Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2756
Revised: December 23, 2003
Accepted: January 12, 2004
Published online: September 15, 2004
AIM: To establish the purification method for Clostridium difficile (C. difficile) toxin A.
METHODS: C. difficile VPI 10463 filtrate was cultured anaerobically by the dialysis bag methods. And then the toxin A was purified by precipitation with 500 g/L (NH4)2SO4 and acid precipitation at pH5.5, followed by ion-exchange chromatography on DEAE-Toyopearl.
RESULTS: Purified toxin A exhibited only one band on native polyacrylamide gel electrophoresis (native-PAGE) and Ouchterlony double immunodiffusion. The molecular weight of toxin A was estimated to be 550000. The purified toxin A had a protein concentration of 0.881 mg/mL. The minimum lethal dose was 1 × 106 MLD/mL (i.p.mice). The cytotoxic titer was 107 CU/mg. The haemagglutinate activity was at a concentration of 1.72 μg/mL. The ratio of fluid volume (mL) accumulated to the length (cm) of the loop was 2.46.
CONCLUSION: The modified method for purification of toxin A of C. difficile was simple and convenient. It may be even more suitable for purification of toxin A on large scales.
-
Citation: Fu SW, Xue J, Zhang YL, Zhou DY. Simplified purification method for
Clostridium difficile toxin A. World J Gastroenterol 2004; 10(18): 2756-2758 - URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2756
Clostridium difficile (C. difficile) is one major causative agent of pseudomembranous colitis (PMC) and a large number of antibiotic-associated diarrhea in humans and animals. It has been reported that this organism produces at least two toxins, designated A (enterotoxin) and B (cytotoxin). Toxin A, a tissue-damaging enterotoxin, causes hemorrhagic fluid accumulation in rabbit ileal loops and is cytotoxic to cultured fibroblasts. Toxin B is an extremely potent cytotoxin for many cultured cells[1]. The importance of toxin A in the pathogenesis of C. difficile enteritis has been documented and several investigators have established the purification method of enterotoxin[2]. In this report, we presented a simple method for purification of toxin A. The highly purified toxin A was obtained and analyzed for its biological and immunological properties.
C. difficile VPI 10463 was grown anaerobically at 37 for 72 h in Brain Heart Infusion (Difco) by the dialysis bag methods.
C. difficile VPI 10463 culture filtrates were centrifuged at 8000 r/min for 20 min and filtrated through a 0.45 μm membrane filter. The culture supernatant of 200 mL was precipitated by 500 g/L saturated (NH4)2SO4 overnight at 4 °C, centrifuged at 8000 r/min for 15 min. The precipitation was dissolved in 50 mmol/L Tris-HCl buffer (pH7.5) and dialyzed in 10 mmol/L acetic buffer(pH5.5) at 4 °C for 24 h, centrifuged twice, The precipitate was dissolved in 50 mmol/L Tris-HCl (pH7.5) and dialysed against the same buffer, concentrated by PEG 10000 at 4 °C. The sample was then loaded onto anion-exchange DEAE-Toyopearl 650 mol/L chromatography column, the sample was equilibrated by 300 mL of 50 mmol/L Tris-HCl buffer (pH7.5, containing 50 mmol/L NaCl), and then eluted first with 200 mL of a linear NaCl gradient (50-250 mmol/L NaCl), followed by 200 mL of 50 mmol/L Tris-HCl buffer (pH7.5) containing 300 mmol/L NaCl. A second elusion using 200 mL of a linear gradient (300-600 mmol/L NaCl) in the same buffer was performed. The toxins that eluted in the first NaCl gradient were designated toxins A. The toxin was pooled and concentrated, and analyzed for its biological and immunological properties.
Cytotoxic activity was determined by using African green monkey kidney (Vero) cells as previously described by Kamiya et al[3]. For the analysis, tenfold dilutions of test samples were prepared and assayed for activity. The cytotoxic titer, which was expressed as the reciprocal of the highest dilution that caused 100% rounding of the cells by 24 h, was determined by serially diluted 100 μL samples transferring to microtiter wells containing Vero cells (105/mL) in 100 μL of MEM medium. The specific activity, which was determined from the cytotoxic titer, was defined as the number of cytotoxic units per microgram.
In reference to the methods described by Meng et al[3], rabbit blood cells were washed 3 times with PBS (0.15 mol/L, pH7.2) and then diluted to 25 mL/L suspension. 2 × serial dilution of specimens (50 μL) was performed with PBS buffer in microplate wells and 50 μL of blood cells were added to each well. The HA titer was expressed as the highest dilution that agglutinated rabbit blood cells at 4 °C for 3 h.
Enterotoxic activity was determined by the rabbit ileal loop assay as previously described[3]. Results were obtained approximately 18 h after injection of 1 mL of test samples and expressed as the ratio of the accumulated fluid (volume) to the length (centimeters) of the loop. Heat-inactivated toxin preparations were used as negative controls.
The minimum lethal dose was determined by intraperitoneally injecting mice (14-16 g) with 0.5 mL aliquots of tenfold serial dilutions of test samples and observing the mice over 72 h for toxicity. Two mice were used for each dose of toxin.
Native polyacrylamide gel electrophoresis was performed in 75 g/L and 40-300 g/L gel. Samples were electrophoresed at 120 V for 15 h after initial electrophoreses at 70 V for 30 min. After electrophoresis, gels were stained with Coomassie brilliant blue R-250 and destained. Molecular weights were estimated by comparison with high molecular weight standards (Pharmacia).
Ouchterlony double immunodiffusion was done in 10 g/L agarose. Crossed immunoelectrophoresis (CIP) was performed as previously described[3]. Goat antiserum against toxin A used in the analyses was prepared in our laboratory.
Protein concentration was determined by the method of Coomassie brilliant blue G-250. Bovine serum albumin was purchased from Sigma Company of USA.
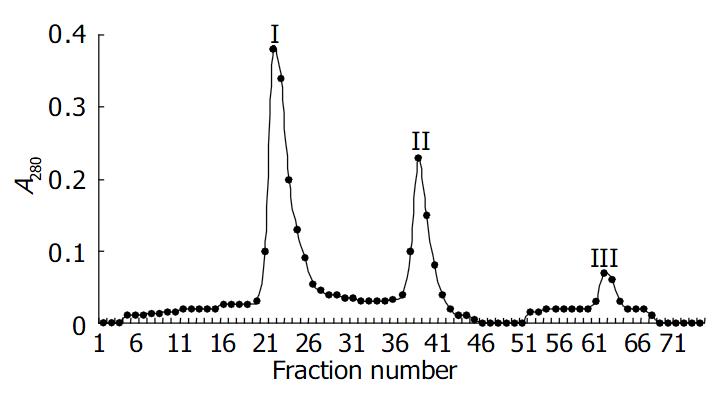
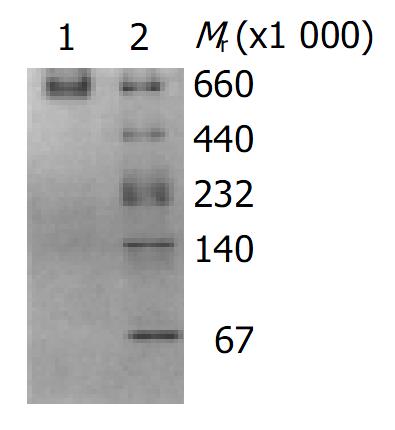
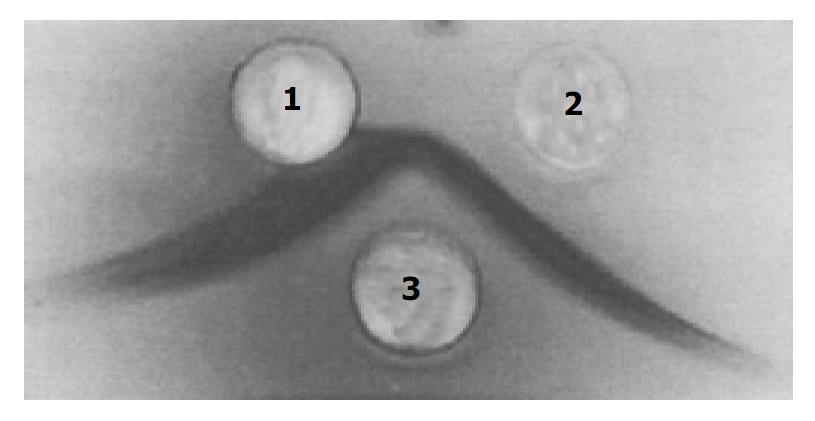
High purified toxin A was obtained by precipitation with 500 g/L (NH4)2SO4 and acid precipitation at pH5.5, followed by DEAE-Toyopearl 650 mol/L column chromatography. The elution profile of toxin A by DEAE-Toyopearl 650 mol/L column chromatography is shown in Figure 1. The purified toxin A exhibited only one band on 40-300 g/L native polyacrylamide gel electrophoresis, the molecular mass of toxin A was estimated to be 550000 (Figure 2). The toxin preparation also gave a single component when analyzed by Ouchterlony double immunodiffusion (Figure 3) and cross immunoelectrophoresis.
The purified toxin A had a protein concentration of 0.881 mg/mL, the cytotoxic titer was 107 CU units per mg. The HA activity was at a concentration of 1.72 μg/mL, the ratio of fluid accumulated volume (mL) to the length (cm) of the loop was 2.46. The minimum lethal dose was 1 × 106 MLD/mL (Table 1). All biological properties of purified toxin A were neutralized by the antiserum of C. difficile toxin A.
| Purification step | Vol (mL) | Protein(mg/mL) | Lethal dose(Mice, MLD/mL) | Cytotoxicity (CU/mg) |
| Crude culture supernatant | 200 | 1.86 | 6 × 104 | 1010 |
| 50% (NH4)2SO4 precipitation | 10 | ND | ND | ND |
| Acid precipitation | 10 | 6.15 | 2 × 106 | 1010 |
| DEAE-Toyopeal 650 mol/L | 6 | 0.881 | 1 × 106 | 107 |
Affinity chromatography used to purify toxin A in the present study was first described by Krivan et al[2] The methods using bovine thyroglobulin for purification of C. difficile toxin A which depends on the temperature was dependent on binding between toxin A and thyroglobulin. A change of the temperature form 4 to 37 toxin A was releases from the glycoprotein molecule. In addition to toxin A, there were trace amounts of toxin B and a few other proteins at low level. As thyroglobulin is an expensive biological reagent, this method might not be suitable for purification of toxin A in some laboratories.
Sullivan et al[4] (1982) described a procedure for preparing milligram amounts of homogeneous toxin A from culture filtrate. The method consisted of ultrafiltration through an Amicon XM100 membrane filter, anion-exchange chromatography on DEAE-Sepharose CL-6B, and precipitation at pH5.5. We purified toxin A from culture filtrate with the method and found that except for a major protein band, there was a faint band as well on native PAGE. High purified toxin A was obtained by precipitation with 500 g/L (NH4)2SO4 and acid precipitation at pH5.5, followed by DEAE-Toyopearl 650 mol/L column chromatography.
In the studies on the toxins of C. difficile, researchers have reported the cytotoxicity of C. difficile toxin A for human colonic and pancreatic carcinoma cell lines[5]. Although there are a lot of problems to be solved before the toxin being used in clinic, the modified method for purification of toxin A of C. difficile was simple and convenient, it may be even more suitable for purification of toxin A on large scale.
Edited by Zhu LH Proofread by Chen WW and Xu FM
| 1. | Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18 Suppl 4:S265-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Kamiya S, Reed PJ, Borriello SP. Purification and characterisation of Clostridium difficile toxin A by bovine thyroglobulin affinity chromatography and dissociation in denaturing conditions with or without reduction. J Med Microbiol. 1989;30:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Meng XQ, Kamiya S, Yamakawa K, Ogura H, Nakamura S. Purification and characterisation of intracellular toxin A of Clostridium difficile. J Med Microbiol. 1993;38:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Sullivan NM, Pellett S, Wilkins TD. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1032-1040. [PubMed] |
| 5. | Kushnaryov VM, Redlich PN, Sedmak JJ, Lyerly DM, Wilkins TD, Grossberg SE. Cytotoxicity of Clostridium difficile toxin A for human colonic and pancreatic carcinoma cell lines. Cancer Res. 1992;52:5096-5099. [PubMed] |