Copyright
©The Author(s) 2025.
World J Gastroenterol. Jun 7, 2025; 31(21): 106964
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.106964
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.106964
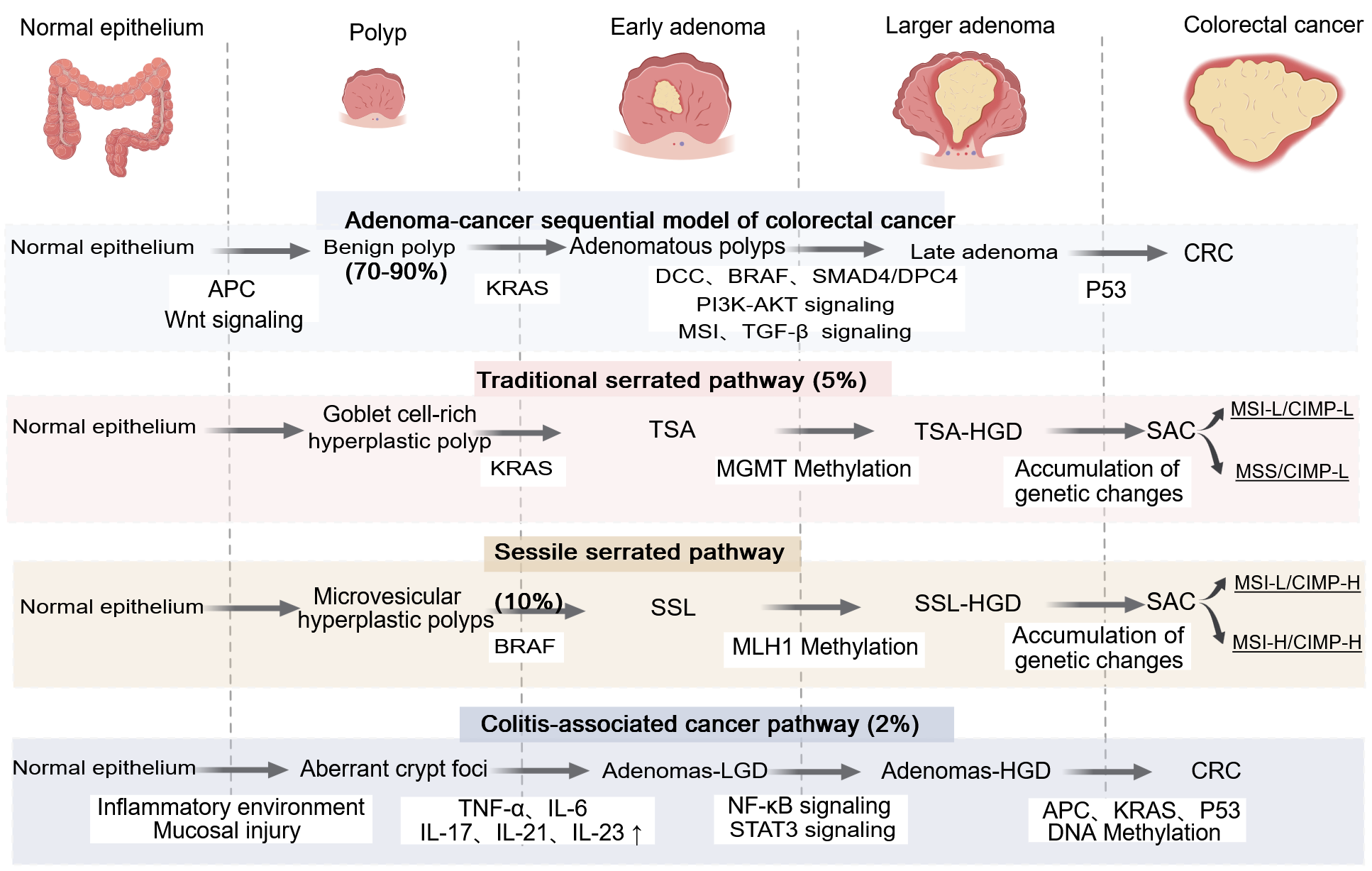
Figure 1 Four distinct pathways of progression from polyps to colorectal cancer, each characterized by specific molecular alterations.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. These pathways include the adenoma-carcinoma-metastasis sequence, the traditional serrated pathway, the sessile serrated pathway, and cancers associated with colitis. DCC: Deleted in colorectal cancer; BRAF: B-raf proto-oncogene; SMAD4: Sma and mad homolog; DPC4: Sma and mad homolog family member 4; MGMT: O-6-methylguanine-DNA methyltransferase; MLH1: MutL homolog 1; AKT: Protein kinase B; PI3K: Phosphatidylinositol 3-kinase; TGF-β: Transforming growth factor beta; MSI: Microsatellite instability; TGF: Transforming growth factor; CRC: Colorectal cancer; CIMP-H: CpG island methylator phenotype-high; CIMP-L: CpG island methylator phenotype-low; MSS: Microsatellite stability; MSI-H: High microsatellite instability; MSI-L: Low microsatellite instability; TSA: Traditional serrated adenomas; TSG: Tumor suppressor genes; TSA-HGD: Traditional serrated adenomas with high-grade dysplasia; SSL: Sessile serrated lesions; SSL-HGD: Sessile serrated lesions with high-grade dysplasia; CAC: Colitis-associated cancer; Adenomas-LGD: Adenomas with low-grade dysplasia; Adenomas-HGD: Adenomas with high-grade dysplasia; SAC: Spindle assembly checkpoint; TNF-α: Tumor necrosis factor-α; IL: Interleukin; NF-Κb: Nuclear factor kappa B; APC: Adenomatous polyposis col; KRAS: Kirsten rat sarcoma viral oncogene homolog; STAT3: Signal transducer and activator of transcription 3.
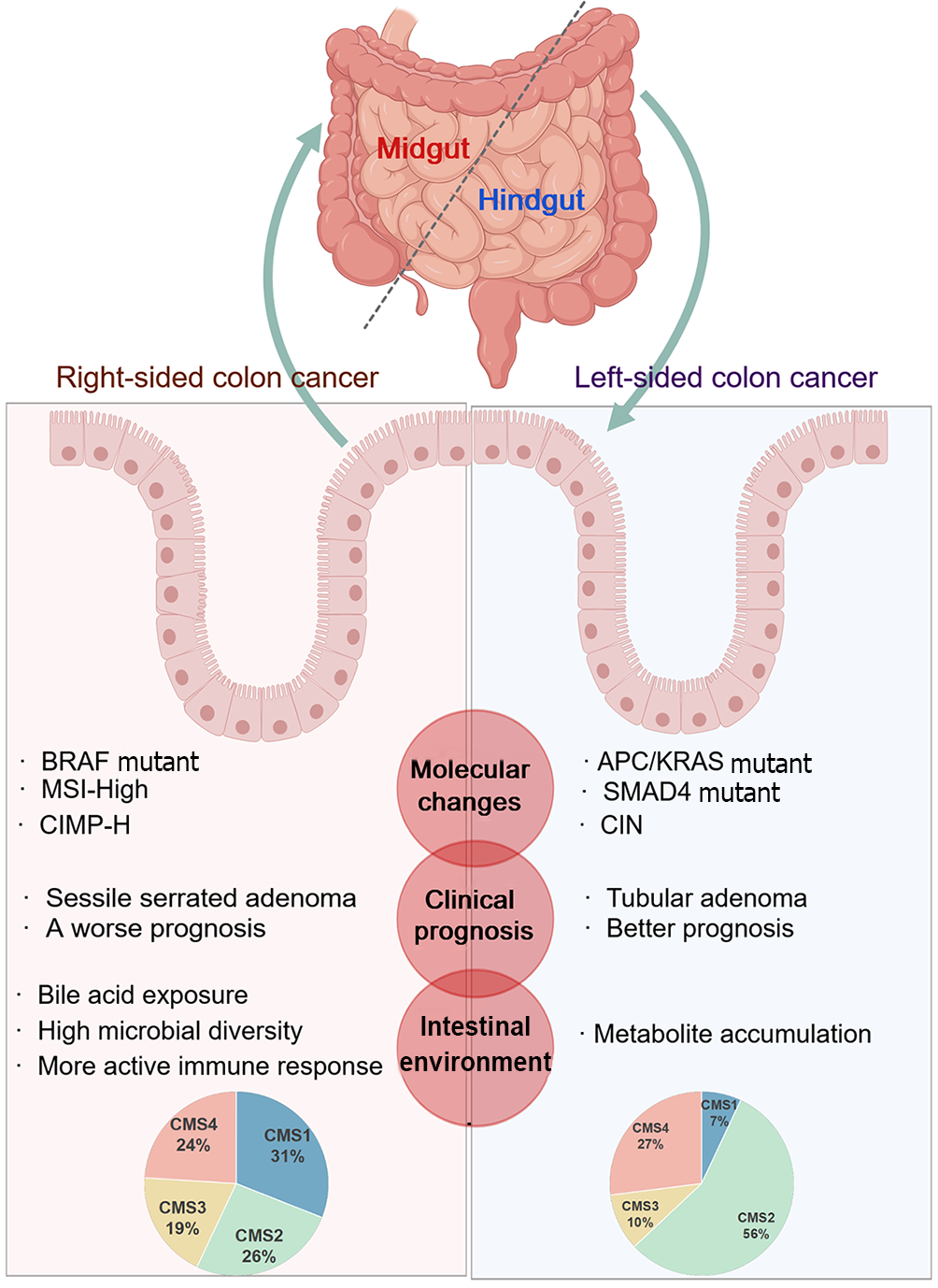
Figure 2 Biological characteristics and molecular distinctions between the left and right - sided colon.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. The figure details the differences in the origin, molecular changes, clinical prognosis, and intestinal environment of the left and right-sided colon cancer. CIN: Chromosomal instability; MSI-H: High Microsatellite instability; CIMP-H: CpG island methylator phenotype-high; CMS: Consensus molecular subtypes; APC: Adenomatous polyposis col; KRAS: Kirsten rat sarcoma viral oncogene homolog; SMAD4: Sma and mad homolog; BRAF: B-Raf proto-oncogene.
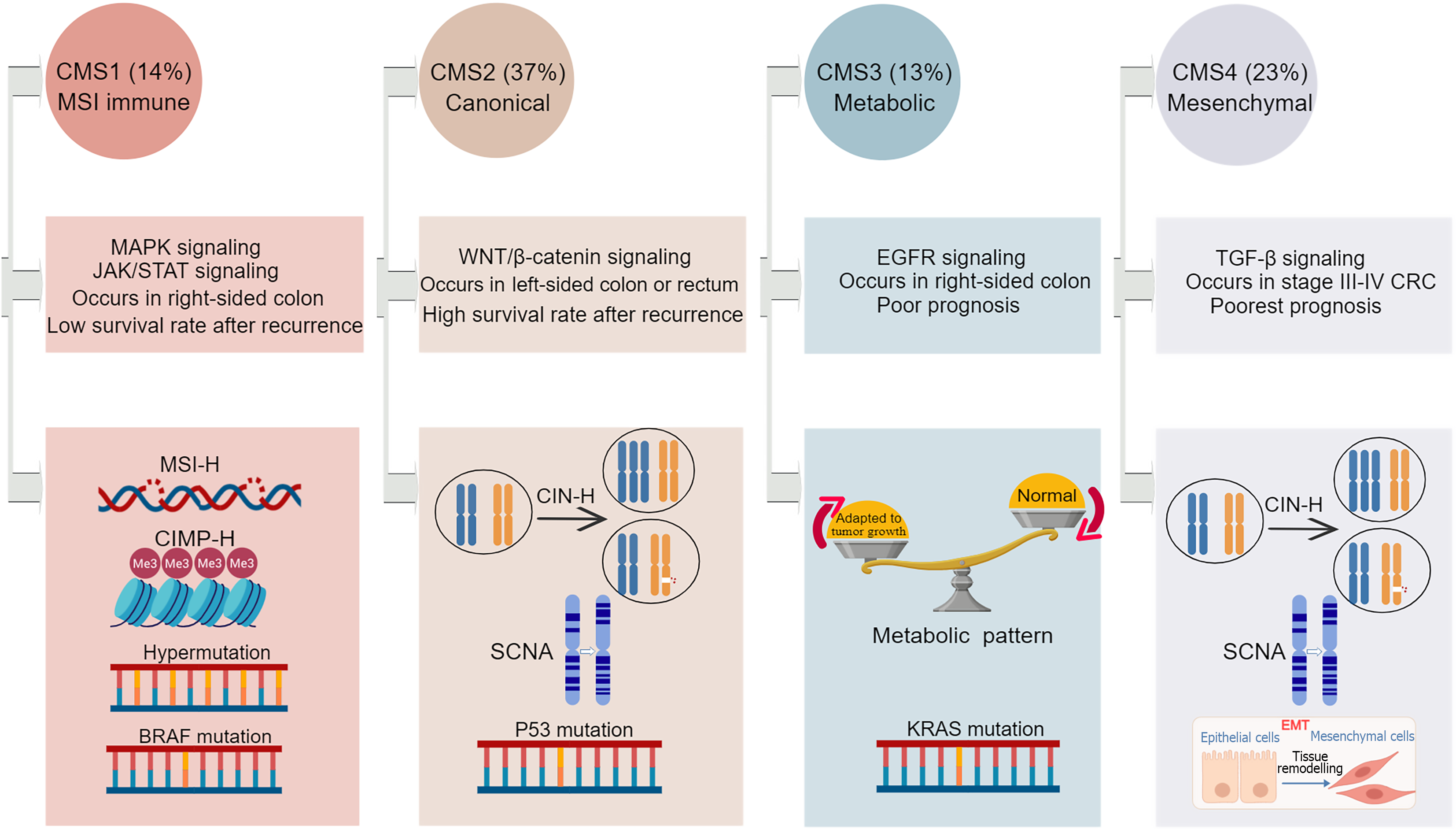
Figure 3 Key features of the four consensus molecular subtypes.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. CRC: Colorectal cancer; CMS: Consensus molecular subtypes; CIN-H: High-level chromosome instability; CIMP-H: CpG island methylator phenotype-high; MSI-H: High microsatellite instability; SCNA: Somatic copy number alteration; EMT: Epithelial-mesenchymal transition; TGF-β: Transforming growth factor beta; MAPK: Mitogen-activated protein kinase; STAT: Signal transducer and activator of transcription; EGFR: Epidermal growth factor receptor; JAK: Janus kinase.
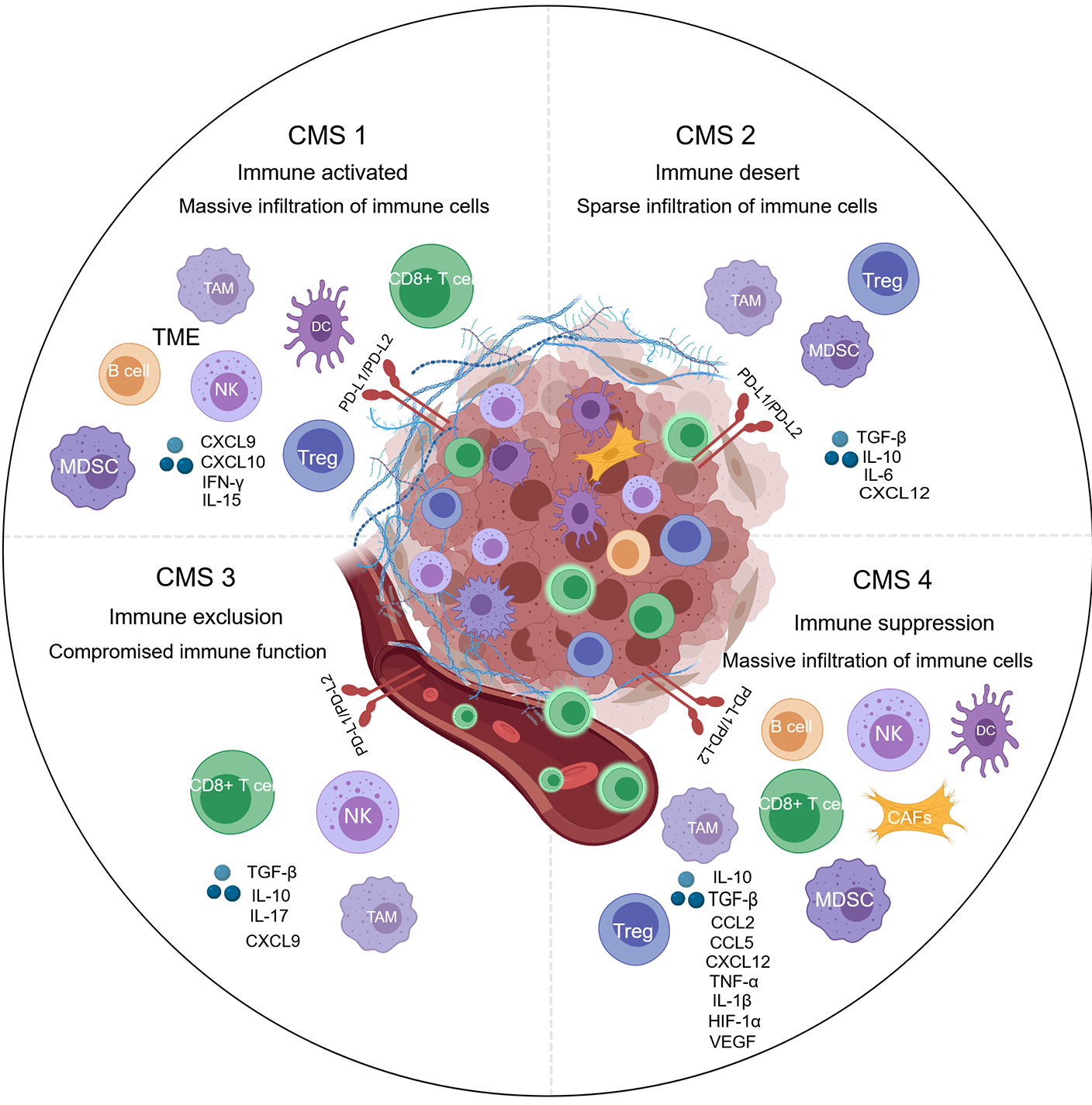
Figure 4 The distinct immune landscapes of the tumor microenvironments across the four consensus molecular subtypes of colorectal cancer.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. Consensus molecular subtypes (CMS) 1 is characterized by an “immune activated” environment with massive immune cells. CMS2 is an “immune desert” with sparse infiltration of immune cells. CMS3 is called “immune exclusion”, in which immune cells compromised are present and located on the periphery of the tumor nest. CMS4 exhibits “immune suppression”, even with an abundance of immune cells. CRC: Colorectal cancer; CMS: Consensus molecular subtypes; DC: Dendritic cells; MDSC: Myeloid-derived suppressor cell; TAM: Tumor-associated macrophages; EMT: Epithelial-mesenchymal transition; PD-L1: Programmed death-ligand 1; PD-L2: Programmed death-ligand 2; IL: Interleukin; TGF-β: Transforming growth factor beta; TME: Tumor microenvironment ; NK: Natural killer; CXCL: C-X-C chemokine ligand; IFN: Interferon; CCL: Chemokine (C-C motif) ligand; HIF-1α: Hypoxia-inducible factor-1α; VEGF: Vascular endothelial growth factor.
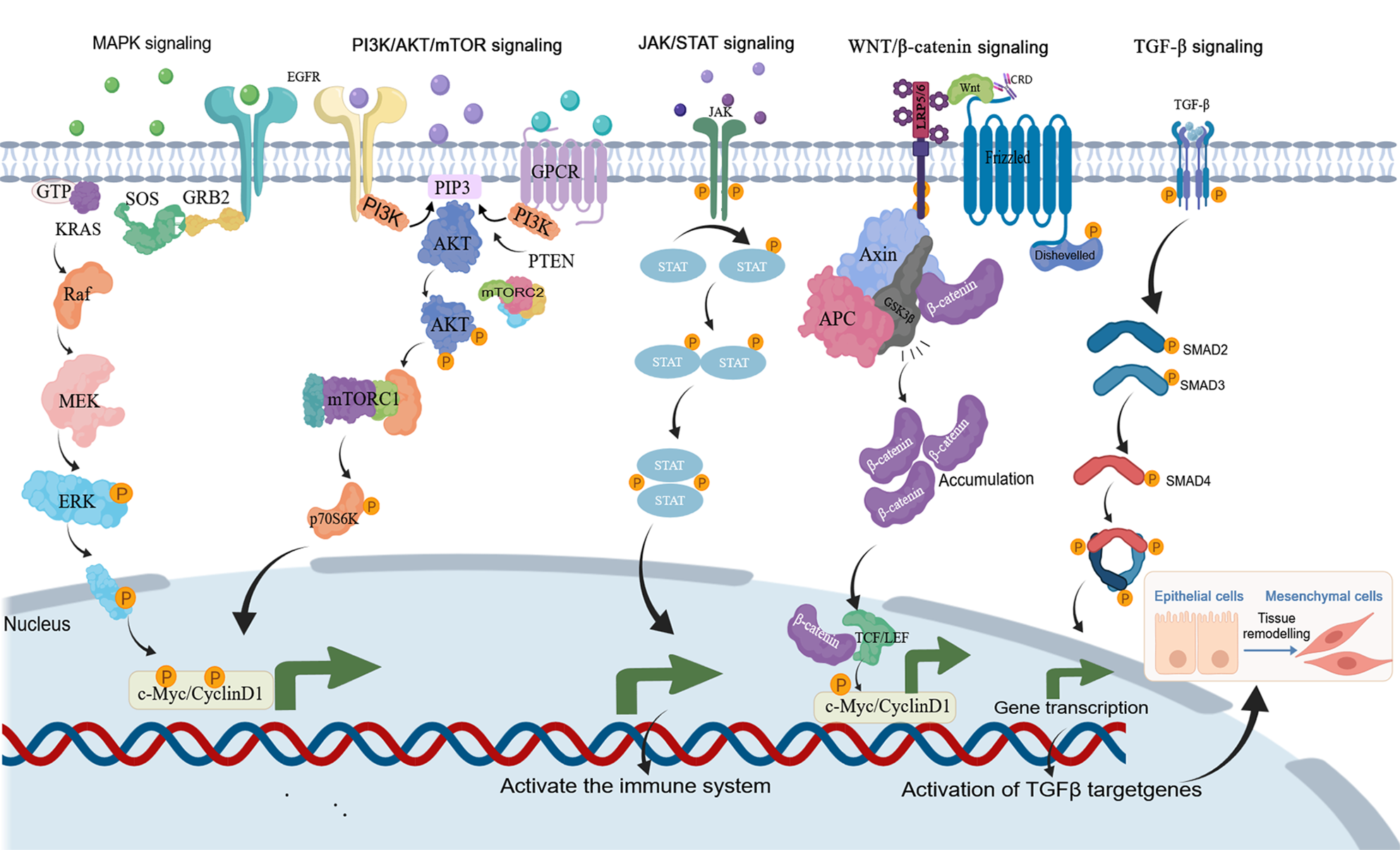
Figure 5 Key signaling pathways in colorectal cancer such as mitogen-activated protein kinase signaling, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling, Janus kinase/signal transducer and activator of transcription signaling, Wnt/β-catenin signaling, transforming growth factor beta signaling.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. MAPK: Mitogen-activated protein kinase; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mechanistic target of rapamycin; JAK: Janus activated kinase; STAT: Signal transducer and activator of transcription; TGF-β: Transforming growth factor beta; SMAD: Sma and mad homolog; GPT: Guanosine Triphosphate; SOS: Son of sevenless; GRB2: Growth factor receptor-bound protein 2; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular signal-regulated kinase; AKT: Protein kinase B; PI3K: Phosphatidylinositol 3-kinase; PIP3: Phosphatidylinositol 3,4,5-trisphosphate; TCF: T-cell factor; LEF: Lymphoid enhancer-binding factor; EMT: Epithelial-mesenchymal transition.
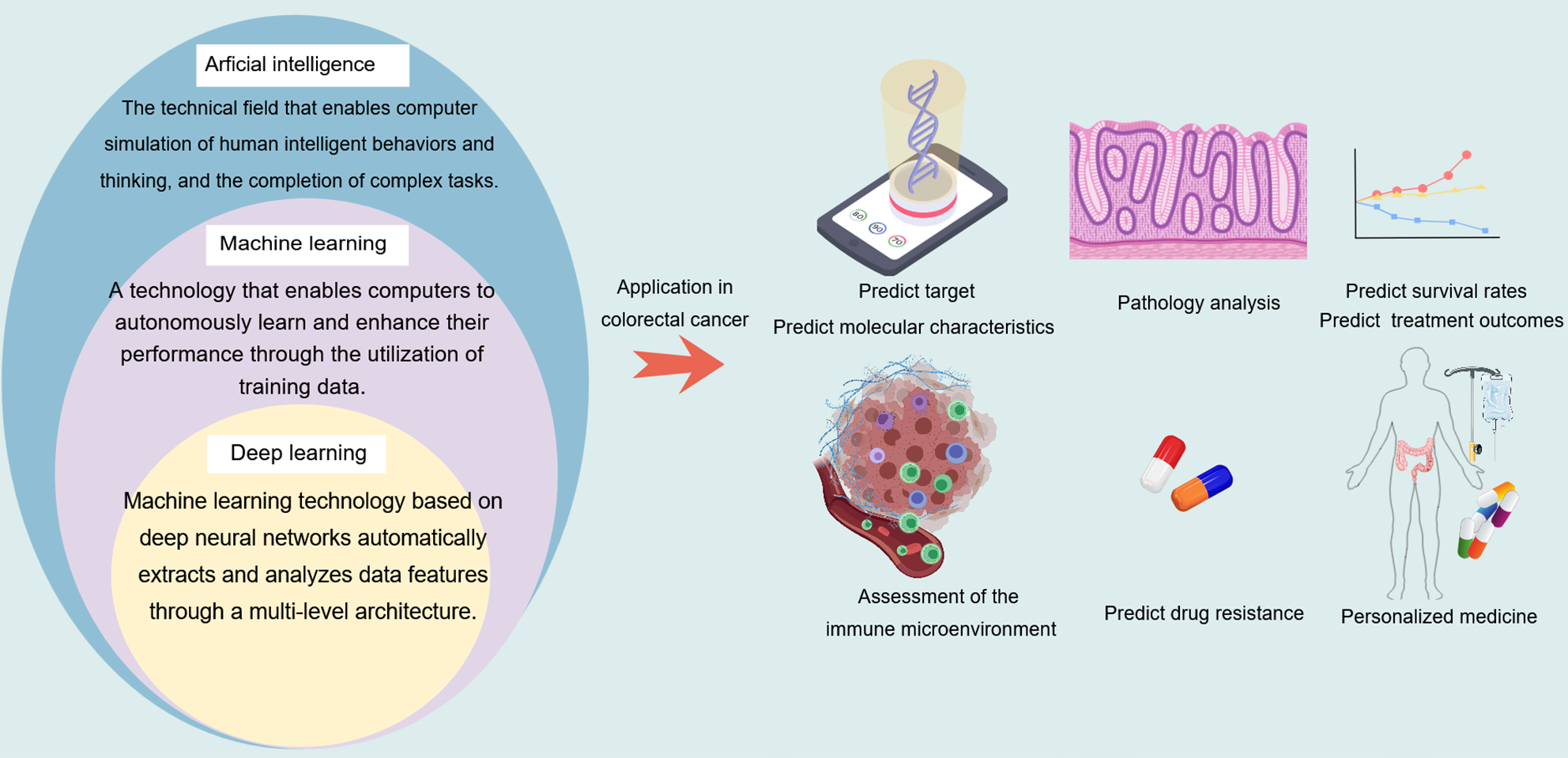
Figure 6 A comparison among artificial intelligence, machine learning, and deep learning, as well as their applications in the molecular diagnosis and treatment of colorectal cancer.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material.
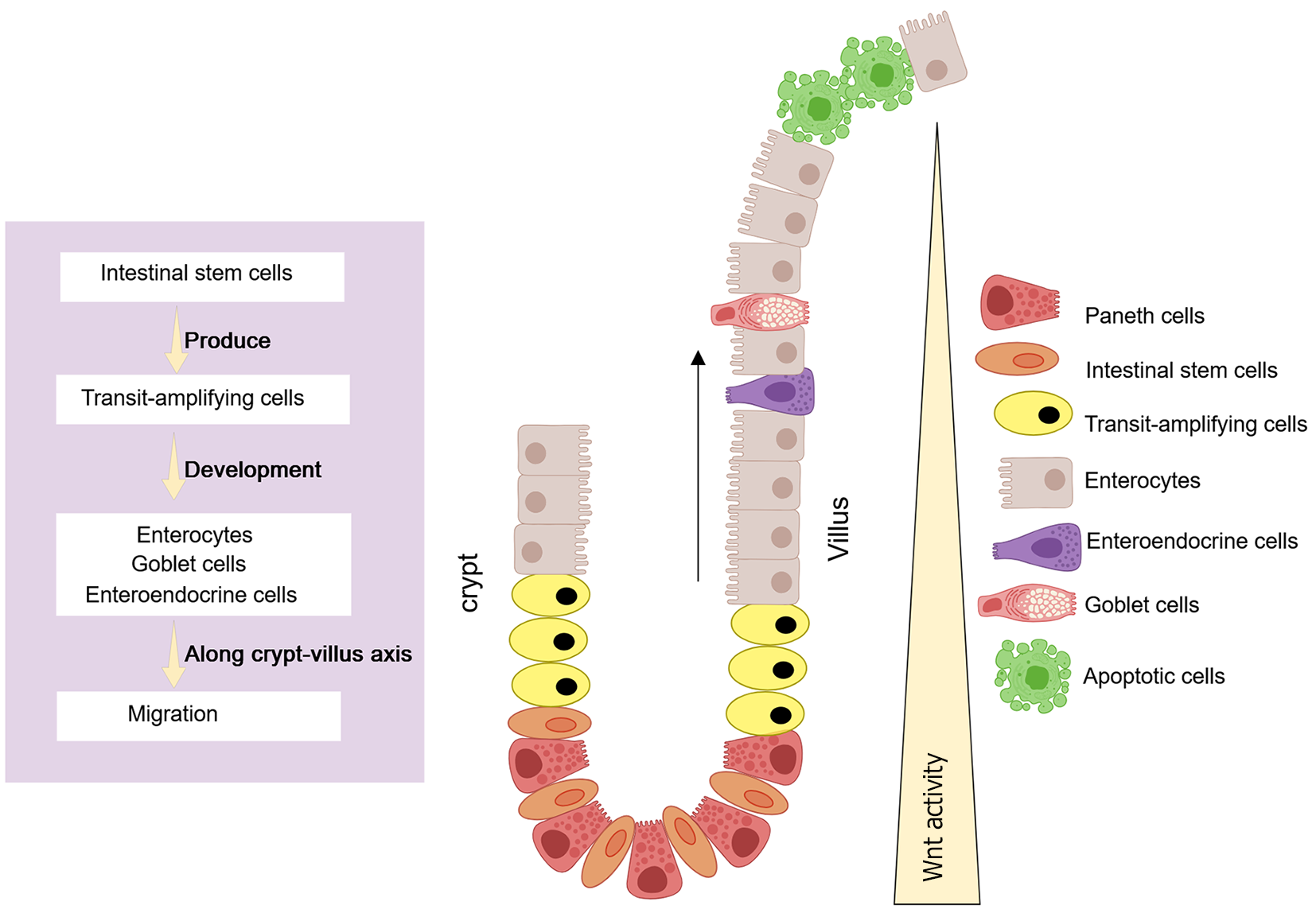
Figure 7 The intestinal crypt is composed of a specialized microenvironment.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. Intestinal stem cells drive crypt renewal and give rise to transamplifying cells, whereas Paneth cells secrete critical niche factors, such as Wnt, which is essential for maintaining the stem cell niche.
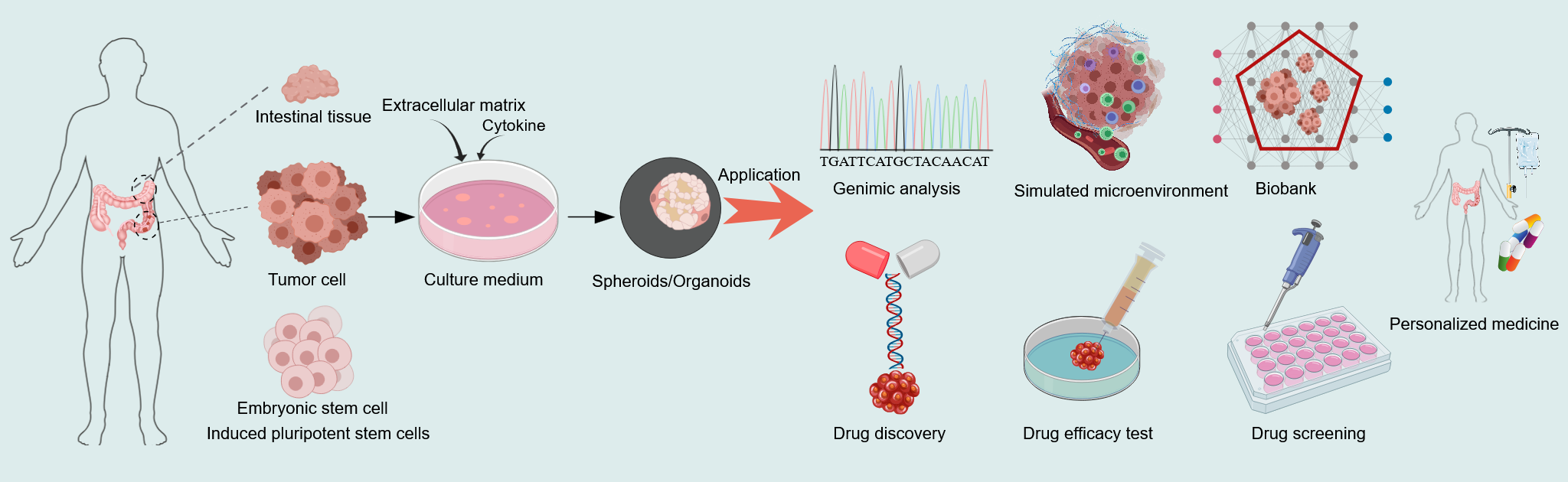
Figure 8 Schematic diagram of the establishment of artificial tissue cultures.
Created with MedPeer (medpeer.cn). Copyright permission has been obtained in the Supplementary material. Tumor tissues from human intestines, stem cells from normal intestinal tissues and embryonic stem cells, as well as induced pluripotent stem cells, are embedded in the basement membrane matrix and maintained in the culture medium containing microenvironment factors crucial for proliferation.
- Citation: Qi GX, Zhao RX, Gao C, Ma ZY, Wang S, Xu J. Recent advances and challenges in colorectal cancer: From molecular research to treatment. World J Gastroenterol 2025; 31(21): 106964
- URL: https://www.wjgnet.com/1007-9327/full/v31/i21/106964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i21.106964