Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6243
Revised: May 21, 2005
Accepted: May 24, 2005
Published online: October 28, 2005
AIM: To study adhesion capacity and CD44 expression of human gastric adenocarcinoma MKN45 cells at different stages of a first cell cycle.
METHODS: MKN45 cells were synchronized by aphidicolin and assayed for adhesion to an endothelial cell (HUVEC) monolayer. Surface expression of CD44 and CD44 splice variants on MKN45 cells was evaluated by flow cytometry. Functional relevance of CD44 adhesion receptors was investigated by blocking studies using anti CD44 monoclonal antibodies or by hyaluronan digestion.
RESULTS: Adhesion of MKN45 to HUVEC was increased during G2/M transit, after which adhesion returned to baseline levels with cell cycle completion. In parallel, CD44 splice variants CD44v4, CD44v5, and CD44v7 were all up-regulated on MKN45 during cell cycle progression with a maximum effect in G2/M. The function of CD44 surface receptors was assessed with specific receptor blocking monoclonal antibodies or removal of hyaluronan by digestion with hyaluronidase. Both strategies inhibited tumor cell adhesion to HUVEC by nearly 50%, which indicates that MKN45-HUVEC-interaction is CD44 dependent.
CONCLUSION: CD44 expression level is linked to the cell cycle in gastrointestinal tumor cells, which in turn leads to cell cycle dependent alterations of their adhesion behaviour to endothelium.
- Citation: Oertl A, Castein J, Engl T, Beecken WD, Jonas D, Melamed R, Blaheta RA. Endothelial adhesion of synchronized gastric tumor cells changes during cell cycle transit and correlates with the expression level of CD44 splice variants. World J Gastroenterol 2005; 11(40): 6243-6248
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6243
Enhanced invasive capacity and proliferative activity are among the array of functions a tumor cell must fulfil to settle as a metastasis in a distant organ. The dynamic process of tumor dissemination requires cooperative activities between the tumor cell itself and the surrounding tissue and involves several molecule classes. The CD44 transmembrane glycoproteins belong to the families of adhesion molecules and were originally thought to mediate lymphocyte homing to peripheral lymphoid tissues. It has now been recognized that CD44 molecules may also initiate metastatic spread of the tumor cells[1]. Still, the precise role of CD44 as a regulatory element of tumor growth and invasion is not clearly understood.
Several clinical studies have been carried out on patients suffering from colorectal neoplasms, but no consistent statement can be drawn about the relevance of CD44 receptors in tumor cell malignancy. Some authors hypothesize that CD44 contributes to both tumor cell proliferation and motility, whilst others do not see any logical link between CD44 expression, proliferative activity and migratory behaviour: Sumiyoshi et al[2], (28 cases) and Yue et al[3], (31 cases) reported a positive relationship between the expression of the proliferating nuclear antigen PCNA, CD44, and metastasis formation in colorectal cancer. In contrast, no statistical correlation was found between CD44 positivity and disease free intervals in studies including a total of 113 colorectal tumor patients[4,5]. Additionally, evaluation of the characteristics of colorectal carcinoma during the metastatic process did not favour an association between CD44 expression, proliferative activity and metastatic ability of tumor cells.
Analyses of surgical specimens of 22 colorectal carci-nomas revealed CD44 expression in tumors associated with proliferative areas. However, there was no apparent association between CD44 positivity and tumor stage, differentiation and progression[6]. In a further study, CD44 expression in a series of 110 colorectal carcinomas correlated well with tumor size, although extensive CD44 expression was also found in the majority of adenomas that were investigated[7]. Based on a topographical investigation, the cellular localization of CD44 and PCNA was found to be different in colorectal tumor cells, and PCNA was present most frequently in cells lacking CD44. The authors concluded that tumors, negative for CD44, have a higher probability of replicating[8].
The conflicting results are partially caused by the retrospective nature of immunohistochemical studies. Furthermore, examination of tissue specimen does not allow unequivocable evaluation of the time-dependent dynamics of tumor-host interactions. There is no doubt that tumor dissemination and malignancy is characterized by a fine-tuned interplay between CD44 expression level, cell cycle activation and migratory capacity. However, there is still little knowledge about how these processes are linked together. To clearly assess if CD44 triggered tumor cell invasion is cell-cycle dependent, we established a cell culture system consisting of aphidicolin-synchronized human gastric adenocarcinoma MKN45 cells. CD44 surface expression was quantified, when MKN45 cells were released into S-phase, G2/M-phase and G0/G1-phase, as well as when adhering to human umbilical vein endothelial cells (HUVEC).
Endothelial cells (HUVEC) were isolated from human umbilical veins and harvested by enzymatic treatment with chymotrypsin. HUVEC were grown in Medium 199 (Biozol, Munich, Germany), 10% fetal calf serum (FCS; Gibco, Karlsruhe, Germany), 10% pooled human serum (Blood Bank of The German Red Cross, Frankfurt am Main, Germany), 20 μg/mL endothelial cell growth factor (Boehringer, Mannheim, Germany), 0.1% heparin (Roche, Basel, Switzerland), 100 ng/mL gentamicin (Gibco) and 2% 1 mol/L HEPES-buffer (Seromed, Berlin, Germany). To control the purity of HUVEC cultures, cells were stained with fluorescein isothioc-yanate (FITC)- labelled monoclonal antibody against Factor VIII-associated antigen (Von Willebrand factor; clone F8/86; Dako, Hamburg, Germany) and analyzed microsc-opically, or by FACscan (Becton Dickinson, Heidelberg, Germany; FL-1H (log) channel histogram analysis; 1×104 cells/scan). Cell cultures with a purity >95% were serially passaged. Subcultures from passages 2-4 were selected for experimental use.
Human gastric adenocarcinoma MKN45 cells were obtained from the tumor cell bank of the Johannes Gutenberg University, Mainz, Germany, and grown in RPMI medium supplemented with 10% FCS, 100 ng/mL gentamicin and 2% 1 mol/L HEPES-buffer. To prepare the tumor cells for the invasion assays, cells were detached from the culture flasks by Accutase (PAA Laboratories).
MKN45 cells were reversibly blocked at the G1/S transition by a 24 h treatment with 5 μg/mL aphidicolin (Sigma, Müchen, Germany). To allow S-phase progression, cells were washed extensively and replated in tumor cell culture medium in the absence of aphidicolin. Cells were sampled at successive time points to assess their position in the cell cycle, their adhesion capacity and their CD44 expression level. Non-synchronized control cell cultures were incubated in aphidicolin free culture medium. Viability of tumor cells was assessed by propidium iodide dsDNA-intercalation (facs-analysis) or by the evaluation of lactate dehydrogenase (LDH) release (Cytotoxicity Detection Kit; Roche) at 490 nm using an automatic microtiter plate reader.
MKN45 cells were reversibly blocked with aphidicolin and subsequently washed and replated in tumor cell culture medium in the absence of aphidicolin. Cells were sampled at successive time points and incubated with propidium iodide and RNAse (Cycle Test Plus; Becton Dickinson). DNA content and cell cycle distribution were analyzed using a Becton Dickinson FACScan flow cytometer. Cytoflu-orometric analysis was performed using CellQuest software (Becton Dickinson), on 10 000 cells per sample. The percentage of cells in S-phase, G2/M-phase and G0/G1-phase was determined using Modfit 2.0 software (Becton Dickinson).
CD44 standard and splice variant (v) expression were determined with anti human CD44 monoclonal antibodies: CD44standard, clone SFF-2; CD44v3, clone VFF-327v3; CD44v4, clone VFF-11; CD44v5, clone VFF-8; CD44v6, clone VFF-7; CD44v7, clone VFF-9; CD44v7+8, clone VFF-17; CD44v10, clone VFF-14 (all: Bender MedSystems, Eching, Germany). Primary antibodies were conjugated with fluorescein isothiocyanate (FITC). CD44 surface expression of MKN45 cells was then measured using a FACscan (Becton Dickinson; FL-1H (log) channel histogram analysis; 1×104 cells/scan) and expressed as mean fluorescence units (MFU). Mouse IgG1-FITC (Dianova-CBL, Hamburg, Germany) was used as an isotype control.
HUVEC were transferred to six-well multiplates (Falcon Primaria; Becton Dickinson). When confluency was reached, MKN45 cells (synchronized versus non-synchronized) were detached from the culture flasks and 0.5×106 cells were then added to the HUVEC monolayer for 60 min. Subsequently, non-adherent tumor cells were washed off using 37 °C Medium 199. The remaining cells were fixed with 1% glutaraldehyde. Adherent tumor cells were counted in five different fields of a defined size (5 mm×0.25 mm) using a phase contrast microscope (20×bjective) and the mean cellular adhesion rate was calculated. Proliferative activity of tumor cells was estimated by the PicoGreen assay[9]. Cells were washed and dried at several time points (0-24 h). Cells were then digested with papain (0.125 mg protein/mL) for 20 h at 60 °C. Thereafter, the fluorescent dye PicoGreen (MoBiTec, Goettingen, Germany), which shows high specificity for dsDNA, was added (1:200 dilution) for 10 min at 20 °C. Fluorescence intensity was determined using a computer-controlled fluorescence reader (Cytofluor 2300 plate scanner; Millipore, Eschborn, Germany) at λex = 485 nm and λem = 530 nm.
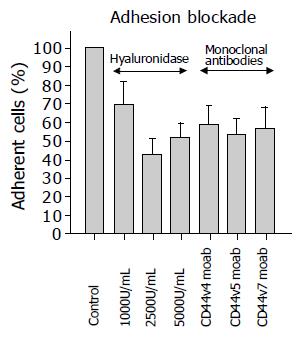
MKN45 cells were preincubated for 60 min with anti CD44v4, anti CD44v5 or anti CD44v7 monoclonal antibodies at 1:80 dilution. MKN45 cells were then added to HUVEC monolayer to evaluate the adhesion capacity as described above. In a second set of experiments, HUVEC were treated with hyaluronidase (Worthington, Lakewood, USA) for 60 min (1 000 U/mL, 2 500 U/mL, or 5 000 U/mL), before MKN45 cells were added. Non treated HUVEC served as the controls. Adhesion capacity of tumor cells was then analyzed as described above.
All studies were performed 3-6 times. Statistical significance was investigated by the Wilcoxon-Mann-Whitney-U-test. Differences were considered statistically significant at a P value less than 0.05.
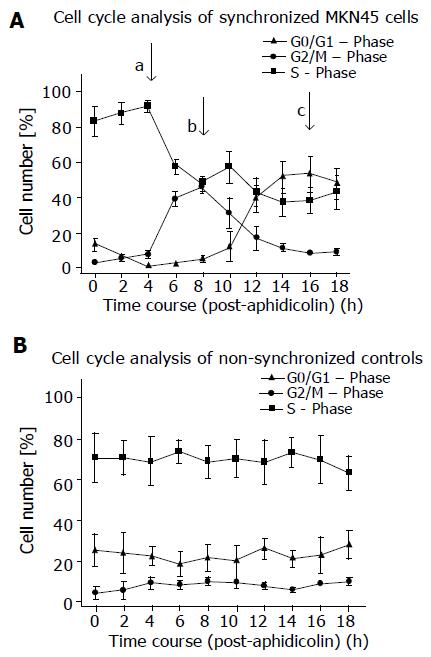
MKN45 tumor cells were synchronized to monitor the changes in adhesion behaviour at various stages during cell cycle transit. The procedure based on treatment with aphidicolin effectively allowed us to follow cells in distinct phases of a single cell cycle prior to any cell division: in G0/G1, at the G2/M transition and in S phase.
Non-synchronized MKN45 reside in the S phase of the cell cycle (Figure 1). However, they were reversibly blocked at the G1/S transition by a 24 h treatment with 5 μg/mL aphidicolin. After extensive washing and replacem-ent in fresh medium to allow cell cycle progression, MKN45 quickly entered S phase after 4 h and reached G2/M after 8 h. MKN accumulated in G0/G1 after 16 h (Figure 1).
Aphidicolin synchronization had no toxic effects on MKN45 because cell viability and adhesion events of synchronized cells to HUVEC were not diminished, compared to untreated controls.
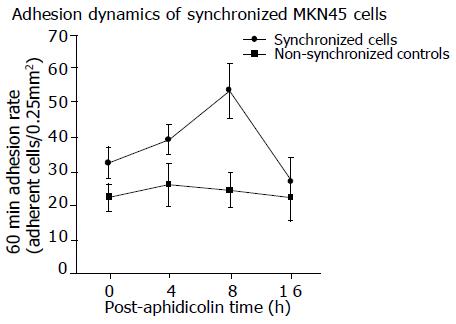
Adhesion of MKN45 to HUVEC was determined at 0 h (immediately after washing out aphidicolin), 4, 8, and 16 h after the end of aphidicolin treatment. This was in accordance to the maximum accumulation of synchronized MKN45 in the S phase, G2/M phase or in G0/G1. Typically, adhesion capacity increased when cells progressed to G2/M (Figure 2; P<0.05). The increased number of adherent MKN45 seen after 8 h was not due to an increase in the total cell number. The PicoGreen assay, which had been carried out as well, revealed no proliferative activity of MKN45 during the experimental procedure. Furthermore, non-synchronous control cultures adhered to HUVEC uniformly at all time points.
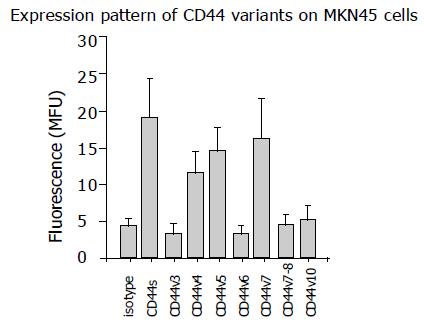
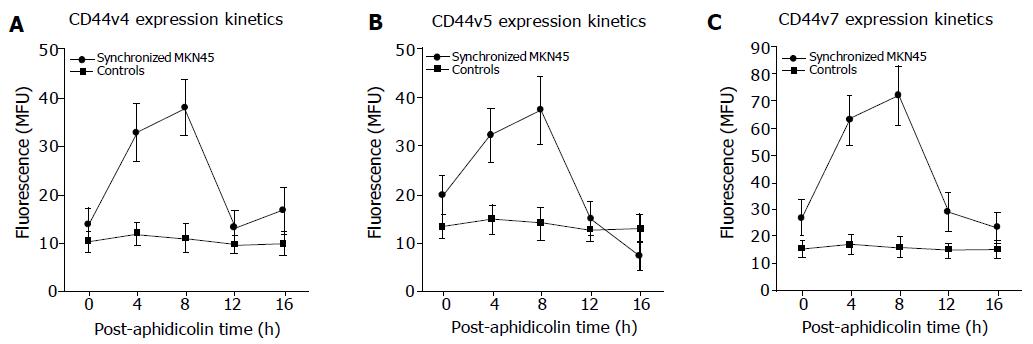
Analysis of CD44 expression in asynchronous control cultures revealed significant expression of CD44 splice variants CD44v4, CD44v5, and CD44v7. CD44 variants CD44v3, CD44v6, CD44v8, and CD44v10 were not detected (Figure 3). Therefore, subsequent studies concentrated on CD44v4, CD44v5, and CD44v7 adhesion receptors. To correlate the differences in CD44 expression level during cell cycle transit with parallel changes of MKN45 adhesion behavio, CD44 splice variants were evaluated 0, 4, 8, 12, and 16 h after the end of aphidicolin treatment. Figure 4 shows that CD44v4, CD44v5 and CD44v7 expression in synchronized cell cultures were all up-regulated during cell cycle progression with a maximum effect 8 h after the experimental onset, i. e. in G2/M (P<0.05). In contrast, non-synchronized MKN45 expressed CD44 variants equally well at all time points.
To evaluate whether CD44v4, v5 and v7 receptors faci-litate adhesion of MKN45 to HUVEC, tumor cells were treated with their respective monoclonal antibodies, or the CD44 ligand hyaluronan on HUVEC was removed by digestion with hyaluronidase. Pretreatment of MKN45 cells with anti CD44 monoclonal antibodies decreased adhesion to HUVEC by nearly 50%, irrespective of the CD44 splice variant used (P<0.05). In good accordance, digestion of hyaluronan on HUVEC blocked MKN45 cell attachment to HUVEC with a maximum effect at a hyaluronidase concentration of 2 500 U/mL (P<0.05; Figure 5).
CD44, an integral membrane glycoprotein expressed by many cell types, serves as the principal receptor which binds hyaluronan and might be a determinant of metastatic and invasive behaviour in carcinomas[10]. One of the basic features of carcinoma cells is their ability to generate a large number of alternatively spliced isoforms of CD44[11,12]. Although the structural characteristics of these isoforms are documented, the functional consequences of CD44 isoform expression on the biology of colon cancer cells still remains unclear.
CD44 surface analysis demonstrated that MKN45 tumor cells strongly express the CD44 splice variants CD44v4, CD44v5 and CD44v7. This is in good accordance with studies on tissue specimens revealing elevated expression of CD44v5 in human colon carcinoma cells which was associated with tumor progression and growth[13]. Mulder and coworkers observed high expression of CD44v5 in most colorectal neoplasms (83%-96%), independent of stage, which indicates that CD44v5 might be an earlier marker of colonic epithelium transformation[14,15]. Using immunohistochemical staining and Northern blotting, Harn HJ et al[16] showed that the expression of CD44 variant encoding exon v5 was up-regulated in human gastric carcinomas. Most notably, CD44v5 was expressed preferentially in poorly differentiated human gastric carcinomas and metastatic lymph nodes[17].
Overexpression of CD44 variants v4 and v7 contributes to the genesis of gastric cancer as well[18,19], although limited data are available and the biological significance of the receptor modulation is not clear[20].
Based on our adhesion-blocking study using specific anti-CD44 monoclonal antibodies, we hypothesize that CD44v4, CD44v5, as well as CD44v7 receptors are important elements which regulate adhesive interactions between gastric tumor cells and the vessel wall. Results of additional experiments, in which endothelial cells were treated with hyaluronidase, further demonstrate that the interaction of CD44 molecules on MKN45 cells with hyaluronan on HUVEC is a critical factor in the adhesion process.
To address the question of whether CD44 expression is linked to the cell cycle, the CD44 splicing isoforms CD44v4, CD44v5 and CD44v7 were evaluated on synchronized MKN45 tumor cells. Intriguingly, CD44 variants increased after cells were released from arrest, showing maximal expression 8 h after the experimental onset. At the same time point, cells passed through G2/M. Therefore, these experiments clearly indicate that CD44 receptor expression “follows” the proliferative activity of tumor cells with an enhanced expression level in G2/M, and subsequent repression in G0/G1 and S.
This is the first report demonstrating a link between cell cycle and CD44 expression in gastric carcinoma cells. However, data derived from other tumor entities support our findings. CD44 ligation with an anti-CD44 monoclonal antibody inhibited the proliferation of human acute myeloid leukemia cells. The effects of anti-CD44 on myeloid cells were associated with specific disruption of cell cycle events and induction of G0/G1 arrest[21]. Experimentally induced proliferation blockade of malignant rhabdoid tumors led to an accumulation in G0/G1, which was accompanied by CD44 down-regulation[22]. In a similar way, CD44 was down-modulated, when human breast cancer cells were blocked in the G1 phase[23]. Interestingly, analysis of 131 patients who underwent surgery for papillary thyroid carcinoma revealed a close relation between extrathyroidal invasion, G2M-phase fraction and CD44 expression[24].
The molecular background of the cell cycle-CD44 axis has not been evaluated in detail. Experiments on myeloid leukemia cells demonstrated that ligation of CD44 with monoclonal antibodies causes growth arrest through the down-regulation of c-Jun expression via AP-1 sites. Decreased JNK expression and a consequent decrease in c-Jun phosphorylation was also assumed to be involved in the down-regulation of the c-Jun promoter activity[21]. Nevertheless, although this is an attractive hypothesis, these events might not necessarily occur in our culture system. Several authors describe progressive changes of the p53 protein and the anti-apoptotic protein bcl-2 in carcinomas, with an inverse relationship between p53, bcl-2 and CD44 expression[7,25]. Both p53 and bcl-2, as well as their downstream targets p21, p27, and/or p130, play key roles in cell proliferation and cell cycle progression[26,27], and therefore might also contribute to CD44 modulation.
The finding that CD44 surface expression was altered during mitosis, together with the fact that CD44 receptors regulate tumor cell endothelial cell interactions, prompted us to speculate that tumor cells change their adhesive behaviour in a cell-cycle dependent manner. Indeed, the number of synchronized MKN45 binding to HUVEC drastically increased in G2/M, and dropped down to basal values in G0/G1 and S. The adhesion dynamics exactly match the cell-cycle dependent expression course of CD44 variants v4, v5, and v7. Based on this, we conclude that CD44 expression level, cell cycle activation and migratory capacity of gastric tumor cells are linked together, with the most aggressive behaviour of tumors in the G2/M phase.
From a clinical viewpoint, therapeutic modulation of the tumor cell cycle might alter the sensitivity of these cells to anti-tumoral compounds and, consequently, might enhance the efficacy of the current treatment protocols.
We would like to thank Karen Nelson for critically reading the manuscript.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Jothy S. CD44 and its partners in metastasis. Clin Exp Metastasis. 2003;20:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Sumiyoshi Y, Yamashita Y, Maekawa T, Sakai N, Shirakusa T, Kikuchi M. Expression of CD44, vascular endothelial growth factor, and proliferating cell nuclear antigen in severe venous invasional colorectal cancer and its relationship to liver metastasis. Surg Today. 2000;30:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Yue SQ, Yang YL, Dou KF, Li KZ. Expression of PCNA and CD44mRNA in colorectal cancer with venous invasion and its relationship to liver metastasis. World J Gastroenterol. 2003;9:2863-2865. [PubMed] |
| 4. | Jüngling B, Menges M, Goebel R, Wittig BM, Weg-Remers S, Pistorius G, Schilling M, Bauer M, König J, Zeitz M. Expression of CD44v6 has no prognostic value in patients with colorectal cancer. Z Gastroenterol. 2002;40:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Indinnimeo M, Cicchini C, Giarnieri E, Stazi A, Mingazzini PL, Stipa V. Evaluation of CD44 variant 6 expression and clinicopathological factors in pulmonary metastases from colon carcinoma. Oncol Rep. 2003;10:1875-1877. [PubMed] |
| 6. | Abbasi AM, Chester KA, Talbot IC, Macpherson AS, Boxer G, Forbes A, Malcolm AD, Begent RH. CD44 is associated with proliferation in normal and neoplastic human colorectal epithelial cells. Eur J Cancer. 1993;29A:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ioachim E, Goussia A, Agnantis NJ. Glycoprotein CD44 expression in colorectal neoplasms. An immuno-histochemical study including correlation with cathepsin D, extracellular matrix components, p53, Rb, bcl-2, c-erbB-2, EGFR and proliferation indices. Virchows Arch. 1999;434:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Furuta K, Zahurak M, Yang XL, Rosada C, Goodman SN, August JT, Hamilton SR. Relationship between CD44 expression and cell proliferation in epithelium and stroma of colorectal neoplasms. Am J Pathol. 1996;149:1147-1155. [PubMed] |
| 9. | Blaheta RA, Kronenberger B, Woitaschek D, Weber S, Scholz M, Schuldes H, Encke A, Markus BH. Development of an ultrasensitive in vitro assay to monitor growth of primary cell cultures with reduced mitotic activity. J Immunol Methods. 1998;211:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Miyake K, Underhill CB, Lesley J, Kincade PW. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990;172:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 470] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Tarin D, Bolodeoku J, Hatfill SJ, Sugino T, Woodman AC, Yoshida K. The clinical significance of malfunction of the CD44 locus in malignancy. J Neurooncol. 1995;26:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Tölg C, Hofmann M, Herrlich P, Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993;21:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 227] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Leister I, Manegold S, Schüler P, Alves F, Becker H, Füzesi L, Markus PM. Effect of laparotomy and CO(2) pneumoperitoneum on tumor growth of human colon carcinoma and expression pattern of tumor-associated proteins in the SCID mouse. Int J Colorectal Dis. 2003;18:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Mulder JW, Wielenga VJ, Polak MM, van den Berg FM, Adolf GR, Herrlich P, Pals ST, Offerhaus GJ. Expression of mutant p53 protein and CD44 variant proteins in colorectal tumorigenesis. Gut. 1995;36:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Mulder JW, Kruyt PM, Sewnath M, Seldenrijk CA, Weidema WF, Pals ST, Offerhaus GJ. Difference in expression of CD44 splice variants between proximal and distal adenocarcinoma of the large bowel. Br J Surg. 1995;82:1468-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Heider KH, Dämmrich J, Skroch-Angel P, Müller-Hermelink HK, Vollmers HP, Herrlich P, Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993;53:4197-4203. [PubMed] |
| 17. | Harn HJ, Ho LI, Chang JY, Wu CW, Jiang SY, Lee HS, Lee WH. Differential expression of the human metastasis adhesion molecule CD44V in normal and carcinomatous stomach mucosa of Chinese subjects. Cancer. 1995;75:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Montgomery E, Abraham SC, Fisher C, Deasel MR, Amr SS, Sheikh SS, House M, Lilliemoe K, Choti M, Brock M. CD44 loss in gastric stromal tumors as a prognostic marker. Am J Surg Pathol. 2004;28:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Torbenson M, Dhir R, Nangia A, Becich MJ, Kapadia SB. Prostatic carcinoma with signet ring cells: a clinicopathologic and immunohistochemical analysis of 12 cases, with review of the literature. Mod Pathol. 1998;11:552-559. [PubMed] |
| 20. | Tempfer C, Lösch A, Heinzl H, Häusler G, Hanzal E, Kölbl H, Breitenecker G, Kainz C. Prognostic value of immunohistochemically detected CD44 isoforms CD44v5, CD44v6 and CD44v7-8 in human breast cancer. Eur J Cancer. 1996;32A:2023-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Zada AA, Singh SM, Reddy VA, Elsässer A, Meisel A, Haferlach T, Tenen DG, Hiddemann W, Behre G. Downregulation of c-Jun expression and cell cycle regulatory molecules in acute myeloid leukemia cells upon CD44 ligation. Oncogene. 2003;22:2296-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Oruetxebarria I, Venturini F, Kekarainen T, Houweling A, Zuijderduijn LM, Mohd-Sarip A, Vries RG, Hoeben RC, Verrijzer CP. P16INK4a is required for hSNF5 chromatin remodeler-induced cellular senescence in malignant rhabdoid tumor cells. J Biol Chem. 2004;279:3807-3816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Righi L, Deaglio S, Pecchioni C, Gregorini A, Horenstein AL, Bussolati G, Sapino A, Malavasi F. Role of CD31/platelet endothelial cell adhesion molecule-1 expression in in vitro and in vivo growth and differentiation of human breast cancer cells. Am J Pathol. 2003;162:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kurozumi K, Nakao K, Nishida T, Nakahara M, Ogino N, Tsujimoto M. Significance of biologic aggressiveness and proliferating activity in papillary thyroid carcinoma. World J Surg. 1998;22:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Jackson PA, Green MA, Pouli A, Hubbard R, Marks CG, Cook MG. Relation between stage, grade, proliferation, and expression of p53 and CD44 in adenomas and carcinomas of the colorectum. J Clin Pathol. 1995;48:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Vairo G, Soos TJ, Upton TM, Zalvide J, DeCaprio JA, Ewen ME, Koff A, Adams JM. Bcl-2 retards cell cycle entry through p27(Kip1), pRB relative p130, and altered E2F regulation. Mol Cell Biol. 2000;20:4745-4753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kaul R, Mukherjee S, Ahmed F, Bhat MK, Chhipa R, Galande S, Chattopadhyay S. Direct interaction with and activation of p53 by SMAR1 retards cell-cycle progression at G2/M phase and delays tumor growth in mice. Int J Cancer. 2003;103:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |