Published online Aug 14, 2009. doi: 10.3748/wjg.15.3757
Revised: July 6, 2009
Accepted: July 13, 2009
Published online: August 14, 2009
AIM: To investigate RNA interference targeting signal transducer and activator of transcription-3 (STAT3) on invasion of human pancreatic cancer cells.
METHODS: We constructed three plasmids of RNA interference targeting the STAT3 gene. After LV (lentivirus)-STAT3siRNA (STAT3 small interfering RNA) the vector was transfected into the human pancreatic cell line, SW1990 and cell proliferation was measured by the MTT assay. Flow cytometry was used to assess cell cycle. Vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) mRNA and protein expression were examined by quantitative PCR and western blotting, respectively. The invasion ability of SW1990 cells was determined by cell invasion assay.
RESULTS: We successfully constructed the LV-STAT3siRNA lentivirus vector and proved that it can suppress expression of STAT3 gene in SW1990 cells. RNA interference of STAT3 by the LV-STAT3siRNA construct significantly inhibited the growth of SW1990 cells, in addition to significantly decreasing both VEGF and MMP-2 mRNA and protein expression. Moreover, suppression of STAT3 by LV-STAT3siRNA decreased the invasion ability of SW1990 cells.
CONCLUSION: The STAT3 signaling pathway may provide a novel therapeutic target for the treatment of pancreatic cancer since it inhibits the invasion ability of pancreatic cancer cells.
- Citation: Yang G, Huang C, Cao J, Huang KJ, Jiang T, Qiu ZJ. Lentivirus-mediated shRNA interference targeting STAT3 inhibits human pancreatic cancer cell invasion. World J Gastroenterol 2009; 15(30): 3757-3766
- URL: https://www.wjgnet.com/1007-9327/full/v15/i30/3757.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3757
Pancreatic cancer is a highly lethal disease that is usually diagnosed at an advanced stage for which there is little or no effective therapy. It remains the fourth most common cause of cancer-related death in the western world[1]. The annual incidence rate of pancreatic cancer is almost identical to the mortality rate; approximately 37 000 new cases are diagnosed each year in the United States, and approximately 33 000 patients die from this disease[2]. Due to the aggressive natural history of this disease, less than 10% of these cases constitute candidates for surgical resection at the time of diagnosis. Although an adjuvant treatment regimen after surgical resection seems to prolong survival, the precise treatment protocol including drug-of-choice is still debated and the focus of several ongoing clinical trials[3]. Effective systemic therapy capable of reversing the aggressive biology of this disease is currently not available. Thus, understanding the molecular mechanisms of pancreatic cancer is one of the most important issues for treatment.
Signal transducer and activator of transcription 3 (STAT3) is an oncogene that is activated by phosphorylation of a conserved tyrosine residue in response to extracellular signals and oncogenes. Once tyrosine is phosphorylated, STAT3 monomers form dimers through reciprocal phosphotyrosine-SH2 interactions. The dimers are phosphorylated STAT3 (P-STAT3), which translocate to the nucleus and bind to cognate DNA sequences, regulate the transcription of target genes and modulate fundamental cellular processes, such as proliferation and differentiation[4]. Inappropriate and constitutive activation of STAT3 may be responsible for pancreatic cancer progression through regulating the expressions of target genes, such as Bcl-xL, Mcl-1, Bcl-2, Fas, cyclin D1, survivin, c-Myc, VEGF, MMP-2 and MMP-9[5–7]. Many studies have indicated that the constitutive activation of STAT3 influences invasion and metastasis. Specifically, the level of activated STAT3 protein has been found to be associated with invasion in thymic epithelial tumors[8], colorectal adenocarcinoma[9], and cutaneous squamous cell carcinoma[10]. Conversely, inhibition of the STAT3 signaling pathway suppresses cancer cell growth, invasion and induces apoptosis in various cancers[11]. Thus, the STAT3 signaling pathway may be one of the common pathways involved in regulating cancer invasion.
Small hairpin RNA (shRNA) expression vector systems have been established to induce RNA interference (RNAi) in mammalian cells[12]. Although these vectors provide certain advantages over chemically synthesized siRNAs, some disadvantages remain, including transient shRNA expression and low transfection efficiency, especially in non-dividing primary cells. To overcome these limitations, shRNA delivery systems using retroviral vectors[13], adenoviral vectors[14] and, more recently, lentiviral vectors[15] have been reported and proven to be safe for humans. Lentivirus vectors encoding antisense targeting sequence have been used in clinical trials with no obvious side effects[1617]. Lentivirus-delivered shRNAs are capable of specific, highly stable and functional silencing of gene expression in a variety of human cells including primary non-dividing cells and also in transgenic mice[1819].
In our study, we constructed a lentivirus vector mediating RNAi targeting of STAT3 (LV-STAT3siRNA). The efficacy of LV-STAT3siRNA plasmids in interference with STAT3 was confirmed by real-time PCR and western blotting. We found that LV-STAT3siRNA suppressed growth and invasion by markedly decreasing the expression of VEGF and MMP-2 in SW1990 cells, but LV-Con (control) had no effect on SW1990 cells. Since the STAT3 signaling pathway is critical for growth and the invasive behavior of pancreatic cancer, silencing of the STAT3 gene with RNAi may provide a novel strategy for investigation of the role of STAT3 gene in the invasion of human pancreatic cancers.
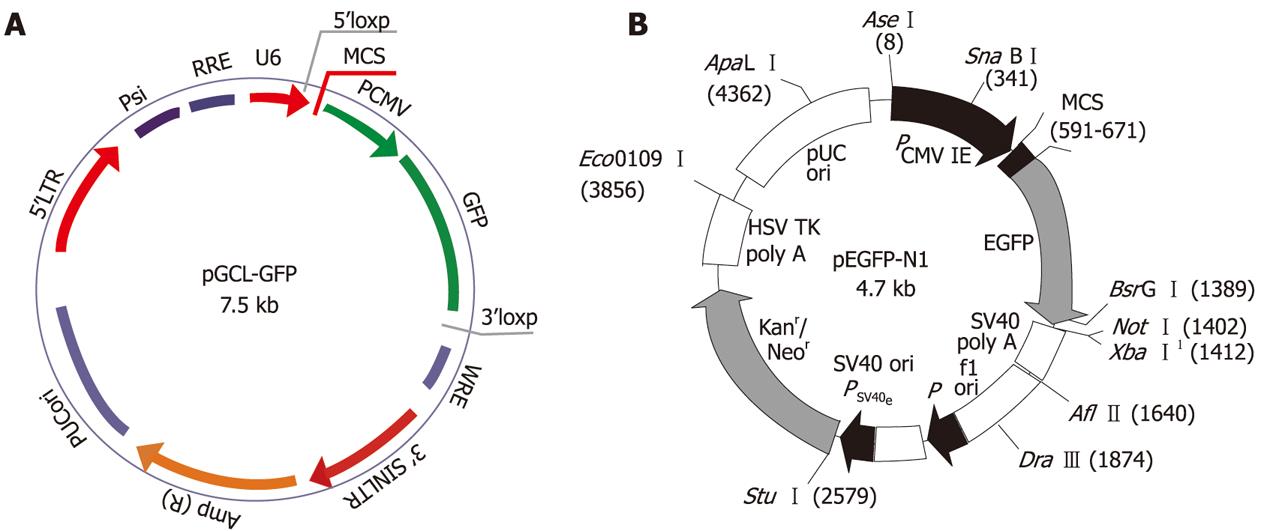
We designed and cloned a shRNA template into a lentivirus vector. A third generation self-inactivating lentivirus vector containing a CMV-driven GFP reporter and a U6 promoter upstream of the cloning restriction sites (Hpa I and Xho I) was used. The introduction of oligonucleotides encoding shRNAs (Figure 1A) between these restriction sites enables the production of the shRNA in vivo. Three coding regions corresponding to targeting human STAT3 starting at positions 466, 1638, and 1061 in the sequence (GenBank Accession: NM 39276) were selected as siRNA target sequences under the guide of siRNA designing software offered by Genscript. We constructed three shRNA-STAT3 lentivirus vectors, namely LV-STAT3siRNA-1, LV-STAT3siRNA-2 and LV- STAT3siRNA-3, respectively (Table 1). Briefly, oligonucleotides were annealed, digested and inserted between the Hpa I and Xho I restriction sites of the plasmid vector. Some mutations were introduced in the sense sequence of the hairpin structure to facilitate sequence and avoid destruction by bacteria during amplification in the bacterial host. Correct insertions of shRNA cassettes were confirmed by restriction mapping and direct DNA sequencing. We constructed a STAT3 over-expression vector, pEGFP-N1-STAT3 (Figure 1B), and co-transfected with recombinant lentivirus vectors into 293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). To detect the interference effects of different target, STAT3 protein expression was determined using western blotting. Recombinant lentivirus vectors and control lentivirus vectors were produced by co-transfecting 293T cells with the lentivirus expression plasmid and packaging plasmids (pHelper 1.0 including gag/pol and pHelper 2.0 including VSV-g). Infectious lentivirus vectors were harvested at 48 h post-transfection, centrifuged to remove cell debris, and filtered through 0.45 μm cellulose acetate filters. The infectious titer was determined by hole-by-dilution titer assay. The virus titers produced were approximately 109 transducing U/mL medium.
| Number | Sequence |
| LV-STAT3siRNA-1 | Oligo1: 5’TaaTGTTCTCTATCAGCACAATTTCAAGAGAATTGTGCTGATAGAGAACATTTTTTTTC3’ |
| Oligo2: 5’TCGAGAAAAAAaaTGTTCTCTATCAGCACAATTCTCTTGAAATTGTGCTGATAGAGAACATTA3’ | |
| LV-STAT3siRNA-2 | Oligo1: 5’TaaCATCTGCCTAGATCGGCTATTCAAGAGATAGCCGATCTAGGCAGATGTTTTTTTTC3’ |
| Oligo2: 5’TCGAGAAAAAAaaCATCTGCCTAGATCGGCTATCTCTTGAATAGCCGATCTAGGCAGATGTTA3’ | |
| LV-STAT3siRNA-3 | Oligo1: 5’TaaCTTCAGACCCGTCAACAAATTCAAGAGATTTGTTGACGGGTCTGAAGTTTTTTTTC3’ |
| Oligo2: 5’TCGAGAAAAAAaaCTTCAGACCCGTCAACAAATCTCTTGAATTTGTTGACGGGTCTGAAGTTA 3’ |
The human pancreatic cancer cell line, SW1990, and 293T cells were purchased from the American Type Culture Collection. Cells were grown in 5% CO2 saturated humidity, at 37°C and cultured as monolayers in RPMI 1640 supplemented with penicillin/streptomycin, 2 mmol/L glutamine and 10% FBS. Cells were subcultured at 1 × 105 cells per well into six-well tissue culture plates. After 24 h culture, cells were infected with recombinant lentivirus vectors at a multiplicity of infection (MOI) of 40.
Cell proliferation was determined by 3-(4,5-dimethylthiazole-2-yl)-2.5-diphenyltetrazolium bromide (MTT) assay. Pancreatic cancer cells were seeded in 96-well culture plates in culture medium at an optimal density (5 × 103 cells per well) in triplicate wells for the LV-STAT3siRNA, LV-Con and parental cells groups. After 1, 2, 3 and 4 d, cells were stained with 20 μL MTT (5 mg/mL) (Sigma, St Louis, MO, USA) at 37°C for 4 h and subsequently made soluble in 150 μL of DMSO. Absorbance was measured at 490 nm using a microtiter plate reader. Cell growth curves were calculated as mean values of triplicates per group.
The cell cycle effects of LV-STAT3siRNA on human pancreatic cancer cells were analyzed by flow cytometry. Briefly, 1 × 105 cells were trypsinized, washed with PBS, centrifuged at 800 r/min and fixed with 70% cold ethanol overnight. Ethanol was aspirated after centrifugation at 1000 r/min, and the cells were incubated in a solution of propidium iodide (PI) (100 mg), 0.5% Triton-X100 and RNase (2000 mg/mL) for 15 min and analyzed by flow cytometry (FCM).
Total RNA was isolated using TRIzol LS (Invitrogen, Carlsbad, CA, USA). The concentration and purity of RNA was determined using a spectrophotometer. M-MLV reverse transcriptase (Promega, Madison, WI, USA) was used to create cDNA for further analyses. Quantitative real-time polymerase chain reaction (RT-PCR) assays were carried out using SYBR Green Real-Time PCR Master Mix (Toyobo, Osaka, Japan) and real-time PCR amplification equipment.
The PCR primers used to detect STAT3 and β-actin were as follows: STAT3, sense strand 5'-CCAAGGAGGAGGCATTCG-3', antisense strand 5'-ACATCGGCAGGTCAATGG-3', with a product length of 147 bp, β-actin, sense strand 5'-TCGTGCGTGACATTAAGGAG-3', antisense strand 5'-AAGGTAGTTTCGTGGATGCC-3', with a product length of 214 bp. The thermal profile consisted of one cycle at 95°C for 15 min, followed by 45 cycles at 95°C for 15 s and 60°C for 30 s. The expression of STAT3 was determined by normalization of the threshold cycle (Ct) of these genes to that of the control housekeeping gene (β-actin). The delta Ct was determined using the following equation: (ΔCt) = (Ct of STAT3) - (Ct of β-actin in each group). The ΔΔCt value obtained was used to find the relative expression of the STAT3 gene according to the following formula: Relative expression = 2-ΔΔCt, ΔΔCt = (mean ΔCt of STAT3 genes in LV-STAT3siRNA groups) - (ΔCt of STAT3 genes in LV-Con group or parental SW1990 group).
The PCR primers used to detect MMP-2, VEGF and β-actin were as follows: MMP-2 sense strand 5'-TAGCATGTCCCTACCGAGTCT-3', antisense strand 5'-ATTGGATGGCAGTAGCTGC-3', with a product length of 151 bp; VEGF sense strand 5'-CTGTCTTGGGTGCATTGGA-3', antisense strand 5'-ATTGGATGGCAGTAGCTGC-3', with a product length of 152 bp; β-actin sense strand 5'-CACCAACTGGGACGACAT-3', antisense strand 5'-ATCTGGGTCATCTTCTCGC-3', with a product length of 138 bp. PCR parameters were as follows: 95°C for 20 min, then 95°C for 30 s, 56°C for 30 s, 72°C for 40 s for 40 cycles, 72°C for 10 min. A standard calibration curve for the expression of each mRNA was generated using 8-fold dilutions of a control RNA sample. MMP-2 and VEGF mRNA expression was calculated as a ratio compared with that of β-actin.
Whole-cell protein extracts and nuclear protein extracts from pancreatic cancer cells were prepared with RIPA Lysis Buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA), respectively, according to the manufacturers’ instructions. Protein concentrations were determined using an assay kit (Bio-Rad, Hercules, CA, USA).
Lysates containing 100 μg of protein were mixed with loading buffer with 5% β-mercaptoethanol and heated for 5 min at 100°C. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes by semi-dry blotting.
Membranes were incubated in blocking buffer [tris-buffered saline (TBS), 0.1% Tween 20, and 5% non-fat dry milk] for 1 h at room temperature, followed by hybridization with anti-P-STAT3 (tyr-705) antibody (Cell Signaling Technology, 1:1000 dilution), anti-STAT3 antibody (Cell Signaling Technology, 1:1000 dilution), anti-MMP-2 antibody (Santa Cruz Biotechnology, 1:500 dilution), anti-VEGF antibody (Santa Cruz Biotechnology, 1:500 dilution), GADPH antibody (Lab Vision, Fremont, CA, USA, 1:100 dilution) or anti β-actin antibody (Lab Vision, Fremont, CA, USA, 1:100 dilution) at 4°C overnight.
After 3 washes in TBS/0.1% Tween 20, the membranes underwent hybridization with a horseradish peroxidase-conjugated secondary antibody rabbit IgG (Santa Cruz Biotechnology, 1:5000 dilution) for 1 h at room temperature. After 3 washes in TBS/0.1% Tween 20, signals were detected by chemiluminescence using luminol reagent (Santa Cruz Biotechnology).
The cell invasion assay was performed using a specialized invasion chamber that included a 24-well tissue culture plate with 12 cell culture inserts (Chemicon, Temecula, CA, USA). The inserts contained an 8 μm pore size polycarbonate membrane with a precoated thin layer of basement membrane matrix (ECMatrix). Briefly, media supplemented with 10% fetal bovine serum was added to the lower chamber as a chemo-attractant. After reaching 60%-70% subconfluence, pancreatic cancer cells were trypsinized, re-suspended in DMEM at 1 × 106 cells/mL, and 300 μL of the cell suspension was added to each upper compartment.
After 24 h incubation at 37°C, non-invasive cells were removed from the upper surface of the membrane using a moist cotton-tipped swab. Invasive cells on the lower surface of the membrane, which had invaded the ECMatrix and had migrated through the polycarbonate membrane, were stained with the staining solution for 20 min and rinsed with distilled water several times. Invasiveness was quantitated by selecting ten different views (400 times) and calculating the number of invading cells.
All assays were conducted three times and found to be reproducible. Data were expressed as mean ± SD and the statistical correlation of data between groups was analyzed by Student’s t test, where P < 0.05 were considered significant. These analyses were performed using SPSS 11.0 software.
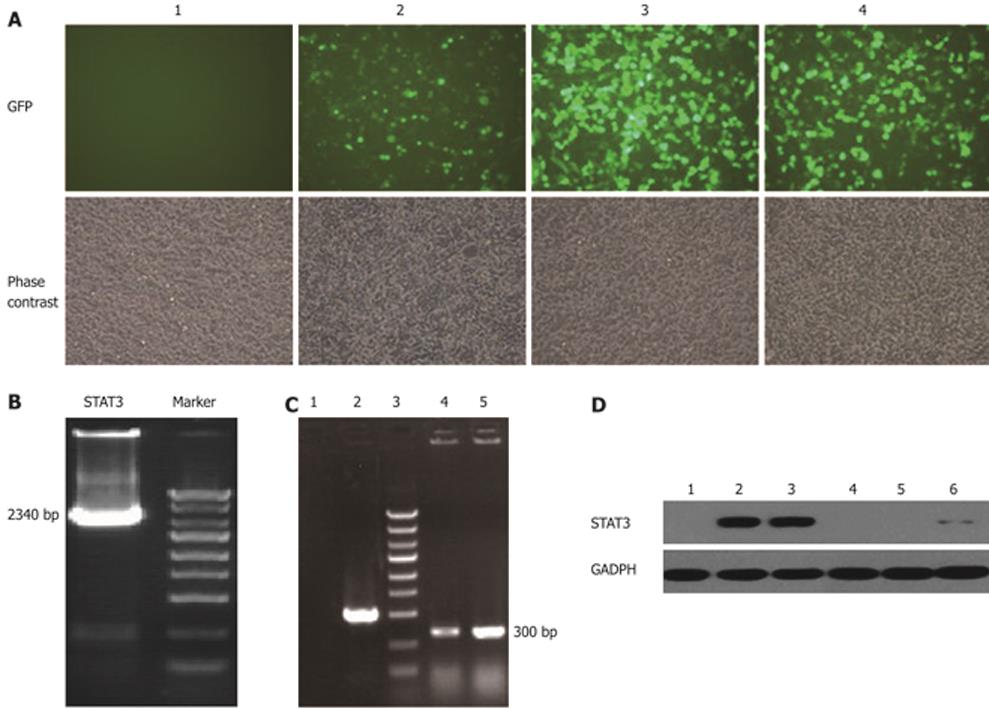
Three plasmids containing shSTAT3 (pGCL-GFP-STAT3siRNA) and pEGFP-N1-STAT3 were co-transfected into 293T cells, respectively. GFP expression in 293T cells was observed under a fluorescent microscope 36-48 h after transfection with pEGFP-N1-STAT3 and pGCL-GFP-STAT3siRNA. Results of the western blotting assay showed that LV-STAT3siRNA-1 and LV-STAT3siRNA-2 could significantly suppress the expression of STAT3 at the protein level in 293T cells. According to the results of western blotting assay, LV-STAT3siRNA-2 was the most effective lentivirus vector and, thus, was used in the following research (Figure 2).
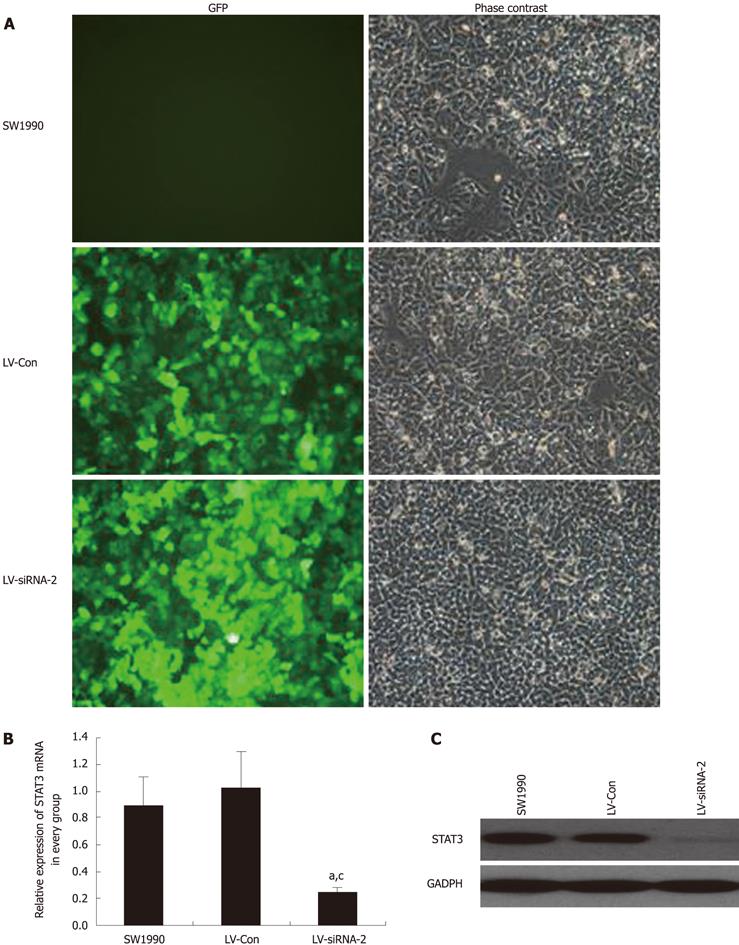
To determine the effect of LV-STAT3siRNA on the expression of STAT3, GFP expression was observed under a fluorescent microscope in SW1990 cells 72 h after infection with LV- siSTAT3-2 at an MOI of 40. Next, real-time PCR and western blotting were performed to determine the mRNA and protein levels of STAT3 in LV-STAT3siRNA-2, LV-Con and parental cell groups. These analyses demonstrated that LV-STAT3siRNA-2 significantly inhibited expression of STAT3 mRNA (P = 0.006, P = 0.007) and protein compared with SW1990 cells and the LV-Con group (Figure 3).
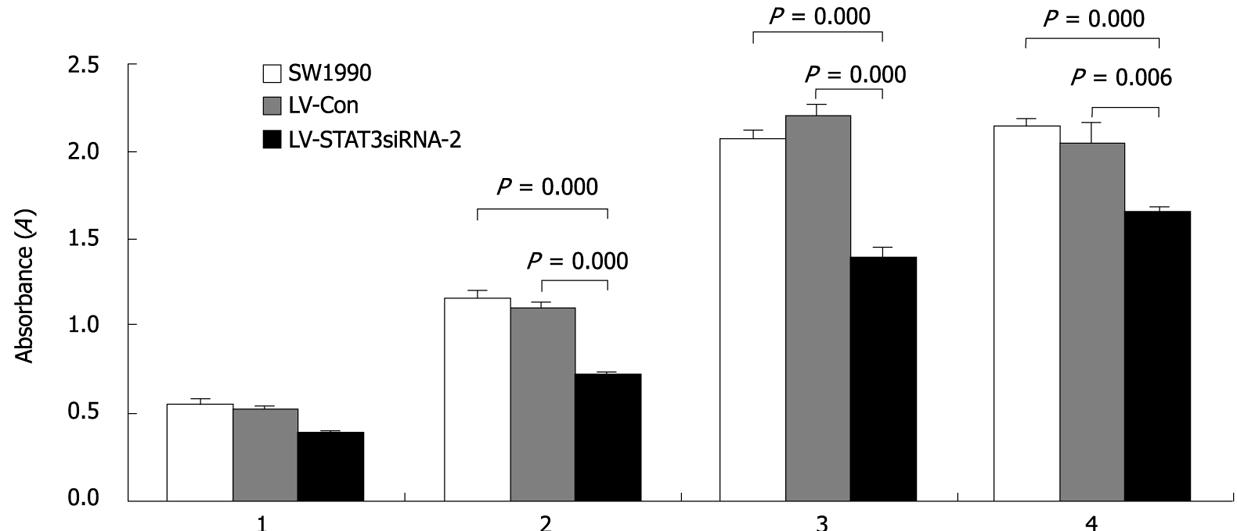
Cell proliferation was monitored for four days after SW1990 cells were infected with LV-STAT3siRNA-2 and LV-Con. The growth of cells infected with LV-STAT3siRNA-2 was markedly inhibited compared with LV-Con and parental SW1990 (Figure 4).
To investigate the effects of LV-STAT3siRNA-2 on cell cycle, G1, G2 and S phase cells were detected by flow cytometric analysis. The total S phase plus G2 phase fraction was used to measure cell proliferation. SW1990 cells were infected with LV-STAT3siRNA-2 and cell proliferation after 72 h was detected by flow cytometry. Consistent with the MTT assay (Table 2), LV-STAT3siRNA-2 caused a significant reduction in cell proliferation compared with the control, LV-Con (P = 0.003) and parental SW1990 groups (P = 0.008).
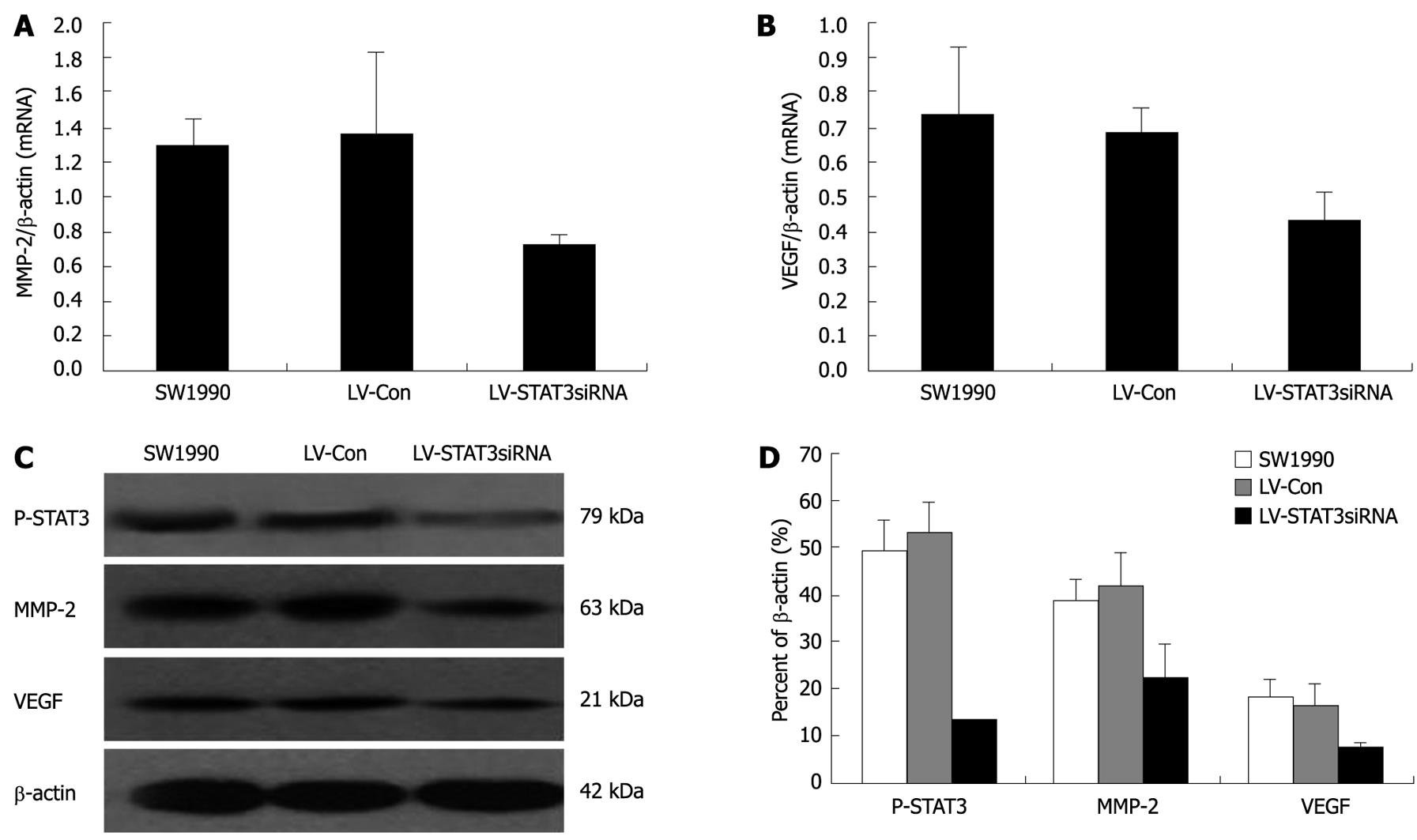
The expression of P-STAT3, MMP-2 and VEGF was analyzed at the mRNA level by real-time PCR (Figure 5A and B). Protein expression was evaluated by western blotting (Figure 5C and D) in the human pancreatic cancer cell lines. Silencing of STAT3 expression by stable transfection of LV-STAT3siRNA-2 significantly decreased the expression of MMP-2 (P = 0.004, P = 0.008) and VEGF (P = 0.006, P = 0.0015) mRNA compared with the control, SW1990 and LV-Con groups. LV-STAT3siRNA-2 could markedly downregulate protein levels of P-STAT3 (P = 0.001, P = 0.000), VEGF (P = 0.031, P = 0.025), and MMP-2 (P = 0.007, P = 0.026) in SW1990 cells compared with the SW1990 and LV-Con groups.
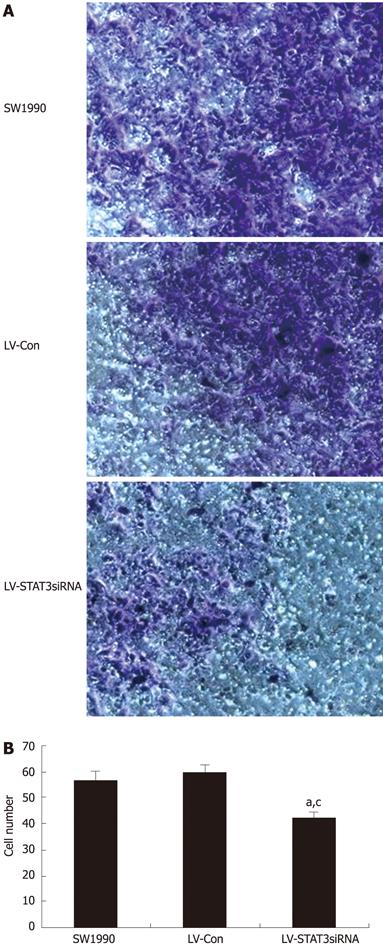
An in vitro cell invasion assay was performed and the number of invading cells counted. The control group, LV-Con, had no effect on invasion of SW1990 cells, however, LV-STAT3siRNA-2 markedly reduced the invasion ability compared with LV-Con (P = 0.004) and parental SW1990 (P = 0.001) (Figure 6).
STAT3 is a key signal transduction protein that mediates signaling by numerous cytokines, peptide growth factors, and oncoproteins. The Janus kinase (JAK)/STAT3 signaling pathway plays a significant role in various physiological processes, including immune function, cell growth, differentiation, and hematopoiesis[20]. However, STAT3 has been implicated in important roles for cell proliferation, invasion and metastasis in diverse human cancers, including pancreatic cancer. Recent studies have also revealed that the promotion of STAT3 activation can contribute to oncogenesis. For example, Huang[1121] and coworkers reported that activation of the STAT3 signaling pathway plays an important role in the progression of pancreatic cancer. These published reports all demonstrated the crucial importance of the JAK/STAT3 signaling pathway in tumorigenesis and progression. In this study, our aim was to determine the role of the JAK/STAT3 signaling pathway in pancreatic cancer progression and to test the hypothesis that the STAT3 gene could serve as a therapeutic target.
RNAi is the process by which double-stranded RNA induces potent and specific inhibition of eukaryotic gene expression through the degradation of complementary messenger RNA, and is functionally similar to the processes of post-transcriptional gene silencing[2223]. In the past few years, RNAi has been widely used by researchers to silence the expression of many target genes because of their high specificity and apparent non-toxicity[24]. Furthermore, systems based on lentiviral vectors have provided new solutions to achieving stable shRNA-mediated knockdown[25]. In this research, we chose a lentivirus vector as our shRNA delivery vehicle because they can infect both dividing and nondividing cells at a high efficiency and sustain long-term gene expression by integrating into the host genome. shRNA was proved to provide long-lasting silencing and maximal inhibition of gene expression at low concentration[26]. Since the potency of the inhibitory effect of shRNA is related to the specificity to its target sequence, we used RT-PCR and western blotting to confirm the efficacy of STAT3siRNA in SW1990 and 293T cells. The greatest STAT3 gene silencing effect was observed when LV-STAT3siRNA-2 was applied and the expression of STAT3 mRNA and protein were markedly inhibited. Given this result, we concluded that LV-STAT3siRNA-2 had a high specificity for STAT3 in SW1990 cells.
Constitutive activation of STAT3 is observed in many types of tumors and promotes cell proliferation and survival[2728]. Inappropriate and constitutive activation of STAT3 may be responsible for pancreatic cancer progression by regulating the expression of target genes, such as c-Myc, Bcl-xL, p21WAF1 and cyclinD1. Moreover, functional inactivation of STAT3 by dominant-negative STAT3 or AG490 (a JAK-specific inhibitor) could inhibit proliferation and promote the apoptosis of pancreatic cancer cells[2930]. In our study, cell cycle and proliferation assays revealed that LV-STAT3siRNA-2 markedly inhibited cell growth and proliferation.
An increasing number of studies suggest that the activation of STAT3 might play an important role in the invasion and metastasis of carcinomas[5]. Furthermore, disruption of the STAT3 signaling pathway has been reported to suppress cell invasion by decreasing cell-cell homotypic adhesions and increasing cell motility and scattering[31]. In the present study, the invasion ability of these cells with a cell invasion assay was examined and found that STAT3 silencing by RNAi in SW1990 cells resulted in a weak level of invasiveness. Therefore, there is a strong relationship between STAT3 and the invasive ability of human pancreatic cancer cells.
Tumor invasion and metastasis is dependent on angiogenesis, the formation of new blood vessels from a pre-existing network of capillaries. VEGF is known to be a potent angiogenic mitogen that plays an important role in tumor angiogenesis, invasion, and metastasis[32]. The role of STAT3 in angiogenesis was first shown when VEGF was found to be a direct target of STAT3 in mouse melanoma cells[6] and then confirmed by a study in a human pancreatic cancer system[33]. According to studies of clinical samples from pancreatic cancer and pancreatic cancer cell lines, MMPs play important roles in tumor cell invasion and metastasis by degrading components of the basement membranes and extracellular matrix[34–36]. Specifically, activated STAT3 regulates tumor invasion of melanoma cells by regulating the transcription of the MMP-2 gene[737]. In the present study, inhibition of the STAT3 gene by RNAi markedly decreased both MMP-2 and VEGF expression in the pancreatic cancer cell line, SW1990. This suggests that silencing of the STAT3 gene could suppress invasion ability based on the down-regulation MMP-2 and VEGF gene expression in SW1990 cells.
Overall, the present study indicates that siRNA targeting of STAT3 mRNA via a lentivirus vector system effectively sustains knockdown of the STAT3 gene expression in SW1990 cells. Here we describe the successful construction of a lentivirus RNAi vector targeting STAT3 that will provide a useful tool to study the function of the STAT3 gene in pancreatic cancer cells. Our findings strongly suggest that the JAK/STAT3 pathway plays a significant role in pancreatic cancer cell invasion. Targeting of STAT3 activation may prove to be a more effective approach to controlling invasion than merely targeting individual molecules, such as VEGF and MMP-2, representing a novel approach to regulating pancreatic cancer invasion.
Signal transducer and activator of transcription 3 (STAT3) is a member of the JAK/STAT signaling pathway. Abnormal activation of STAT3 plays a critical role in metastasis and invasion in a variety of human tumors including pancreatic cancer. The authors aim was to study the effect of silencing of STAT3 on invasion in human pancreatic cancer cells.
Activated STAT3 has been shown to promote cell proliferation, metastasis, and angiogenesis, as well as protect tumor cells from apoptosis by regulating associated genes, such as Bcl-xL, Mcl-1, Bcl-2, Fas, cyclin D1, survivin, c-Myc, VEGF, MMP-2, and MMP-9. The authors sought to determine whether the STAT3 signaling pathway regulates the invasive potential of pancreatic cancer cells. The inhibition of STAT3 may offer a novel strategy for pancreatic cancer intervention.
The authors successfully constructed the lentivirus LV-STAT3siRNA vector. Expression of LV-STAT3siRNA can suppress expression of STAT3 gene in SW1990 cells. Flow cytometry analysis showed that the cell cycle of SW1990 cells was inhibited by LV-STAT3siRNA compared with controls. Moreover, LV-STAT3siRNA significantly suppressed the invasion ability of SW1990 cells by down-regulating the VEGF and MMP-2 genes.
Lentivirus vectors are safe for human use. Lentivirus vectors encoding the antisense targeting sequence have been used previously for treatment in clinical trials with no obvious side effects. Most recently, a lentivirus vector containing β-globin gene has been approved in phase I/II clinical trials for human β-thalassemia and sickle cell anemia gene therapy. Targeting of STAT3 activation may prove to be an effective approach to controlling invasion in pancreatic cancer cells.
This is an interesting manuscript. The authors utilized three different siRNAs against STAT3 and infected the pancreatic cell line SW1990. They show that siRNA is the most effective at suppressing protein levels of VEGF, MMP-9 downstream effector molecules of STAT3 signaling pathway as well as STAT3 itself. They also show siRNA suppressed the proliferative capacity of SW1990 cells and also its invasive ability.
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. |
| 2. | Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Metastasis Rev. 2008;27:495-522. |
| 3. | Boeck S, Ankerst DP, Heinemann V. The role of adjuvant chemotherapy for patients with resected pancreatic cancer: systematic review of randomized controlled trials and meta-analysis. Oncology. 2007;72:314-321. |
| 5. | Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13:1362-1366. |
| 6. | Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-2008. |
| 7. | Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550-3560. |
| 8. | Chang KC, Wu MH, Jones D, Chen FF, Tseng YL. Activation of STAT3 in thymic epithelial tumours correlates with tumour type and clinical behaviour. J Pathol. 2006;210:224-233. |
| 9. | Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Nagayasu T, Sekine I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833-838. |
| 10. | Suiqing C, Min Z, Lirong C. Overexpression of phosphorylated-STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma. J Dermatol. 2005;32:354-360. |
| 11. | Huang C, Cao J, Huang KJ, Zhang F, Jiang T, Zhu L, Qiu ZJ. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006;97:1417-1423. |
| 12. | Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550-553. |
| 13. | Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243-247. |
| 14. | Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006-1010. |
| 15. | Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183-188. |
| 16. | Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM, Schonely K, Ni Y, Binder GK, Levine BL. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Hum Gene Ther. 2005;16:17-25. |
| 17. | Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308-316. |
| 18. | Nishitsuji H, Ikeda T, Miyoshi H, Ohashi T, Kannagi M, Masuda T. Expression of small hairpin RNA by lentivirus-based vector confers efficient and stable gene-suppression of HIV-1 on human cells including primary non-dividing cells. Microbes Infect. 2004;6:76-85. |
| 19. | Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401-406. |
| 20. | Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406-6417. |
| 21. | Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li H, Jiang T, Huang K, Cao J. RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci. 2007;98:1099-1106. |
| 23. | Lee SH, Sinko PJ. siRNA--getting the message out. Eur J Pharm Sci. 2006;27:401-410. |
| 24. | Gartel AL, Kandel ES. RNA interference in cancer. Biomol Eng. 2006;23:17-34. |
| 25. | Li M, Rossi JJ. Lentiviral vector delivery of siRNA and shRNA encoding genes into cultured and primary hematopoietic cells. Methods Mol Biol. 2005;309:261-272. |
| 26. | Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222-226. |
| 27. | Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502-2512. |
| 28. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. |
| 29. | Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891-905. |
| 30. | Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M, Tanaka M. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107-116. |
| 31. | Rivat C, De Wever O, Bruyneel E, Mareel M, Gespach C, Attoub S. Disruption of STAT3 signaling leads to tumor cell invasion through alterations of homotypic cell-cell adhesion complexes. Oncogene. 2004;23:3317-3327. |
| 32. | Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592-1598. |
| 33. | Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319-329. |
| 34. | Bloomston M, Zervos EE, Rosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668-674. |
| 35. | Matsuyama Y, Takao S, Aikou T. Comparison of matrix metalloproteinase expression between primary tumors with or without liver metastasis in pancreatic and colorectal carcinomas. J Surg Oncol. 2002;80:105-110. |
| 36. | Tan X, Egami H, Ishikawa S, Sugita H, Kamohara H, Nakagawa M, Nozawa F, Abe M, Ogawa M. Involvement of matrix metalloproteinase-7 in invasion-metastasis through induction of cell dissociation in pancreatic cancer. Int J Oncol. 2005;26:1283-1289. |
| 37. | Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188-3196. |