cell growth.
- Citation: Tsai CY, Chen YH, Chien YW, Huang WH, Lin SH. Effect of soy saponin on the growth of human colon cancer cells. World J Gastroenterol 2010; 16(27): 3371-3376
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3371
Colon cancer is one of the major causes of cancer mortality worldwide, which results from interactions of different factors such as aging, family history and dietary style. It has been suggested that consumption of higher levels of soy foods can lead to a lower incidence of acquiring colon cancer[1,2]. The most discussed compounds related to colon cancer in soy are isoflavone and saponins[3]. Saponins have been found to have biological benefits and have been utilized pharmaceutically[4]. Soy saponins are amphiphilic compounds and categorized as triterpenoic saponins. They are able to interact with the cancer cell membranes that are rich in phospholipids and cholesterol and with the hydroxyl groups on the aglycone moiety[5]. Research has found that steroid saponins extracted from fenugreek reduce the number of aberrant crypt foci in azoxytethane-induced rat colon cancer, and induce apoptosis of HT-29 human colon cancer cells[6]. However, whether soy saponins affect the growth of cancer cells by causing apoptosis or necrosis is still not clear.
In this study, we investigated the in vitro physical and biological effects of soy saponins on WiDr colon cancer cells, the same cell line as HT-29[7] (American Type Culture Collection, Rockville, MD, USA; Catalogue 1988), by examining the number of living cells, cell morphology, alkaline phosphatase (AP) and protein kinase C (PKC) activities, and the expression of c-Jun, c-Fos, and P53 protein, and cell apoptosis.
Saponin extraction was performed according to the method of Berhow et al[8]. The purity of crude saponin extracted was examined by high performance liquid chromatography (HPLC) (TSP, Germany) using commercial soy saponin as a standard (Wako, Japan). The HPLC conditions were as follows: C18 column (Vercopak, ODS-3, 4.6 mm × 250 mm); UV absorbance: 190-350 nm; analyzing temperature 30°C; flow rate: 1 mL/min; gradient solvent system: solvent A, 0.05% trifluoroacetic acid in water, solvent B, acetonitrile; 63% A to 52% A in 38 min.
Cells were cultivated in minimal essential medium that contained 10% fetal bovine serum, sodium bicarbonate (1.5 g/L), and 1.0 mmol/L sodium pyruvate at 37°C and 5% CO2. Cells were subcultured into a new medium (100 mm diameter dish) when they reached a high density, at a series of dilutions from 1:3 to 1:6. When they reached 2 × 103 cells/well, cells were cultivated in each well of a 96-well plate. After stable attachment in the medium (day 0), cells were treated with five different concentrations (0, 150, 300, 600, 1200, 2400 ppm) of soy saponins (16 wells/concentration). CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, USA) was used to measure the viability of cells every 24 h for 3 d.
After the cells were treated with different concentrations of soy saponins for 24 h, they were examined by electron microscopy. Scanning electron microscopy (SEM; S-2400; Hitachi, Japan) was performed to observe the differences in cell morphology. Transmission electron microscopy (TEM; H600; Hitachi) was used to investigate intracellular morphology.
Cells were cultivated in a 10-cm Petri dish. After the cells were stable, fresh medium with 0, 150, 300, 600, and 1200 ppm of soy saponins was added. Cells were cultivated for another 72 h before being subjected to tests for proliferation and differentiation. Sodium butyrate (2.5 mmol/L) was used as a positive control for detecting AP activity. The level of differentiation was measured using Alkaline Phophatase Liquicolor (Human, Germany). The activity of PKC was measured using Peptag® Assay (Promega, USA). c-Jun, c-Fos, and wild-type P53 protein expression in WiDr cells was analyzed using SDS-PAGE and western blotting. A 12% resolving gel and a 5% stacking gel were applied. β-actin (43 kDa) was used as the internal control. The antibodies used were rabbit anti-c-Jun polyclonal antibody (Stressgen, Canada), rabbit anti-c-Fos polyclonal antibody, mouse anti-P53 monoclonal antibody, and mouse anti-β-actin monoclonal antibody (Sigma, USA).
Data were expressed as mean ± SD. One-way analysis of variances and Fisher’s least significant difference were performed using SAS 8.13. Differences were significant at P < 0.05.
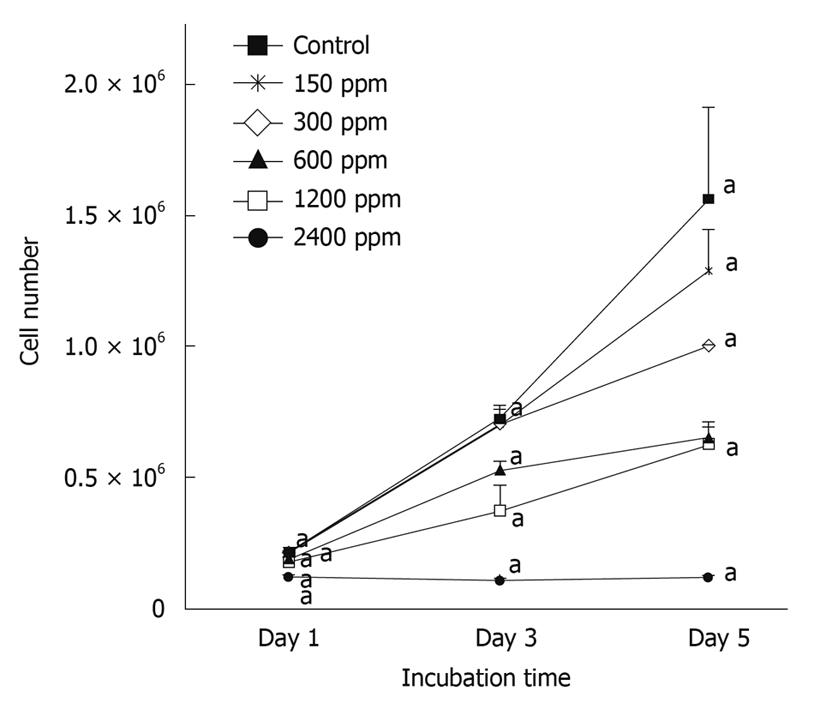
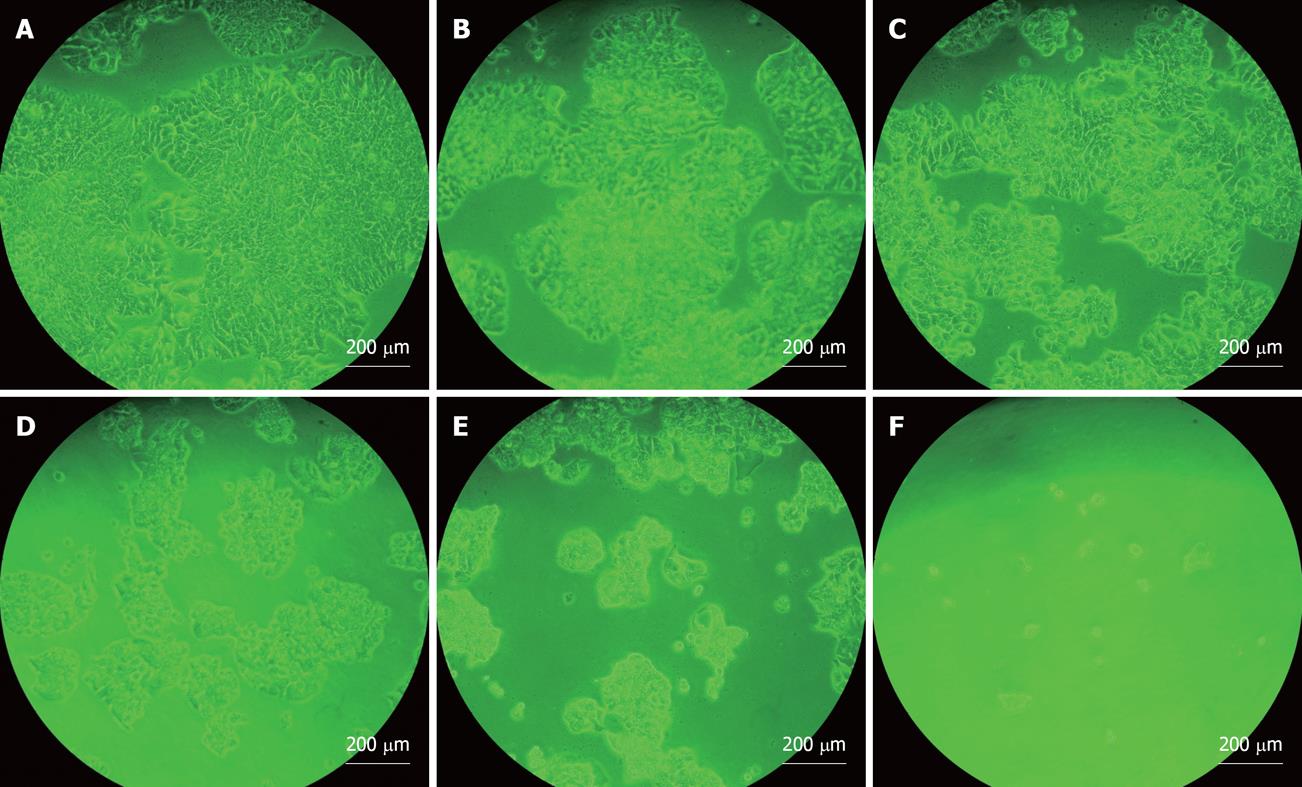
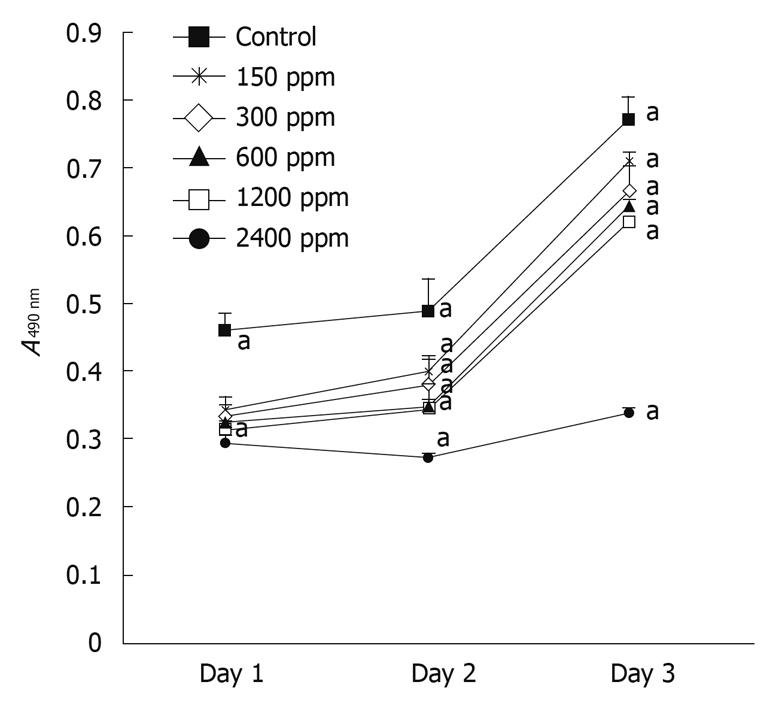
Figure 1 illustrates the dose-dependent inhibitory effect of soy saponins on the number of WiDr cells. At the end of day 1, the cell count was significantly lower in the group treated with 2400 ppm saponins compared to that in the control group (P < 0.05). The percentage inhibition was 40.7%. At the end of day 3, compared to the control group, the number of cells in the groups treated with 600, 1200 and 2400 ppm was significantly lower (P < 0.05), with percentage inhibition of 27.4%, 56.6% and 84.8%, respectively. At the end of day 5, the groups treated with 300, 600, 1200 and 2400 ppm of soy saponins had a lower cell count than the control group (P < 0.05), with percentage inhibition of 36.0%, 57.9%, 59.7% and 92.2%, respectively. Under light microscopic observation at the end of day 5, it was shown that cell density in the medium decreased in parallel with the increase of soy saponins in the medium (Figure 2). Figure 3 shows that under treatment with soy saponins (150-2400 ppm), the percentage cell survival was decreased in a reversed dose-dependent manner (P < 0.05) at each time point.
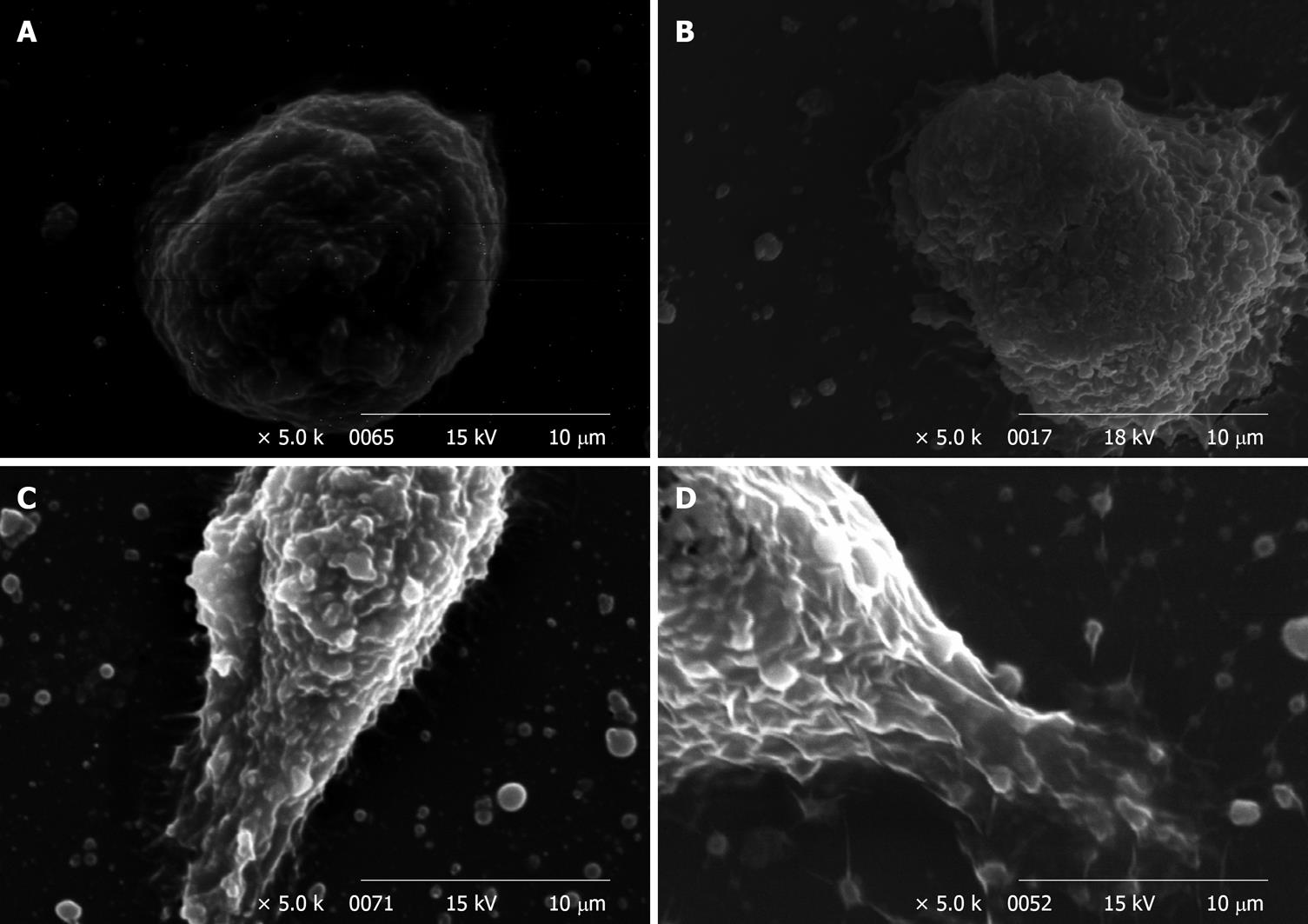
Figure 4A-D shows the SEM observation of WiDr cells treated with 0, 300, 600, 1200 and 2400 ppm of soy saponins. When the dose of soy saponins increased, the surface of WiDr cells became rougher, and the cell shape changed from round to irregular. As the dose reached 1200 ppm (Figure 4C), breaks were seen on the surface of WiDr cells. At 2400 ppm soy saponin, complete deformation of WiDr cells was observed (Figure 4D).
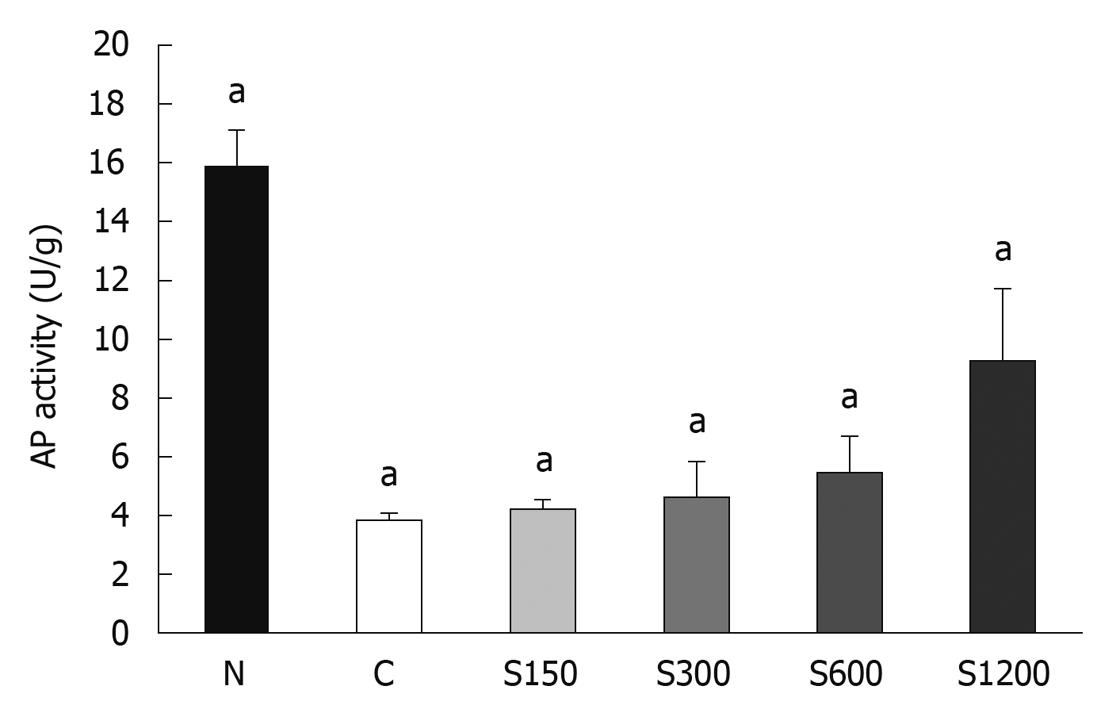
Figure 5 shows that soy saponins induced AP activity in WiDr cells in a dose-dependent manner (P < 0.05). The WiDr cell line is one of the colon cancer cell lines without AP activity. The control sample with sodium butyrate showed increased AP activity, while the one without sodium butyrate did not. The activated AP indicated that WiDr cancer cells might slow down the proliferation process but shift toward the differentiation process.
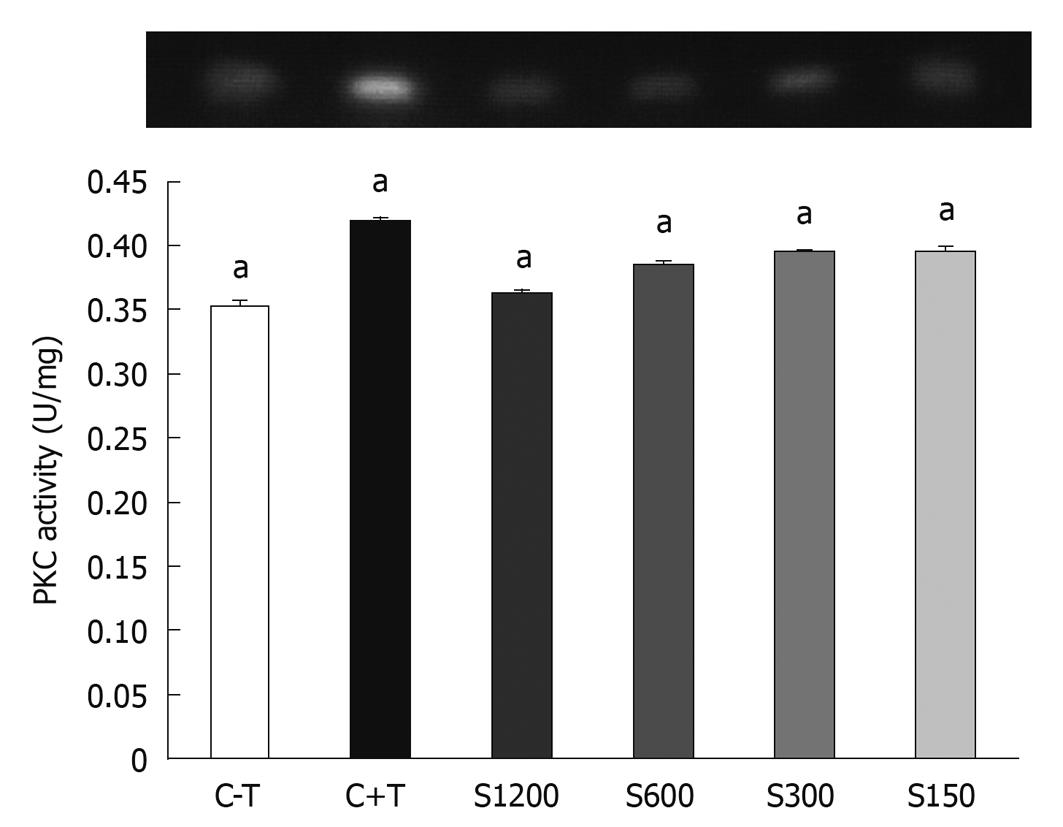
The effect of soy saponin on PKC activity is shown in Figure 6. 12-O-tetradecanoyl phorbol-13-acetate (TPA) was added to the medium to induce PKC activity. The medium without TPA showed no PKC activity. As the dose of saponins in the medium increased, the inhibitory effect on PKC increased.
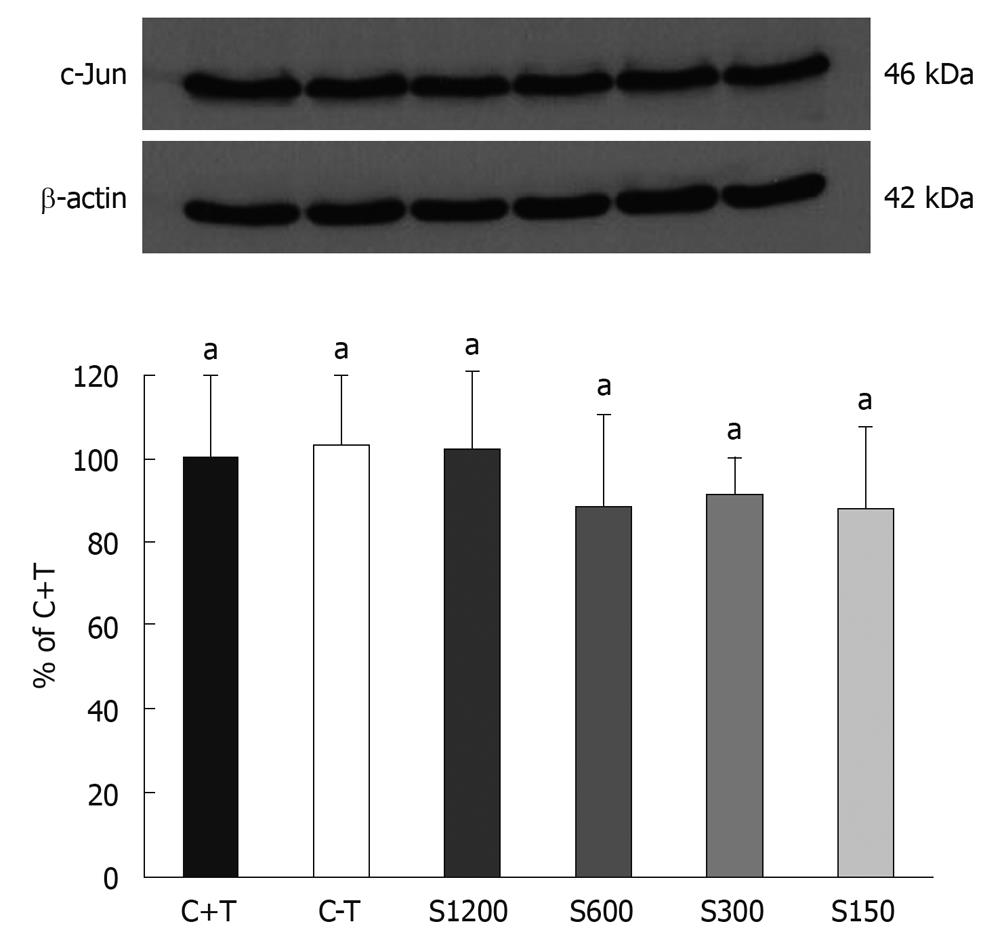
There was no significant difference in the expression of P53 and c-Fos proteins between the groups with/without soy saponin treatment (data not shown). On the other hand, Figure 7 shows a trend towards an inhibitory effect of saponins on the expression of c-Jun after 3 d of incubation.
In this study, we investigated the effects of soy saponin on cell growth, proliferation/differentiation-related enzyme activities, and the expression of apoptosis-related proteins of WiDr human colon cancer cells. We found that soy saponins effectively inhibited the growth rate and survival of human colon cancer cells in a dose-dependent manner. Soy saponins are amphiphilic compounds that can be used as bio-surfactants. They are structurally similar to oleanolic acid and ursolic, which have been shown to be glucocorticoid receptors with anti-carcinogenic activity[9,10].
PKC is one of the markers for cell proliferation. PKC activity increases as the cells undergo the proliferation process. As shown in our study, the addition of soy saponin effectively inhibited the activity of PKC in a dose-dependent manner. On the other hand, P53 protein is responsible for regulating some reactions such as the cell cycle, DNA repair, and apoptosis[11,12]. The relationship between P53 protein and the HT-29 cell death is still not clear[13]. Shen et al[14] have found that 2’-OH flavanone inhibits the growth of HT-29 cells via increasing the expression of P21, but it has no effect on P53 protein. In our study, we did not find any inhibitory effect of soy saponin on the P53 protein of WiDr cells.
Under normal conditions, cells proliferate to a certain level and then differentiate to different kinds of cells. If the cells are stimulated by some exogenous factors, for example, carcinogens, cells may not differentiate, proliferate abnormally, and form tumors. In our study, compared to the control group, the cell number was decreased and AP activity was increased by addition of soy saponins, which is an indication of cell differentiation. It has been shown that materials such as vitamin D3, with membranolic actions, can regulate the transportation of Ca2+ ions through the membrane and induce cell differentiation[15]. Our SEM results showed that cell morphology was changed by saponins, with a similar membranolic effect. Soy saponins may also promote cell differentiation if the cell membrane has not been destroyed by too high a concentration.
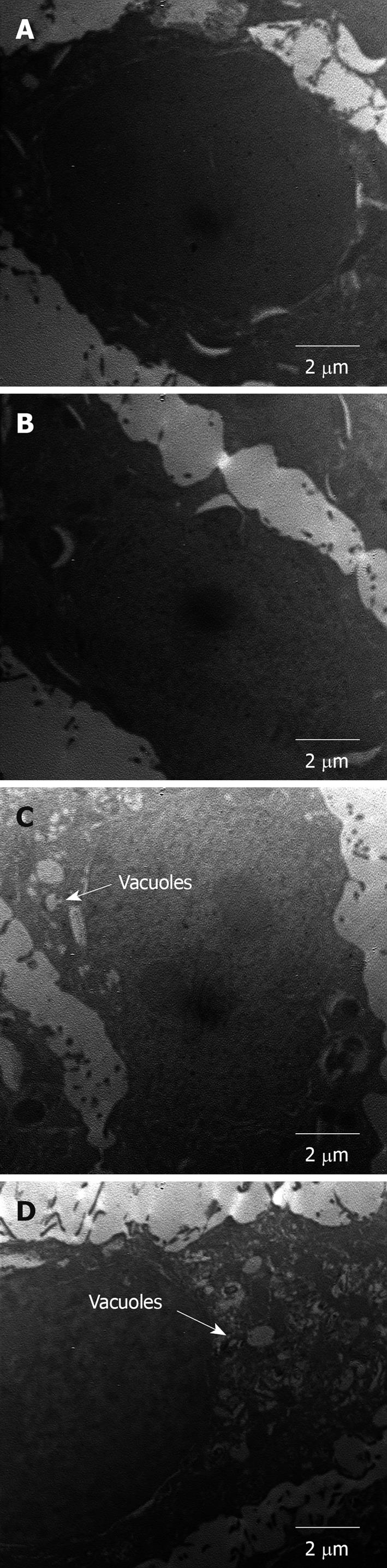
Programmed cell death can be categorized into two types, type I apoptosis and type II autophagic death[16-18]. The major differences in these two types are that, in apoptosis, cells die individually, and phagocytes are necessary for cell degradation. On the other hand, in autophagic death, cells die in groups through a lysosomal mechanism, in which vacuoles are observable in cells[17]. In our SEM study, cell membranes became rough and wrinkled when treated with high concentrations of saponins. In addition, under TEM observation (Figure 8), vacuoles appeared in the cells that had been treated with higher concentrations of saponins, which may be an indication of type II autophagic death. It has been found that the level of microtubule-associated protein light chain 3 is increased after saponin treatment[19], which is an indication of type II autophagic death. For these reasons, the inhibitory effect of soy saponins on WiDr cells may not be apoptosis, but rather autophagic death at higher concentrations.
In conclusion, we found that soy saponins changed the membrane structure and affected the growth of WiDr cells in two different ways; by increasing the AP activity while reducing PKC activity to induce cell differentiation at lower concentrations, or by inducing type II autophagic death at higher concentrations. This may need further investigation.
Colon cancer is one of the major causes of cancer mortality worldwide. Soy saponins are categorized as amphiphilic compounds, and may be able to react with the phospholipids and cholesterol on the membrane of cancer cells, and with the hydroxyl groups on the aglycone moiety.
Steroid saponins extracted from fenugreek reduced the number of colon aberrant crypt foci in azoxymethane-induced rat colon cancer and induced apoptosis of HT-29 human colon cancer cells. However, how soy saponins affect the growth of cancer cells is still not clear. In this study, the authors investigated the in vitro physical and biological effects of soy saponins on WiDr colon cancer cells.
Recent studied have suggested that saponins affect the growth of colon cancer cells. This is believed to be the first thorough study that has focused on the relationship between biomarkers of apoptosis, such as expression of c-Jun, c-Fos, and P53 protein, and cell morphology, proliferation, and differentiation.
By understanding how soy saponins affect colon cells, this study may represent a future strategy for prevention or treatment of colon cancer.
The authors investigated the inhibitory effects of soy saponins on colon cancer cells. Soy saponins inhibited the growth of colon cancer cells by reducing protein kinase C activity, while the features of type II programmed cell death (autophagic death) was observed. It is a well written paper with promising results that may be the basis of forthcoming research in cancer biology and therapy.
Peer reviewer: Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46., Budapest 1088, Hungary
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
| 1. | Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, Gao YT, Zheng W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89:577-583. |
| 2. | Oba S, Nagata C, Shimizu N, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S. Soy product consumption and the risk of colon cancer: a prospective study in Takayama, Japan. Nutr Cancer. 2007;57:151-157. |
| 3. | MacDonald RS, Guo J, Copeland J, Browning JD Jr, Sleper D, Rottinghaus GE, Berhow MA. Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer. J Nutr. 2005;135:1239-1242. |
| 4. | Raju J, Mehta R. Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr Cancer. 2009;61:27-35. |
| 6. | Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392-1398. |
| 7. | Francí C, Egea G, Arribas R, Reuser AJ, Real FX. Lysosomal alpha-glucosidase: cell-specific processing and altered maturation in HT-29 colon cancer cells. Biochem J. 1996;314:33-40. |
| 8. | Berhow MA, Cantrell CL, Duval SM, Dobbins TA, Maynes J, Vaughn SF. Analysis and quantitative determination of group B saponins in processed soybean products. Phytochem Anal. 2002;13:343-348. |
| 9. | Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, Kim KW. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771-778. |
| 10. | Lee HY, Chung HY, Kim KH, Lee JJ, Kim KW. Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids. J Cancer Res Clin Oncol. 1994;120:513-518. |
| 12. | Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594-604. |
| 13. | Kobayashi H, Tan EM, Fleming SE. Sodium butyrate inhibits cell growth and stimulates p21WAF1/CIP1 protein in human colonic adenocarcinoma cells independently of p53 status. Nutr Cancer. 2003;46:202-211. |
| 14. | Shen SC, Ko CH, Tseng SW, Tsai SH, Chen YC. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol Appl Pharmacol. 2004;197:84-95. |
| 15. | Tanaka Y, Bush KK, Klauck TM, Higgins PJ. Enhancement of butyrate-induced differentiation of HT-29 human colon carcinoma cells by 1,25-dihydroxyvitamin D3. Biochem Pharmacol. 1989;38:3859-3865. |
| 16. | Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero S. Senescence, apoptosis or autophagy? When a damaged cell must decide its path--a mini-review. Gerontology. 2008;54:92-99. |
| 17. | Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol. 2002;3:779-787. |
| 18. | Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl). 1990;181:195-213. |
| 19. | Ellington AA, Berhow M, Singletary KW. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis. 2005;26:159-167. |