Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6338
Revised: December 21, 2004
Accepted: December 23, 2004
Published online: October 28, 2005
AIM: Recent studies demonstrating the direct involvement of dendritic cells (DC) in the activation of pathogenic T cells in animal models of inflammatory bowel disease identify DC as important antigen presenting cells in the colon. However, very little is known about the properties of colonic DC.
METHODS: Using immunohistochemistry, electron microscopy and flow cytometry we have characterized and compared colonic DC in the colon of healthy animals and interleukin-2-deficient (IL2-/-) mice that develop colitis.
RESULTS: In the healthy colon, DC resided within the lamina propria and in close association with the basement membrane of colonic villi. Type 1 myeloid (CD11c+, CD11b+, B220-, CD8α-) DC made up the largest (40-45%) population and all DC expressed low levels of CD80, CD86, and CD40, and had high endocytic activity consistent with an immature phenotype. In colitic IL2-/- mice, colonic DC numbers increased four- to five-fold and were localized within the epithelial layer and within aggregates of T and B cells. They were also many more DC in mesenteric lymph nodes (MLN). The majority (>85%) of DC in the colon and MLN of IL2-/- mice were type 1 myeloid, and expressed high levels of MHC class II, CD80, CD86, CD40, DEC 205, and CCR5 molecules and were of low endocytic activity consistent with mature DC.
CONCLUSION: These findings demonstrate striking changes in the number, distribution and phenotype of DC in the inflamed colon. Their intimate association with lymphocytes in the colon and draining lymph nodes suggest that they may contribute directly to the ongoing inflammation in the colon.
- Citation: Cruickshank SM, English NR, Felsburg PJ, Carding SR. Characterization of colonic dendritic cells in normal and colitic mice. World J Gastroenterol 2005; 11(40): 6338-6347
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6338
There is increasing evidence that inflammatory bowel disease (IBD) is the result of dysregulated immune and T cell responses to the intestinal bacterial microflora resulting in chronic intestinal inflammation[1,2]. However, it is not clear how antigen is taken up and processed in the colon. In the small intestine antigen uptake is believed to occur via specialized epithelial cells, or M cells, located in Peyer’s patches[3]. Peyer’s patches represent a relatively small area of the small intestine and no equivalent to the M cell has yet been described in the colon resulting in the speculation as to whether cells such as epithelial cells and lamina propria (LP) dendritic cells (DC) may contribute to antigen uptake and presentation. However, little is known about the nature of antigen presentation in the colon.
It is likely that DC play a pivotal role in both the initiation and regulation of immune responses in the intestinal tract. Our recent studies, using the interleukin 2-deficient (IL2-/-) mouse model of ulcerative colitis (UC), demonstrated the direct involvement of colonic DC in the activation of pathogenic T cells[4]. Both colitis as well as spontaneous bone loss in these animals were caused by increased production of the TNF family member, receptor activator of NFκB ligand (RANKL) by activated CD4+ T cells. Modulation of the interaction of RANKL with its receptor RANK by the administration of osteoprotegerin, a soluble decoy receptor for RANKL, resulted in reduced intestinal inflammation and ablation of colonic DC. However, a detailed analysis of the phenotype and function of these cells in the murine colon in healthy and inflamed tissue is lacking.
DC derived from murine Peyer’s patches have been extensively characterized and comprise at least three populations: (1) MAC-1+ (CD11b) myeloid DC in the subepithelial dome; (2) CD8α lymphoid DC in the interfollicular T cell regions and: (3) CD11b- CD8α- in both areas[5,6]. In contrast, little is known about the phenotype of colonic DC. DC from human colonic biopsies of normal and inflamed colon have been described as a heterogenous population of immature cells[7]. In contrast, preliminary analyses of DC from normal and colitic mice have suggested that DC are immature in the normal colon but mature in the diseased colon[3,8].
The observation that DC are key to the development of colitis in IL2-/- model[3] makes it important to elucidate their phenotype and function to gain a greater understanding of their role in driving immune responses within the colon. In the current study, we have used immunohistochemistry, electron microscopy and flow cytometry to characterize and compare the DC present in colonic tissue from healthy and inflamed murine colon. This study represents the first attempt to characterize colonic DC in the IL2-/- model of UC.
Animals C57BL/6 and C57BL/6-IL2-/- transgenic mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen free conditions at the University of Leeds. IL2-/- mice were obtained from hetero-zygote matings and were identified by PCR analysis of tail DNA samples[2]. Mice were killed at 7-8 wk of age. Sentinel mice were routinely screened for common mouse pathogens and none were detected.
Immunohistochemistry Colonic tissue from the distal, mid, and proximal colons of C57BL/6- IL2-/- mice and age-matched littermate controls were snap frozen in OCT medium. Sections (6 μm) were fixed in cold acetone and stained for cell-specific markers using the tyramide amplification method (Perkin Elmer, Berkshire) as described previously[6]. Sections were stained with antibodies to F4/80, CD11c, MAC-1, CD4, B220, (all supplied by Becton Dickinson, Cowley, Oxford), and cytokeratin (Sigma, Poole, Dorset) and then counterstained with 4-6-diamidino-2-phenolinodole dihydrochloride (DAPI) to visualize cell nuclei. Slides were mounted with Mowiol and analyzed with a Zeiss Axioplan u.v. microscope.
Cell isolation Colonic LP cells were isolated as described previously[4]. Briefly, colons were opened longitudinally, washed, and finely minced. LP cells were released from the tissue by incubation for 2 h under gentle agitation at 37 °C with collagenase I (4 mg/mL) in RPMI containing 10% FBS. The resultant cells were washed, resuspended in a 40% solution of Percoll (Amersham, Buckinghamshire) and gently layered onto a 60% Percoll gradient (400 g, 30 min). The cells were collected at the gradient interface, washed and prepared for analyses by flow cytometry or electron microscopy as described below. Mesenteric lymph node (MLN) cells were excised from C57BL/6 and C57BL/6- IL2-/- mice and homogenized to obtain a suspension of mononuclear cells.
Flow cytometry MLN and colonic LP cells were analyzed by flow cytometry on a FACScan flow cytometer using CELLQuest software (Becton Dickinson). Cells were stained with fluorochrome and/or biotin conjugated antibodies to CD45, B7.1 (CD80), B7.2 (CD86), CD40, CD8α, CD4, B220 (CD45R), GR-1, F4/80, MAC-1 (CD11b), I-A/I-E, CD11c, CCR5 (Becton Dickinson), DEC205 (Serotec Ltd, Oxford) and streptavidin conjugated to Alexa Fluor 633 (Molecular Probes, OR, USA). Isotype-matched antibodies of irrelevant specificity were used to determine the level of non-specific staining and frequency of cells stained with test antibodies.
Electron microscopy CD11c+ LP cells from C57BL/-IL2-/- and C57BL/6 littermate controls were enriched by negative immunomagnetic selection to deplete the cell preparation of T (CD3+) and B (CD19+) cells prior to preparing the samples for electron microscopy. Between 1-5×105 cells were fixed in 0.1 mol/L sodium phosphate buffer, pH 7.4 containing 3% glutaraldehyde for at least 12 h at 4 °C. After washing in sodium phosphate buffer, the cells were embedded as a pellet in low gelling temperature agarose (Sigma). The pellet was washed thoroughly and post-fixed for 1 h in 1% osmium tetroxide, washed and left overnight in dH2O prior to being stained for 2-4 h in 2% uranyl acetate. After washing in dH2O, the cells were dehydrated using an acetone gradient, embedded in araldite resin prior to curing for 18 h at 65 °C. Ultrathin sections (100 nm thick) were cut on a Reichert-Jung Ultracut E microtome and collected on a 200 mesh copper grid. After staining with Reynold’s lead citrate, the grid was carbon coated and visualized using a JEOL 1200 EX electron microscope. All samples analyzed by electron microscopy were also analyzed by flow cytometry to confirm the presence of DC in the cell samples.
Endocytosis assay Endocytic activity was assessed by measuring the uptake of FITC-dextran (Sigma). Freshly isolated MLN and LP cell preparations (2105 cells/mL) from C57BL/6 and C57BL/6-IL2-/- mice were incubated at 37 °C with FITC-DX (1 mg/mL, Sigma) for 1 h. Control samples were incubated at 4 °C. Incubation was stopped by washing in ice-cold PBS, stained with antibodies to CD11c or F4/80 as described above, fixed in 1% paraformaldehyde and permeabilized in 0.1% saponin and analyzed by flow cytometry as described above. Results are expressed as the proportion of cells (F4/80+ or CD11c+) which have specifically taken up the FITC-DX minus the background staining (cells incubated at 4 °C).
Data are presented as mean±SE. Statistical significance was evaluated using a Mann-Whitney test.
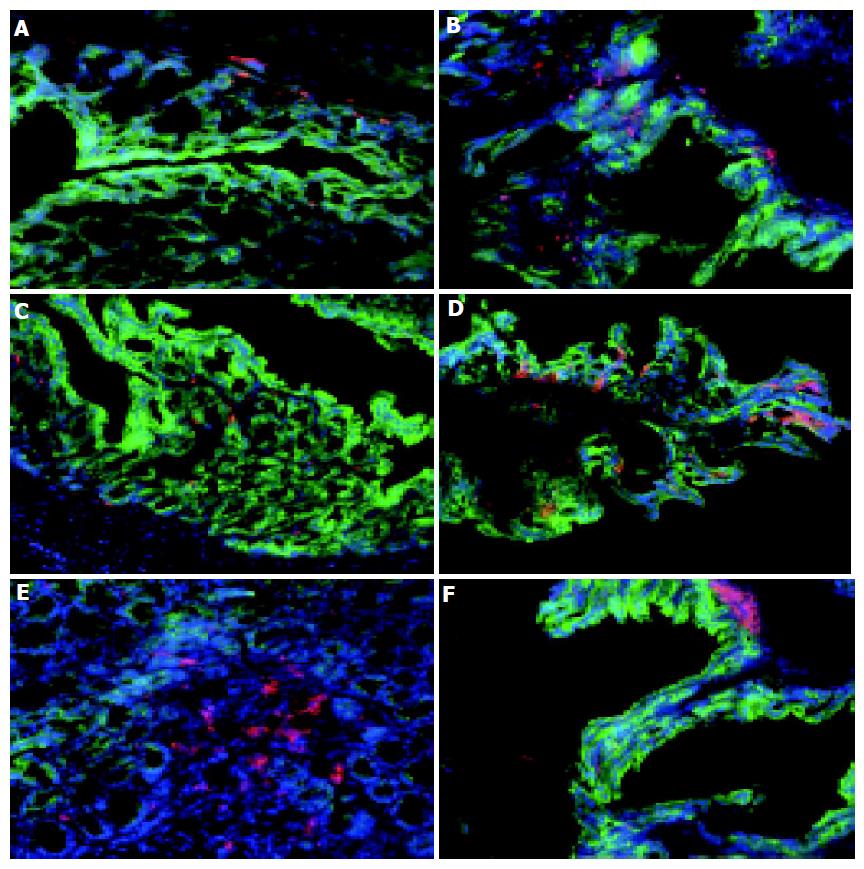
A comparative analysis of the localization and type of DC and macrophages within the colons of normal (7 wk old C57BL/6) and colitic (7 wk old C57BL/6-IL2-/-) mice was carried out using a three fluorochrome immunohistochemical procedure. The relatively few CD11c+ DC present in the normal colon (Figure 1C) were mostly found within the LP and in close association with the basement membranes of the colonic villi (Figure 1C). Similarly, F4/80+ macrophages were also found located within the LP and basement membrane of the normal colon (Figure 1A). F4/80+ macrophages outnumbered CD11c+ DC in the normal, healthy colon.
In contrast, there were changes in both the number and distribution of F4/80+ macrophages and CD11c+ DC in the colon of colitic IL2-/- mice. C57BL/6-IL-2-/- mice spontaneously develop a progressive form of intestinal inflammation that is restricted to the colon and histopat-hologically resembles human UC[9], and has been shown to be triggered by commensal gut bacteria[9,10]. There were more macrophages (82.540.2 compared with 106.127.1 per field of view) and colonic DC (52.55.2 compared with 80.216.1 per field of view) in the inflamed colons of IL2-/- mice compared to the normal healthy colon (Figures 1B and D). Furthermore, the distribution of colonic CD11c+ DC was altered in colitic animals with the majority of DC found in close association with the luminal surface, adjacent to the colonic epithelia (Figure 1D). This suggests that in colitic animals, colonic DC are mobile and migrate to the epithelial layer perhaps in response to epithelial derived signals or microbial antigens from the gut lumen. Of note, in the inflamed colon was the accumulation of CD11c+ in aggregates, or lymphoid follicle like structures, at the base of crypts beneath the epithelial layer (Figure 1F). Although, similar clusters of colonic DC were also found in the colons of normal animals (Figure 1E), they were less well organized and much rarer.
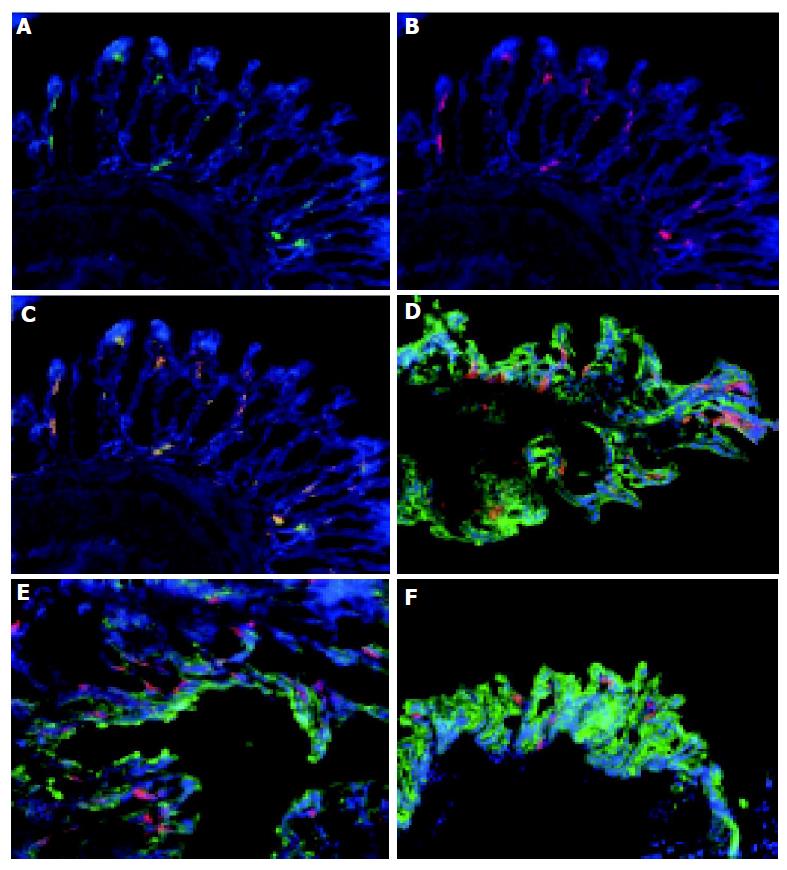
To ascertain if colonic DC were restricted to specific parts of the colon, sections of the distal, mid, and proximal colon of both normal and colitic animals were analyzed. CD11c+ cells were detected throughout the entire colon (Figures 2D-F) in both normal and colitic animals. Of note, a greater proportion of the aggregates of CD11c+ were observed in the mid and distal areas compared to the proximal area of the colon in IL2-/- mice. The number of DC aggregates varied from mouse to mouse with 2-4 being observed in the sections of tissue from IL2-/- mice.
The phenotype of colonic DC was determined by immunolabeling with antibodies specific for MAC-1 (CD11b) and B220. The majority of CD11c+ cells co-expressed MAC-1+ (Figures 2A-C) consistent with them being myeloid DC. A small proportion (approximately 5%) of the colonic CD11c+ cells within the lymphoid aggregates of IL2-/- animals co-expressed B220 (see below, Figures 3G-I) suggesting that they may be plasmacytoid DC. The majority of CD11c+ cells in the lymphoid aggregates were MAC-1+ myeloid DC (data not shown).
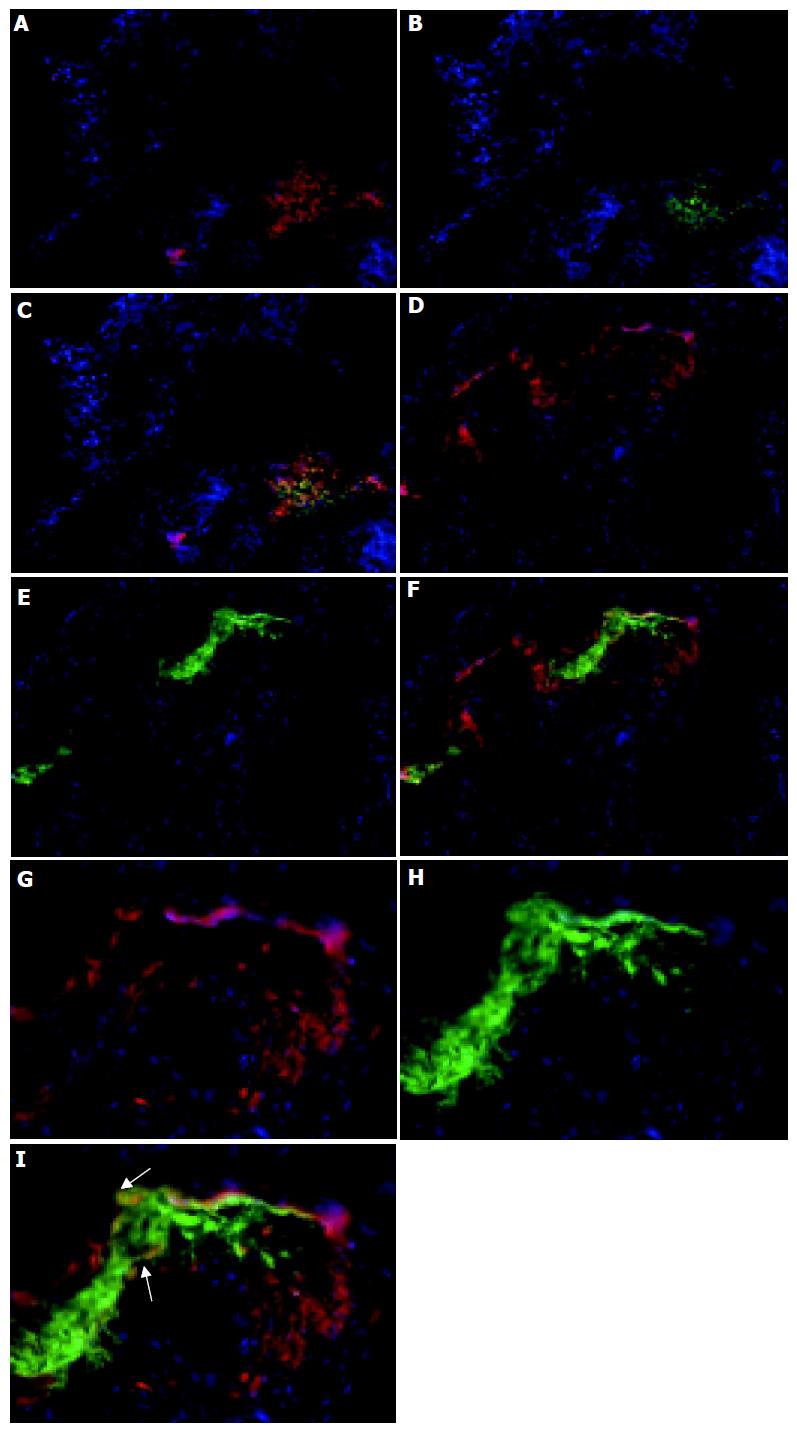
The distribution of CD11c+ cells in lymphoid aggregates within the colons (Figure 3) of colitic animals was analyzed next. These follicular-like structures were found at the base of crypts, with up to four being found per section with the exact number varying between individual mice. In IL2-/- mice these follicular structures were comprised of tightly packed groups of CD11c+ cells which were particularly evident at the margins of these follicular structures (Figures 1F and 3). The cellular composition of these follicle-like structures was determined by immunolabeling with antibodies specific for B220 and CD4. Whereas, CD11c+ cells were primarily localized to the outer edges of the structures, B220+ B cells and CD4+ T cells were found in the centre (Figure 3). There was also evidence of co-localization of CD4 and CD11c antibody staining (Figures 3A-C) indicating that either some CD11c+ cells were also CD4+, as described in the small intestine[11] and colon[8], or that some CD11c+ cells are in intimate contact with CD4 T cells. Such close contact of CD11c+ cells with CD4+ T cells would suggest that local activation of T cells may be occurring within these sites. Although the majority of CD4+ cells were found in the follicular structures, smaller numbers of positive cells were also seen in the LP of the villi and in blood vessels. B220+ B cells were almost entirely restricted to the lymphoid follicle-like structures (Figures 3D-F). A small proportion of B220+ cells co-localized with CD11c+ cells within the lymphoid follicle (Figures 3G-I), which may represent plasmacytoid DC. CD11c+, B220+ cells were found primarily at the interface of the B220+ cells in the centre of the lymphoid follicle and CD11c+ cells at the aggregate margin.
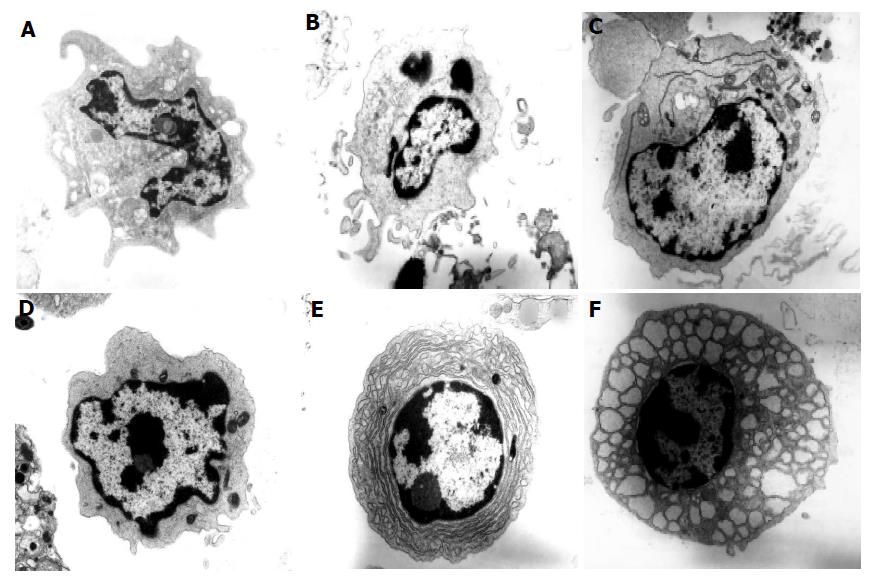
The identity and type of DC present in the colons of normal C57BL/6 and colitic C57BL/6 IL2-/- mice was confirmed by electron microscopy (Figure 4). Of the total cells analyzed from C57BL/6 mice, DC represented approximately 2-4% of the cells counted. DC have been classified into three types by electron microscopy[12,13]. Type 1 DC are small, irregularly shaped cells with small cell surface projections and heterochromatic nuclei with the chromatin found in a thick band at the nuclear margin and also in small condensed areas. Type 2 DC are larger than type 1 cells and have fewer projections and a euchromatic nucleus with disaggregated chromatin which is also found as a thin band at the nuclear margin. DC especially type 2 DC can have a prominent rough endoplasmic reticulum. Some cells appear to have features of both types 1 and 2 DC and have been termed type 1-2. Type 3 DC have a veiled appearance and euchromatic nuclei.
Colonic DC from normal C57BL/6 mice were mostly Type 1 (Figure 4A) with approximately 1% representing type 3 DC. However, some DC type 1 can have long projections making them appear similar to type 3 DC so this small subset of cells may in fact represent DC type 1. In comparison with normal colonic DC, many more colonic DC were identified in IL2-/- mice (13% compared with 2%) confirming our immunohistochemical findings. Colonic DC in the IL2-/- mice were comprised of type 1 DC (7-9%), type 1-2 (2%) and type 2 (2-3%, Figures 4B-D). Some of the DC (Figure 4C) had a prominent rough endoplasmic reticulum characteristic of plasmacytoid DC[14]. This would appear to confirm our immunohistochemical finding that there are small numbers of B220+ CD11c+ plasmacytoid DC within the colonic mucosa of IL2-/- mice. No type 3 DC were detected in the IL2-/- sample. Of the type 1 DC, half had undeveloped cytoplasm and may therefore represent a more immature population. Overall, these results confirm and extend our immunohistochemical findings that the majority of CD11c+ DC detected by immunohistochemistry were CD11b+ myeloid DC.
The presence of B220+ B cells within the lymphoid follicular structures in the colons of IL2-/- mice was also confirmed by electron microscopy. Plasma cells represented approximately 10% of cells counted (Figure 4E) in the IL2-/- sample. The majority of plasm a cells detected had a dilated rough endoplasmic reticulum with vacuoles containing protein (Russell bodies). These are known as Mott cells and make more immunoglobulins than they are able to secrete[15]. The presence of plasma cells within the colonic lymphoid follicles suggests that the follicles are functional and that lymphocyte activation may occur locally within the colon, contributing to the chronic inflammation seen in these animals.
The phenotype of DC was further analyzed by flow cytometry. There was approximately a five fold increase in the number of colonic DC in colitic animals with colonic CD11c+ DC representing 3.01.3105 of the total cells in IL2-/- mice compared with 6.01.4104 cells in normal colons. These numbers were calculated by evaluating the CD45+ CD11c+ cells as a proportion of the absolute cell numbers and were generated from four different age- and sex-matched pairs of mice. These results confirm the increase in numbers of colonic DC in colitic mice seen by both electron microscopy and immunohistochemistry.
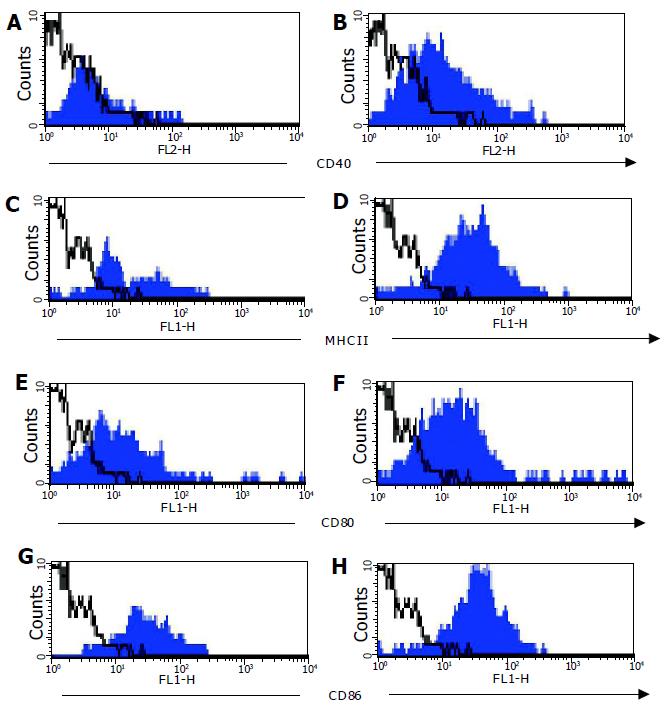
Expression of co-stimulatory molecules (CD40, CD80, and CD86) on CD11c+ cells was compared on DC from C57BL/6 and C57BL/6-IL2-/- mice. Cell surface expression of MHCII and CD40 was low, and CD80 and CD86 was low to moderate on CD11c+ cells derived from normal colons (Figure 5). In contrast, expression of CD40, MHCII, CD80, and CD86 were all higher on DC derived from colitic animals (Figure 5). Furthermore, DC from colitic animals also expressed high levels of DEC205 (data not shown). CD11c+ cells from both C57BL/6 and C57BL/6-IL2-/- mice all expressed high levels of the chemokine receptor CCR5 (data not shown).
The myeloid phenotype of the majority of colonic DC was also confirmed by flow cytometry with CD11c+ cells being uniformly MAC-1+ and CD8α. A small proportion of CD11c+ DC were B220+ confirming the immunohistochemical observations that there were small numbers of plasmacytoid DC present in the colons of colitic animals.
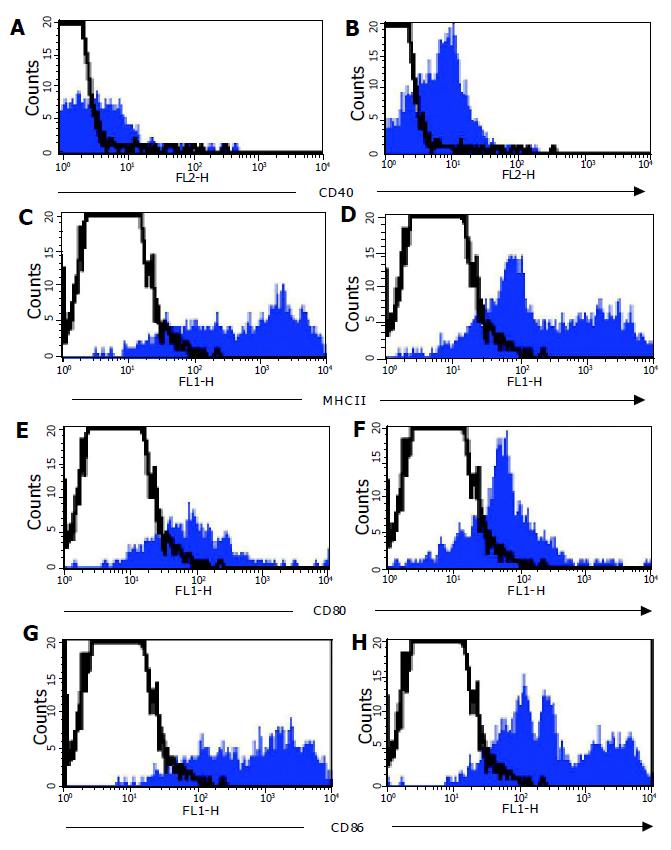
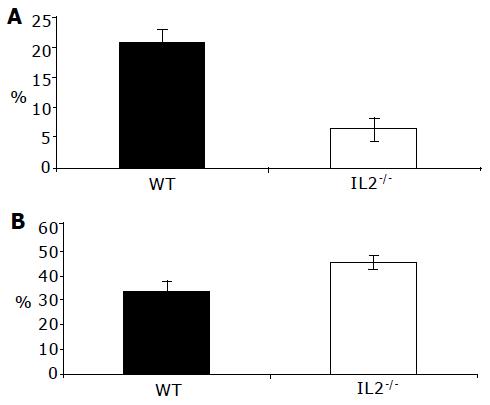
In parallel to analyzing DC from the colonic LP, MLN cells were analyzed from normal and colitic animals. There were many more DC in the MLN of colitic C57BL/6-IL2-/- (3.00.9105) compared with normal C57BL/6 (9.72104) mice. MLN DC expressed high levels of MHCII and CD86, and moderate levels of CD40 and CD80. Furthermore, MLN DC from both C57BL/6 and C57BL/6-IL2-/- animals all expressed the marker DEC205. It was interesting to note that in the MLN of IL2-/- animals, there was an enrichment of DC populations, which expressed lower levels of MHCII (Figure 6B) and CD86 (Figure 6D). In normal mice, myeloid DC represented approximately 40-45% of DC detected in the MLN whereas in IL2-/-, they represented up to 87% of the total DC detected. As for LP DC, all MLN DC expressed CCR5.
That colonic DC from normal C57BL/6 and colitic C57BL/6-IL2-/- animals are immature and mature, respectively, was confirmed by analyzing their endocytic activity (Figure 7). Colonic DC from normal animals were significantly more endocytically active (P<0.001) compared with DC from colitic animals (Figure 7A) consistent with their immature and mature status, respectively. Compared to DC, colonic macrophages were more endocytically active and took up a greater proportion of the FITC-dextran (Figure 7B). Macrophages from colitic animals endocytosed slightly more FITC-dextran than those from normal C57BL/6 animals although this was not statistically significant. Contaminating epithelial cells present in the colonic LP cell preparations all demonstrated endocytic activity. Consistent with MLN DC being mature cells, they had low endocytic activity (data not shown).
This study represents the first to attempt to quantify and characterize colonic DC in the normal, healthy colon and in the colon of colitic mice. We have shown that DC in the normal colon are primarily myeloid of an immature phenotype with high endocytic activity. In contrast, colitic animal, colonic DC are mature and poor at taking up antigen. Furthermore, the redistribution and migration of DC in C57BL/6-IL2-/- mice and the accumulation of DC, B and T cells within lymphoid follicle-like structures suggests that activation of lymphocytes may occur locally within the colon.
There is a profound increase in the number of DC present in the inflamed colon as shown by three independent approaches. This contrasts, however, with a previous study that attempted to quantify DC in colonic biopsies from normal, UC and Crohn’s patients in which it was not possible to identify any differences in the number of DC in the normal vs diseased colon[16]. In using, a murine model of colitis, it was possible to enumerate DC throughout the entire colon including cells within the lymphoid follicles in which many of the DC appeared to reside. By their very nature, biopsy samples may not be representative of the distribution of different cell types throughout the colon and may exclude some or all of these cells. Alternatively, these different findings may simply reflect species-specific differences.
Colonic DC are primarily myeloid DC with a small proportion of plasmacytoid DC present in the inflamed colon. This contrasts with a previous study using rag-1-/- mice transfused with CD4 T cells to induce intestinal inflammation in which CD8α CD11c+ lymphoid DC were detected in the colon, which expanded two- to three-fold during inflammation[8]. This apparent difference may reflect differences in the properties of colonic DC in lymphocyte-replete vs lymphocyte-deficient mice, and different genetic backgrounds[17]. Alternatively, since some plasmacytoid DC have been reported to co-express CD8α the expanded population seen in CD4 T cell-transfused rag-1-/- mice may also represent an expansion of resident plasmacytoid DC which we have identified here. Although expression of the GR-1 antigen has been used to identify plasmacytoid DC in somestrains of mice[17], it was not possible to attempt this analysis here since plasmacytoid DC in C57BL/6 mice do not express GR-1.
The significance of different DC populations in normal and colitic colons is not clear. Plasmacytoid DC are associated with inflammatory responses and can secrete cytokines such as IFNα and IFNβ in response to viruses or bacterial antigens (CpG-DNA)[17,18]. Their role as APC in vivo, however, remains controversial with evidence of their involvement in the induction of tolerance[18-20]. Interestingly, they have also been associated with human plasma cell differentiation[21] and our observation that they localize to lymphoid follicles suggests that they may also be involved in plasma cell differentiation in the mouse. Myeloid DC may be important in promoting T regulatory/tolerogenic responses. Peyer’s patch myeloid DC preferentially produce IL-10 in response to S. aureus and prime T cells to synthesize IL4 and IL-10 whereas lymphoid and CD8α-CD11b- DC primed IFNγ production by T cells[11]. However, our findings that myeloid DC are present in lymphoid follicle structures in the colon and comprise the major DC population in colitic animals suggests that they may promote local immunogenic or pathogenic rather than tolerogenic responses. Their ability to promote Th2 CD4 T cell responses[11] may be important for B cell class switching and contribute to the activation of the plasma cells observed in the lymphoid follicles of colitic IL2-/- mice. It is important to note, however, that DC from the colon and small intestine may not possess the same functional properties. For example, it has been shown that treatment of mice with RANKL promotes tolerogenic T cell responses in the small intestine[22] whereas excessive RANKL in the colon drives colonic inflammation by promoting DC maturation and survival[23,24]. Furthermore, the nature of the signals that promote DC maturation can have a significant impact on the resultant immune response. Signaling through different toll-like receptors (TLR) generates distinct biological responses and differential expression of TLR by different DC subsets enables them to respond to distinct microbial structures in a specific manner[25,26]. Therefore, ligation of pattern recognition receptors by different bacteria may promote different types of T cell responses, including tolerogenic responses, and differences in small intestine and colonic DC function may therefore be due to different environmental influences[22,27,28]. For example, the interaction of DC with other cell types in the colon may be of significance, since colonic epithelial cells (CEC) can suppress T-cell activation even in the presence of professional APC[29]. Thus, the interactions of different cell types in the colon and the influence of the cell environment may have profound effects on the immune response generated.
The localization of colonic DC was altered in colitis. DC in IL2-/- animals were found adjacent to the colonic epithelium and may therefore access the lumen. This is not the case in normal animals, in which the colonic DC were found exclusively within the LP. DC have been reported as forming tight junctions with epithelial cells[30] enabling them to sample luminal antigens. However, our observation that, DC in the normal colon although capable of endocytosis are excluded from the epithelial layer, suggests that colonic DC may not normally sample luminal antigens in healthy mice. By contrast, in colitic mice DC are well positioned to take up luminal antigen although as mature cells they had low endocytic activity. Interestingly, we also found that CEC are strongly endocytic which may support the hypothesis that they are the primary cells involved in sampling luminal antigens within the colon. Based on this assumption we would propose that DC interact directly with CEC. Indeed, we have found that colonic DC express CCR5 and CEC secrete a variety of chemokines including ligands for CCR5 (MCP-1, MIP1α, and MIP1β) in response to different bacterial stimuli and may therefore play a role in attracting DC to the epithelial layer[31].
The number of CD11c+ DC in the MLN of colitic mice was increased compared to normal mice and a population of MHCIIint, CD86int DC were expanded in the MLN, corresponding to the phenotype of mature LP DC which suggested that some colonic LP DC may have migrated from the colonic mucosa to the MLN. In addition to T-cell activation occurring within the MLN, there may also be local activation of lymphocytes within the colon as we found organized aggregates of DC at the base of the crypts in the inflamed colon, which primarily comprised DC, B cells, and T cells. The presence of plasma cells including Mott cells, which were identified by electron microscopy, in the colonic LP of IL2-/- animals adds weight to this hypothesis. Of note, similar aggregates of DC, B, and T cells have been described in human UC[32]. The formation of lymphoid aggregates may also contribute significantly to the pathology of IBD as DC which mature in inflamed tissue and fail to migrate to the lymph nodes are thought to act as nucleation sites to organize lymphoid structures which can sustain chronic inflammation[33].
In conclusion, we have characterized the phenotype of colonic DC in normal mice and mice with colitis. There is a dramatic increase in DC numbers within the colonic LP of the inflamed colon which have an altered phenotype and localization. Furthermore, we have described the formation of lymphoid aggregates which may contribute directly to the ongoing inflammation. Further study of these cells may be informative in understanding the etiology of colitis and persistence of chronic inflammation.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, and Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;102:448-455. [RCA] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 630] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Contractor NV, Bassiri H, Reya T, Park AY, Baumgart DC, Wasik MA, Emerson SG, Carding SR. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385-394. [PubMed] |
| 3. | Garside P, Mowat AM. Oral tolerance. Semin Immunol. 2001;13:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Kelsall BL, Stüber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996;795:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381-1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 446] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958-4967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Krajina T, Leithäuser F, Möller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1257] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 10. | Contractor NV, Bassiri H, Reya T, Park AY, Baumgart DC, Wasik MA, Emerson SG, Carding SR. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385-394. [PubMed] |
| 11. | Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884-4890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 303] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Patterson S, Gross J, Bedford P, Knight SC. Morphology and phenotype of dendritic cells from peripheral blood and their productive and non-productive infection with human immunodeficiency virus type 1. Immunol. 1991;72:361-367. |
| 13. | Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Jiang Y, Hirose S, Hamano Y, Kodera S, Tsurui H, Abe M, Terashima K, Ishikawa S, Shirai T. Mapping of a gene for the increased susceptibility of B1 cells to Mott cell formation in murine autoimmune disease. J Immunol. 1997;158:992-997. [PubMed] |
| 16. | Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 365] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Hochrein H, O’Keeffe M, Wagner H. Human and mouse plasmacytoid dendritic cells. Hum Immunol. 2002;63:1103-1110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Kelsall BL, Biron CA, Sharma O, Kaye PM. Dendritic cells at the host-pathogen interface. Nat Immunol. 2002;3:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 816] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 22. | Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol. 2002;169:3606-3612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. 2000;191:495-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1490] [Cited by in RCA: 1439] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 26. | Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692-37699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 500] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 29. | Cruickshank SM, McVay LD, Baumgart DC, Felsburg PJ, Carding SR. Colonic epithelial cell mediated suppression of CD4 T cell activation. Gut. 2004;53:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Lan JG, Cruickshank SM, Singh JC, Farrar M, Lodge JP, Felsburg PJ, Carding SR. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J Gastroenterol. 2005;11:3375-3384. [PubMed] |
| 32. | Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarström S, Hammarström ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |