Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7380
Revised: November 28, 2005
Accepted: October 23, 2006
Published online: December 7, 2006
AIM: To recognize cystic neoplasia of the pancreas and thus to identify a panel of curable diseases.
METHODS: Sixty-four cases of cystic neoplasia of the pancreas, including 28 cases of intraductal papillary mucinous neoplasia (IPMN), 12 cases of serous cystic neoplasia (SCN), 11 cases of mucinous cystic neoplasia (MCN), 11 cases of solid pseudo-papillary neoplasia (SPN), and 2 cases of solid tumor with cystic degeneration were examined immunohistochemically for their expression of MUC1, MUC2, MUC4, MUC5AC, and MUC6, as well as other related antigens.
RESULTS: Adenoma type of IPMN and borderline lesions exhibited high expressions of MUC2, and MUC5AC. In contrast, IPMN with invasive carcinoma component showed MUC1 immunoreactivity. SCN was mainly positive for MUC1 and MUC6, while negative for MUC2, MUC4 and MUC5AC. Noninvasive MCN, regardless of its cellular atypia degree, was positive for MUC5AC and negative for MUC1. MUC1 expression was only observed in patients with an invasive component. No mucin expression was found in SPN.
CONCLUSION: Mucin profile may, in conjunction with histologic study, provide important information on tumor types and patient treatment of cystic neoplasia of the pancreas.
- Citation: Ji Y, Lou WH, Jin DY, Kuang TT, Zeng MS, Tan YS, Zeng HY, Sujie A, Zhu XZ. A series of 64 cases of pancreatic cystic neoplasia from an institutional study of China. World J Gastroenterol 2006; 12(45): 7380-7387
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7380.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7380
Cystic neoplasia of the pancreas accounts for about 10%-15% of all cystic pancreatic lesions[1]. The majority of cystic lesions of the pancreas are pseudocysts. Although cystic neoplasia of the pancreas is rare, it encompasses a spectrum of benign, borderline and malignant neoplasiae. In contrast to solid tumors of the exocrine pancreas, which are exclusively malignant (85%-90%), the clinical challenge is the differential diagnosis and management of cystic neoplasia.
The broad spectrum of pancreatic neoplasia and tumor-like lesions with cystic features has been recently reviewed[1-3]. The incidence or frequency of pancreatic cystic neoplasia varies among different institutes. Because there has been no comprehensive study in a large series of cases from China comparing the incidence and biology to Western series, we reviewed all cystic neoplasiae collected in pathological files of Zhongshan Hospital over five years. Most of the neolpasms in our series were well documented and have been included in the recent World Health Organization (WHO) classification[4]. The four most common cystic neoplasiae of the pancreas are intraductal papillary mucinous neoplasia (IPMN), serous cystic neoplasia (SCN), mucinous cystic neoplasia (MCN), and solid pseudo-papillary neoplasia (SPN).
Mucins are a group of high molecular weight glycoproteins that are widely expressed in epithelial cells. Fourteen mucin genes have been identified thus far[5-8]. They can be further grouped into two subfamilies: secreted and transmembrane mucin genes. Among these, MUC2, MUC5AC and MUC6, known as the gel-forming mucins, are expressed in the pancreas either under normal physiologic or tumoral conditions. Among membrane-bound mucins, MUC1 and MUC4 are the two main mucins associated with pancreas[7,8].
In the present study, we examined the expression of the well-characterized MUCs (MUC1, 2, 4, 5AC and 6) in pancreatic cystic neoplasia and compared the expression profiles with those observed in adenocarcinoma and endocrine tumor of pancreas with cystic feature. The overall goal was to identify the mucin profile that is potentially a specific marker for the differential diagnosis of cystic neoplasia of the pancreas.
Pancreatic cystic tumors diagnosed between January 1999 and June 2005 in the Department of Pathology of Zhongshan Hospital, Fudan University were identified, reviewed, and classified according to the recent World Health Organization classification of pancreas neoplasia[6]. During this period, 248 patients were operated on in our hospital for pancreatic lesions. Clinical information was obtained from the patient records or the clinicians in charge. The tumors were removed surgically from all the patients. All specimens were fixed in buffered-formalin and embedded in paraffin. Deparaffinized sections were stained with haematoxylin and eosin, Alcian blue and periodic acid-Schiff. One or two representative blocks from each case were selected. Immunohistochemical analysis was carried out on serial sections cut from the cystic epithelial neoplasia using the Envision (DAKO, Carpiteria, CA) method with several antisera (Table 1).
| Antibody | Clone | Type | Producer | Dilution | Commence |
| PanCK | AE1/AE3 | MoAb | DAKO | 1:100 | Membrane |
| CK 7 | OV-TL | MoAb | DAKO | 1:200 | Membrane |
| CK 8/18 | RCK108 | MoAb | DAKO | 1:200 | Membrane |
| CA19-9 | 116-NS-19-9 | MoAb | DAKO | 1:50 | Cytoplasmic |
| CEA | A0115 | PoAb | DAKO | 1:200 | Cytoplasmic |
| EMA | E29 | MoAb | DAKO | 1:100 | Membrane |
| MUC1 | Ma695 | MoAb | Novacastra | 1:100 | Apical membrane |
| MUC2 | Ccp58 | MoAb | Novacastra | 1:1000 | Cytoplasmic |
| MUC4 | MoAb | Zymed | 1:100 | Apical membrane | |
| MUC5AC | CLH2 | MoAb | Novacastra | 1:1000 | Cytoplasmic |
| MUC6 | CLH5 | MoAb | Novacastra | 1:100 | Cell membrane |
| Synaptophysin | MoAb | DAKO | 1:100 | Cytoplasmic | |
| Chromogranin-A | DAK-A3 | MoAb | DAKO | 1:20 | Cytoplasmic |
| NSE | PoAb | DAKO | 1:200 | Cytoplasmic | |
| α1-AT | PoAb | DAKO | 1:500 | Cytoplasmic | |
| α1-ACT | PoAb | DAKO | 1:250 | Cytoplasmic | |
| Vimentin | V9 | MoAb | DAKO | 1:100 | Cytoplasmic |
| α-inhibin | MoAb | DAKO | 1:50 | Nuclear | |
| β-catenin | MoAb | DAKO | 1:100 | Membrane/ nuclear |
All cases were analyzed by 2 pathologists in accordance with the WHO criteria. In the current study, SCN, MCN, IPMN were classified into three classes according to the degree of cytologic and structural atypia including increased nuclear-to-cytoplasmic ratio, loss of polarity, pleomorphism, hyperchromatism, prominent nucleoli, abnormal mitosis, cribriform pattern and multilayer, and presence of invasion. Immunohistochemical evaluation was independently performed by two authors (Ji Y, and Zhu XZ) without knowledge of the clinical data. For each marker, the positive cell distribution in tumor tissue and staining pattern (cytoplasmic, membranous, or nuclear) were recorded. For mucins, we evaluated the staining of cytoplasm and luminal surface. The cells were considered positive when at least one of the components was positive. Percentages of the positively stained neoplastic cells were as follows: -: < 5% of neoplastic cells stained; +: 5%-20% of neoplastic cells stained; ++: 20%-50% of neoplastic cells stained; +++: > 50% of neoplastic cells stained.
This series was comprised of 64 cases, including 28 cases of IPMN, 12 cases of SCN, 11 cases of MCN, 11 cases of SPN, 1 case of ductal adenocarcinoma with cystic feature (cDAC), and 1 case of endocrine tumor with cystic feature (cPET). All cystic neoplasiae of the pancreas in order of their relative frequency are listed in Table 2.
| Type | n | Percentage | |
| Intraductal papillary mucinous neoplasia | 28 | 43.8 | |
| Adenoma | 5 | ||
| Borderline | 4 | ||
| Carcinoma | 19 | ||
| Serous cystic neoplasia | 12 | 18.8 | |
| Adenoma | 11 | ||
| Borderline | 0 | ||
| Carcinoma | 1 | ||
| Mucinous cystic neoplasia | 11 | 17.3 | |
| Adenoma | 8 | ||
| Borderline | 1 | ||
| Carcinoma | 2 | ||
| Solid pseudo-papillary neoplasia | Borderline (Uncertain malignant potential) | 11 | 17.3 |
| Cystic ductal adenocarcinoma | High-grade malignant | 1 | 1.6 |
| Cystic pancreas endocrine tumor | Low-grade malignant | 1 | 1.6 |
| Total | 64 | 100 |
The details of the cases included in the study are shown in Table 3. The immunostaining findings are listed in Tables 4 and 5.
| Clinical feature | Type | IPMN n = 28 | SCN n =12 | MCN n =11 | SPN n =11 | cDAC n =1 | cPET n =1 |
| Age (yr) | Mean (range) | 65 (45-80) | 52.7 (36-69) | 51.7 (29-72) | 39 (22-52) | 58 | 45 |
| Sex | Male:Female | 4:3 | 3:10 | 1:10 | 1:10 | 1:0 | 1:0 |
| Location | Head | 16 | 5 | 2 | 3 | 1 | 1 |
| Body/tail | 12 | 7 | 9 | 8 | |||
| Tumor size | Range (mean) | 2-7.5 (4.5) | 3-12 (6) | 2-13 (7.8) | 2-21 (6.5) | 5 | 4.5 |
| Type | IPMNn = 28 | SCNn = 12 | MCNn = 11 | SPNn = 11 | cDACn = 1 | cPETn = 1 |
| CK8/18 | + | + | + | - | + | + |
| CK19 | ++ | + | + | - | + | +/- |
| CA19-9 | + | - | +/- | - | + | - |
| CEA | + | - | -/+ | - | + | - |
| SYN | +/- | - | +/- | +/- | - | + |
| CHG-A | +/- | - | +/- | - | - | + |
| NSE | + | +/- | +/- | + | ||
| α-AT | - | - | - | + | - | - |
| α-ACT | - | - | - | + | - | - |
| Vim | - | - | + | - | - | |
| α-inhibin | - | + | +1 | +/- | ||
| β-catenin | - | + | - | +/- | - | - |
| Type | IPMNn = 28 | SCNn = 12 | MCNn = 11 | SPNn = 11 | cDACn = 1 | cPETn = 1 |
| MUC1 | 7/28 + | 1/12 +/- | 2/11 + | - | ++ | - |
| MUC2 | 15/28 ++ | 1/13 +/- | - | - | - | - |
| MUC4 | - | - | -- | - | ++ | |
| MUC5AC | 28/28 + | 2/12 + | 10/11 ++ | - | + | - |
| MUC6 | - | 11/12 ++ | - | - | + | - |
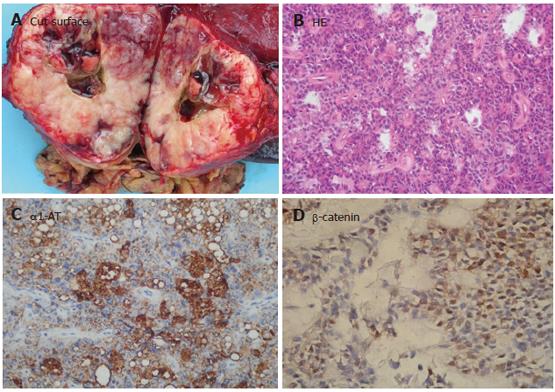
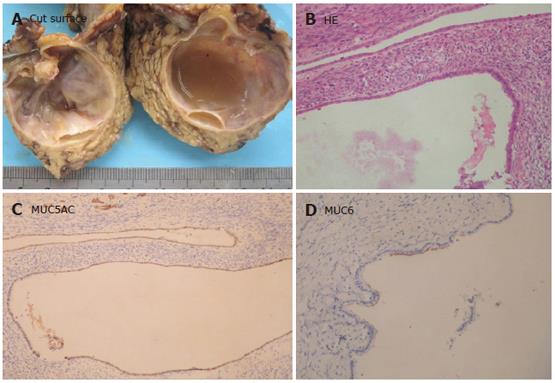
Macroscopically, neoplasia could be classified as main pancreatic duct type in 15 (50.4%) patients (Figure 1A), and branch type in 5 patients, both main and branch duct types in 8 patients, respectively. On histologic analysis of the entirely resected specimens, IPMN showed simple hyperplasia (adenoma) in 5 patients, atypical hyperplasia (borderline lesion) in 4 patients, in situ carcinoma in 6 patients, and invasive carcinoma in 13 patients. The epithelial proliferative lesions in all patients involving the main or secondary pancreatic duct showed a papillary architecture (Figure 1B). Moderate to severe fibroatrophic changes of obstructive pancreatitis were observed in 21 patients.
Immunochemically, neoplasia could be classified as intestinal type (MUC1-, MUC2+) in 11 patients (Figure 1C), pancreaticobiliary type (MUC1+, MUC2-) in 9 patients, gastric type (MUC1-, MUC2-, MUC5AC+) in 6 patients (Figure 1D), and oncocytic type in 2 patients. Of the 13 IPMN patients with invasive carcinoma, 5 showed the features of mucinous carcinoma in their invasive component and 8 showed ductal carcinoma in their invasive component with MUC1+. The mucin profiles of the mucinous or ductal component corresponded to the intestinal or pancreaticobiliary type, respectively. All IPMN samples stained for CEA, CK8/18, and CK19 were negative for CK20, α1-AT. However, single endocrine cells were detected by SYN and CHG-A in 10 intestinal types and 4 pancreaticobiliary types of IPMN. They expressed both gastric hormones (serotonin and gastrin) and/or pancreatic hormones (glucagon, PP) focally.
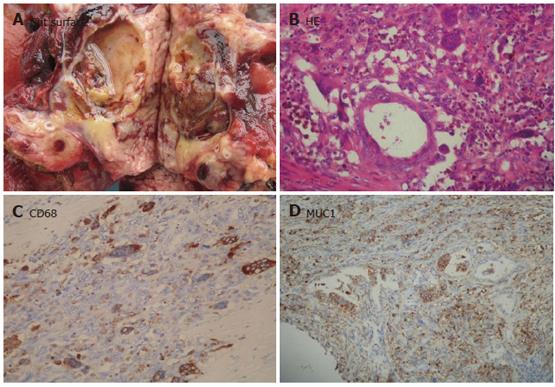
The most common subtype of SCN was serous microcystadenoma (SMA, 8 cases), characterized by a central stellate scar, sharp demarcation from surrounding pancreatic tissue, and its predominant occurrence in women. SMA was comprised of numerous small cysts with a diameter ranging from 0.01 to 0.5 cm and a few larger cysts up to 1.5 cm in diameter. The cysts contained eosinophilic fluid and were lined by a single layer of cuboidal or flattened epithelial cells with pale to clear cytoplasm. The tumor cells were centrally located with round to oval nuclei. The cytoplasm was positive for granular periodic acid-Schiff. The usually thin fibrous septae were acellular. The central scar was composed of hyalinized tissue. Three cases of serous oligocystic ill-defined adenoma (SOIA) showed only a few, usually rather large cysts (0.2-2.5 cm in diameter) filled with clear or brown fluid (Figure 2A). The cyst epithelium was cytologically identical to that of SMA (Figure 2B). However, unlike SMA, SOIA was not well-defined due to the extension of small cysts into the adjoining pancreatic tissue. The tumors also lacked a central fibrous scar. One serous cystadenocarcinoma (SCC) was found to have a liver metastatic lesion during operation. The primary tumor of pancreas, which was similar to SMA, showed no distinct atypia.
Besides stained for CK8/18, CK19, the cells were also positive for neuron specific enolase (NSE) and negative for alpha-1-antitrypsin (α1-AT) and alpha-1-antichymotrypsin (α1-ACT). Of the mucins tested, MUC1 (75%) (Figure 2D) and MUC6 (66%) (Figure 2C) had the highest positive rates. MUC2 and MUC5AC staining was completely lacking. In the normal pancreatic tissue entrapped into the tumors, small duct cells and centroacinar cells were apically positive for MUC6 and MUC1. In addition, the epithelial cells were stained for α-inhibin.
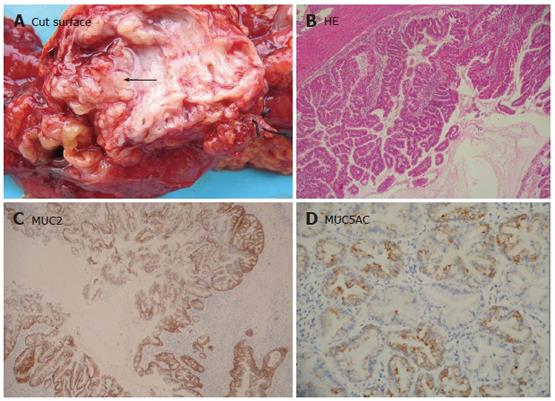
MCN was studied in 11 patients. The tumors presented as a large round cystic mass with a unilocular or multilocular cut surface (Figure 3A). Micorscopically, the cysts were lined by mucin-producing epithelial cells with variant degrees of dysplasia supported by an ovarian-like stroma (Figure 3B). MCN was classified as adenomas (MCA) in 8 patients, borderline tumor (MCB) in 1 patient, and carcinomas (MCC) in 2 patients with invasive component. The epithelial component of MCN was stained for CK8/18, CK19, and CEA. MUC1 positivity was, however, found in the invasive components of MCC. All of our MCN patients were negative for MUC2, except for goblet cells, which were quite numerous in MCB and rare in MCA. MUC5AC expression was found in all the 11 MCN patients. The cytoplasmic staining intensity increased with progressive nuclear atypia and was the strongest in invasive carcinomas (Figure 3C). A few cells were stained faintly for MUC6 (Figure 3D). In addition, endocrine cells detected by CHG-A, were found in 3 MCN patients, of them 2 also had expression of gastrin and serotonin, 1 had expression of only serotonin.
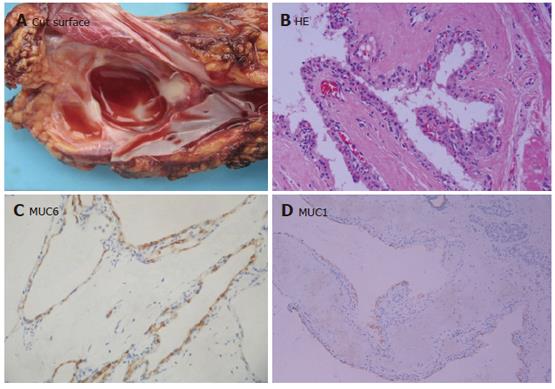
The extent of cystic changes in 11 SPN patients varied from tumor to tumor, affecting either part of the tumor tissue, or almost the entire tumor tissue. The cystic structures contained hemorrhagic debris and were usually surrounded by hemorrhagic-necrotic tissues (Figure 4A). Microscopically, SPN showing solid areas was composed of monotonous polygonal epithelioid cells, often with minimal intervening stroma accompanied with innumerable capillary-sized vessels (Figure 4B). Some areas showed more extensive stromal fibrosis, with round aggregates of perivascular hyalinized stroma imparting a cylindromatous appearance. In the pseudopapillary regions, the cells located away from the small vessels appeared to have dropped away, leaving an irregular cuff of cells surrounding each vascular core.
Immunohistochemical studies revealed that SPN showed a consistent pattern of reactivity for vimentin, α-1-antitrypsin (α-1-AT) (Figure 4C) and α-1-antichymotrypsin (α-1-ACT), but an inconsistent positive pattern for CD10 and S-100 protein. None of these proteins was reactive for mucins and pancreatic hormones. Staining of the pancreatic enzyme trypsin was consistently negative, as the specific endocrine marker chromogranin. Seven cases were focally positive for synaptophysin and the less specific marker NSE. Positive β-catenin (Figure 4D) and progesteron receptors were limited to the nuclei of tumor cells.
MUC1 and MUC4 were observed in invasive component. CEA, CK7, 19 and CA19-9 with focal staining of MUC5AC did not exceed 30% of the tumor, in which MUC2 was absent (Figure 5A-5D).
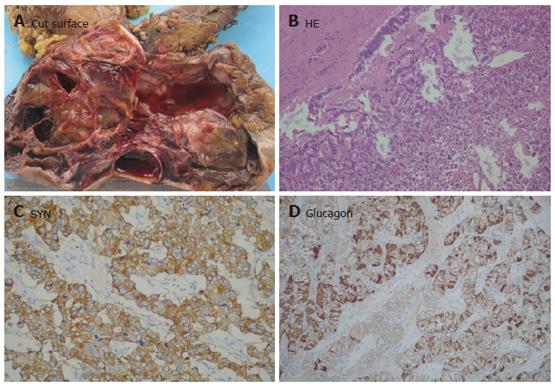
The NET which was not encapsulated in the pancreatic head, infiltrated the duodenal wall and was considered malignant. As the typical NET had no cystic change, NET was diffusely stained for SYN, CHG-A and NSE, as well as pancreatic hormones and glucagons (Figure 6A-6D).
Cystic tumor of the pancreas accounts for 10%-15% of pancreatic cystadenomas and 1% of pancreatic carcinomas[1-3]. With the increased availability of imaging techniques and improved feasibility of surgical resection, an increasing number of cystic pancreatic neoplasiae have been identified in several institutions, thus their diagnosis can be established more easily.
The clinical challenge is the differential diagnosis and management of cystic neoplasm, which represents a wide range of biologic behavior. A variety of biochemical, genetic, and imaging techniques have been developed for the diagnosis and monitoring of the outcome of cystic neoplasia of the pancreas. In consideration of the potentials and limitations inherent in each of these methods, the need for efficient differential diagnostic markers that specifically discriminate pancreatic cystadenocarcinoma from pancreatic carcinoma cannot be overemphasized. In this regard, mucins may represent potential candidates for such a purpose, with respect to their biochemical properties relating to malignant conditions[9,10]. Analysis of mucin expression profiles in PCN patients has brought some light on their prognosis[5,6]. MUC1 is thought to have an inhibitory role in cell-cell and cell-stroma interactions as well as in immunoresistance[5]. MUC1also acts as a signal transducer, interacting with and promoting the activities of EGFR, MAP kinase and Wnt signaling pathway[11]. In pancreatic neoplasia, MUC1 has been found to be a marker of an aggressive phenotype expressed in high-grade PanINs, and more importantly, presents uniformly in infiltrating conventional ductal adenocarcinoma[5,6,9]. On the other hand, MUC2 functioning as a protective barrier in the intestinal epithelium[12,13], appears to be a marker of an indolent phenotype in the pancreas[5]. MUC2 is not expressed in the normal pancreas, PanINs or ductal adenocarcinoma, but is often detectable in IPMNs and uniformly presents in colloid carcinomas.
IPMN is a mucin-producing epithelial neoplasia involving the main and/or branch pancreatic ducts. Recent data suggest that a more heterogeneous profile of IPMN has different mucin patterns and clinical prognosis[11,12]. In consistent with previous reports, the intestinal type of our IPMN patients expressed MUC2, MUC5AC and MUC4, but not MUC1. It had a lower invasive and metastatic potential in comparison to DACs and therefore had a better clinical outcome. The pancreatobiliary type of IPMN is MUC1 positive and MUC2 negative with an increased tendency to invade, and seems to be related to DAC[13,14]. The third group with a gastric foveolar differentiation which is MUC5AC positive but usually does not express either MUC1 or MUC2, was not identified in this study. The lack of this type in our series may be due to the misdiagnosis of MCN for their similar mucin profile.
Pancreatic SCN is usually considered benign. Whether SCN is malignant is still controversial. However, the malignancy of pancreatic SCN has been recognized since 3% of reported SCNs are cancerous in nature[15]. In many reported cases of malignant SCN, there are multifocal lesions in the liver or additional lesions, but pancreatic tumor itself shows no signs of malignancy. Amorphous nuclei, irregular nuclear margins, and coarse nuclear chromatin in SCN suggest that it has a malignant potential. In the present study, SCN exhibited a similar if not identical immunoprofile-positivity for MUC1 and MUC6, whereas no SCN expressed MUC5AC, although mucin production is not a histologic feature of SCN. The mucin profile of SCN that was positive for MUC6 supports the hypothesis of a direction of differentiation toward centroacinar cells, since the ultrastructural features of tumor cells are similar to those of centroacinar cells[15]. This hypothesis is further supported by the centroacinar localization of incipient serous cystic tumors observed incidentally[16]. In immunostaining, helpful markers for SCN are α-inhibin and MUC6.
MCN was first considered as a premalignant or overtly malignant cystic neoplasia. Because of the reputed difficulty in classifying MCN as a “benign” cystadenoma, some pathologists classify MCN of the pancreas with only small areas of epithelial hyperplasia or dysplasia as grade 1 cystadenocarcinoma, thereby implying a more aggressive natural history and predetermining their future management and follow-up as a malignancy despite successful resection. When these neoplasiae become malignant, only portions of the tumor dedifferentiate (i.e., become proliferative, develop nuclear dysplasia, and eventually invade the stroma). These areas may not be sampled unless the entire neoplasm is examined. In addition, separation of cystic mucinous neoplasia from non-invasive IPMN and high-grade ductal adenocarcinoma that incidentally have cystic areas (related to necrosis or ductal obstruction) requires both adequate sampling and clinicoradiologic and pathologic correlation. Mucin gene expression may discriminate between MCN and DAC[7]. In this study, noninvasive MCN, regardless of the degree of cellular atypia, was positive for MUC5AC and negative for MUC1. MUC1 expression was observed only in patients with an invasive component. MUC2 perinuclear expression was restricted to goblet cells scattered within the epithelium of MCN, often accompanied with endocrine cells, a further indication of intestinal differentiation. As MUC5AC is a gastric foveolar mucin, and expressed during intestinal ontogenesis, further investigation is required before we understand the role of MUC5AC in MCN. In addition, the expression of α-inhibin in stromal cells of MCN may be useful in the diagnosis of cystic pancreatic lesions.
SPN of the pancreas is a rare neoplasia with distinctive pathologic features and low-grade malignant potential that preferentially affects young women. SPN is composed of uniform and polygonal epithelioid cells, which are arranged in a discohesive pattern. SPN can be readily diagnosed by routine histologic examination, but immunohistochemical studies are frequently performed to confirm the diagnosis. Tumor cells of SPN are characteristically positive for vimentin and α-1-antitrypsin, and may also show focal immunoreactivity for cytokeratin and synaptophysin and abnormal nuclear localization of β-catenin. Staining of chromogranin and the acinar markers trypsin and chymotrypsin is consistently negative. SPN is composed of monomorphous cells with uncertain lineage or histogenesis[17]. The phenotype of SPN surprisingly does not resemble that of any normal epithelial cell lines of the pancreas.
Cystic NET is relatively easy to be distinguished from other epithelial cystic neoplasiae by the lack of mucin expression and presence of neuroendocrine markers[18].
In general, mucins follow a defined spatial and temporal pattern of expression throughout the development of an organ. However, recent studies demonstrate that deregulated expression of mucins is associated with various types of neoplasia. In the normal adult pancreas, MUC1 expression is confined to the apical membrane of intralobular ductules, possibly a manifestation of the "lumen maintenance" role of MUC1. MUC1 expression is not detectable in larger ducts or other pancreatic elements (islets or acini)[19]. MUC6 mRNA is also detectable in acini, in which the signal is largely restricted to centroacinar cells (Table 5). MUC2 or MUC5AC is not expressed in normal adult pancreas[20,21]. In pancreatic adenocarcinoma, tumors and tumor cell lines can overexpress MUC1. Additionally, an aberrant expression of MUC4 in pancreatic tumor and cell lines has been reported, a MUC that is usually undetectable in normal pancreas.
It is important to distinguish between a pseudocyst tumor and a cystic tumor because they are frequently confused with one another and the management of these entities is entirely different.
In summary, mucin profile may lead not only to the early diagnosis of pancreatic tumors but also to the accurate diagnosis of neoplasia. Mucin alteration may, in conjunction with histologic study, provide important information on tumor type and patient treatment.
S- Editor Wang GP L- Editor Wang XL E- Editor Bi L
| 1. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 3. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Klöppel G, Lüttges J. WHO-classification 2000: exocrine pancreatic tumors. Verh Dtsch Ges Pathol. 2001;85:219-228. [PubMed] |
| 5. | Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Chhieng DC, Benson E, Eltoum I, Eloubeidi MA, Jhala N, Jhala D, Siegal GP, Grizzle WE, Manne U. MUC1 and MUC2 expression in pancreatic ductal carcinoma obtained by fine-needle aspiration. Cancer. 2003;99:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633-1638. [PubMed] |
| 9. | Kosmahl M, Pauser U, Anlauf M, Klöppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Ringel J, Löhr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, Carraway KL 3rd, Kufe D. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276:35239-35242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Klöppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology. 1998;45:1981-1985. [PubMed] |
| 13. | Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 717] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Egawa N, Maillet B, Schröder S, Mukai K, Klöppel G. Serous oligocystic and ill-demarcated adenoma of the pancreas: a variant of serous cystic adenoma. Virchows Arch. 1994;424:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Kosmahl M, Wagner J, Peters K, Sipos B, Klöppel G. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Bektas H, Werner U, Kaaden S, Philippou S, Klöppel G, Klempnauer J. Solid-pseudopapillary tumor of the pancreas--a rare and frequently misdiagnosed neoplasm. Langenbecks Arch Surg. 1999;384:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Rindi G, Klöppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80 Suppl 1:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Nakajima K, Ota H, Zhang MX, Sano K, Honda T, Ishii K, Nakayama J. Expression of gastric gland mucous cell-type mucin in normal and neoplastic human tissues. J Histochem Cytochem. 2003;51:1689-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal" pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Lüttges J, Beyser K, Pust S, Paulus A, Rüschoff J, Klöppel G. Pancreatic mucinous noncystic (colloid) carcinomas and intraductal papillary mucinous carcinomas are usually microsatellite stable. Mod Pathol. 2003;16:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |