Copyright
©The Author(s) 2015.
World J Gastroenterol. Mar 28, 2015; 21(12): 3499-3508
Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3499
Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3499
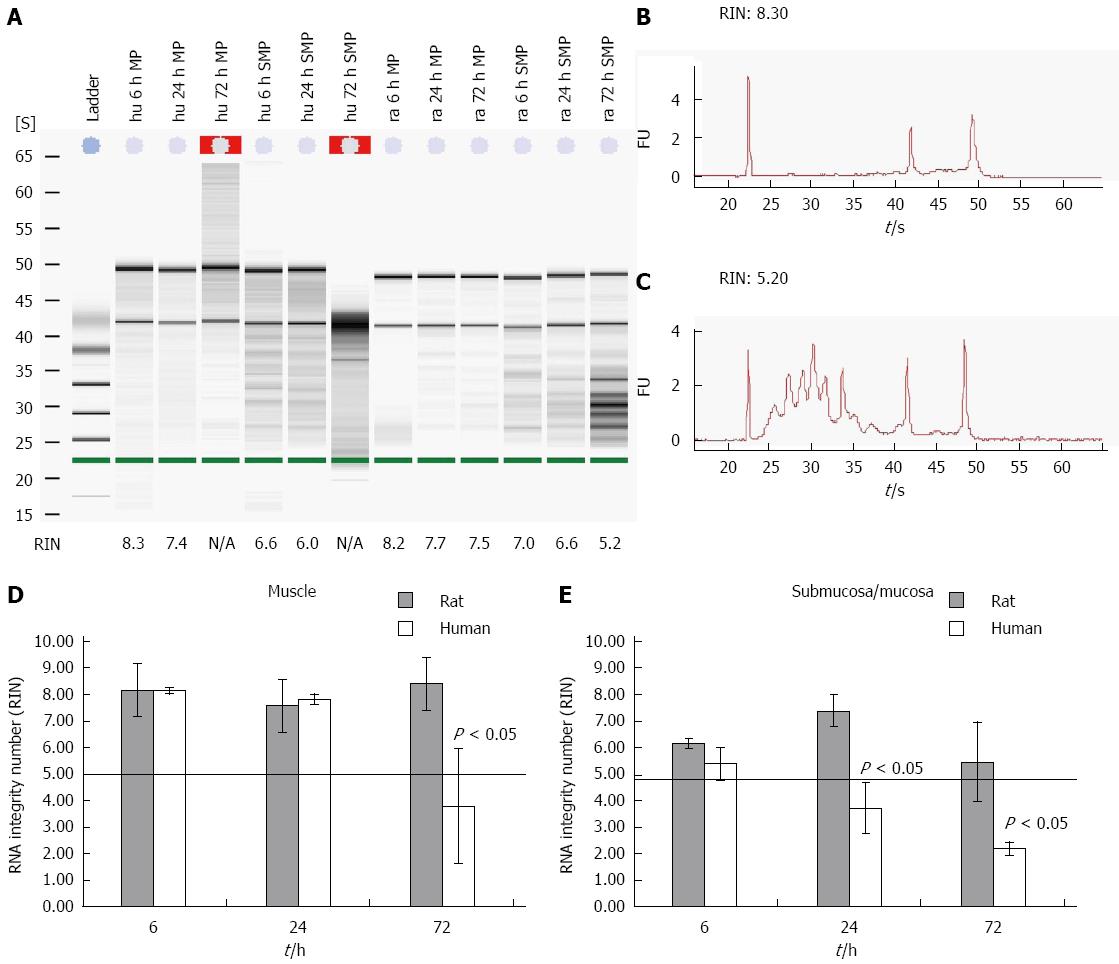
Figure 1 Time course of degradation effect on muscle and submucosal/mucosal human and rat tissue.
Human and rat gut samples were stored in MEM-Hepes on ice and separated in smooth muscle and submucosal/mucosal layer (muscle, mucosa) at different points in time. RNA from the tissues was isolated and degradation (RIN) of the different samples was examined. A: The figure shows a typical gel for human and rodent samples with different integrities. First the ladder, followed by 3 human muscle samples (hu MP), another 3 samples from human mucous layer (hu SMP) all from different points in time. The last 6 samples were comparable samples from rat (ra); B and C: Typical electrophoretic RNA measurements obtained from an Agilent 2100 bioanalyzer displaying different RIN values for intact (8 and 3 respectively; B) and partly degraded RNA (5 and 2 respectively; C); RIN values from both rat and human tissues from different compartment and time points are depicted in (D) and (E). Data are presented as the mean ± SE. n = 10.
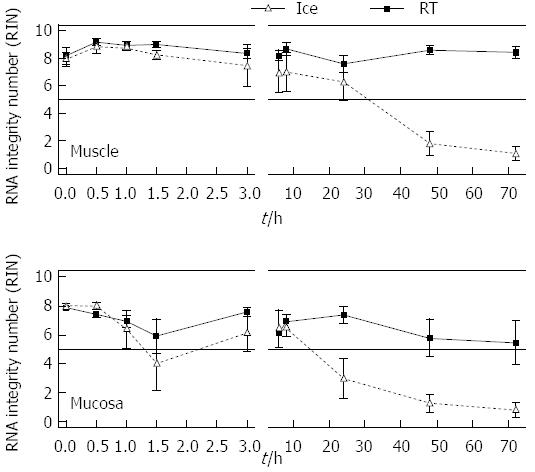
Figure 2 Time and temperature based measurement of RNA integrity of isolated RNA from rat muscle and submucosal/mucosal tissue of the gut.
Pieces of rat gut were stored in MEM-Hepes on ice or at room temperature (RT) and at different points in time samples were separated into smooth muscle and submucosal/mucosal layer (muscle, mucosa). The RNA integrity number was assessed for the individual samples. Data are presented as the mean ± SE. aP < 0.05 and n = 6.
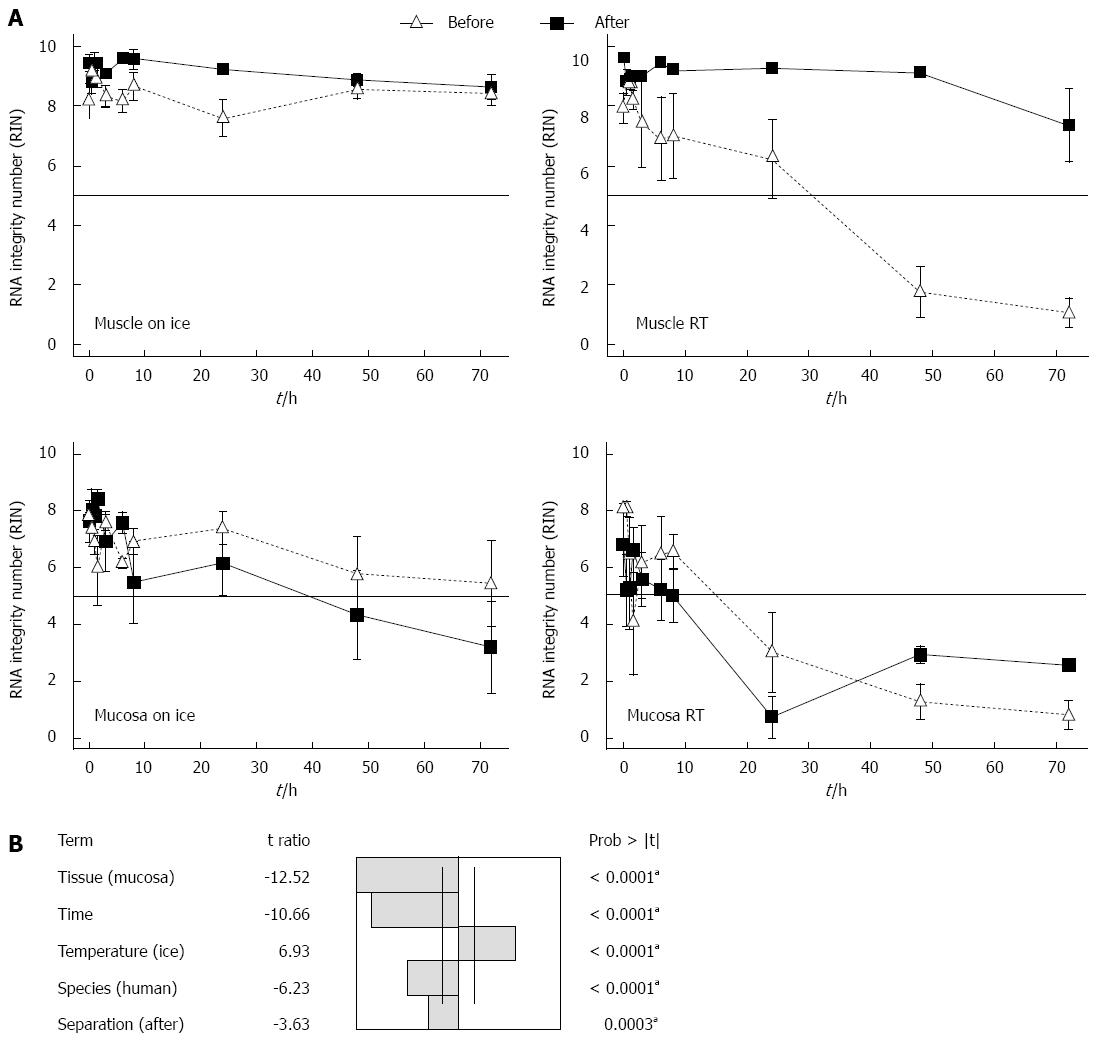
Figure 3 Time course of RNA integrity from isolated RNA from muscle and submucosal/mucosal tissue with an initial separation or with separation after storage.
A: In the experiment “before” the gut from rat was first rinsed with PBS and separated into muscle and submucosal/mucosal layer and then the samples were stored in medium on ice or at room temperature (RT). In the experiment “after” the tissue of the gut was stored in medium on ice or at RT and at different points in time samples were washed with PBS and separated in muscle and submucosal/mucosal layer. The RNA integrity number was measured. Data are presented as the mean ± SE; aP < 0.05 and n = 6; B: A fitting of the factors: temperature, tissue, time, species and tissue separation of the gut in muscle and submucosal/mucosal layer before or after incubation was calculated. To identify the impact factors upon the RIN, an analysis of variance (ANOVA) at aP < 0.05 significant level was used and n = 6. RIN: RNA integrity number.
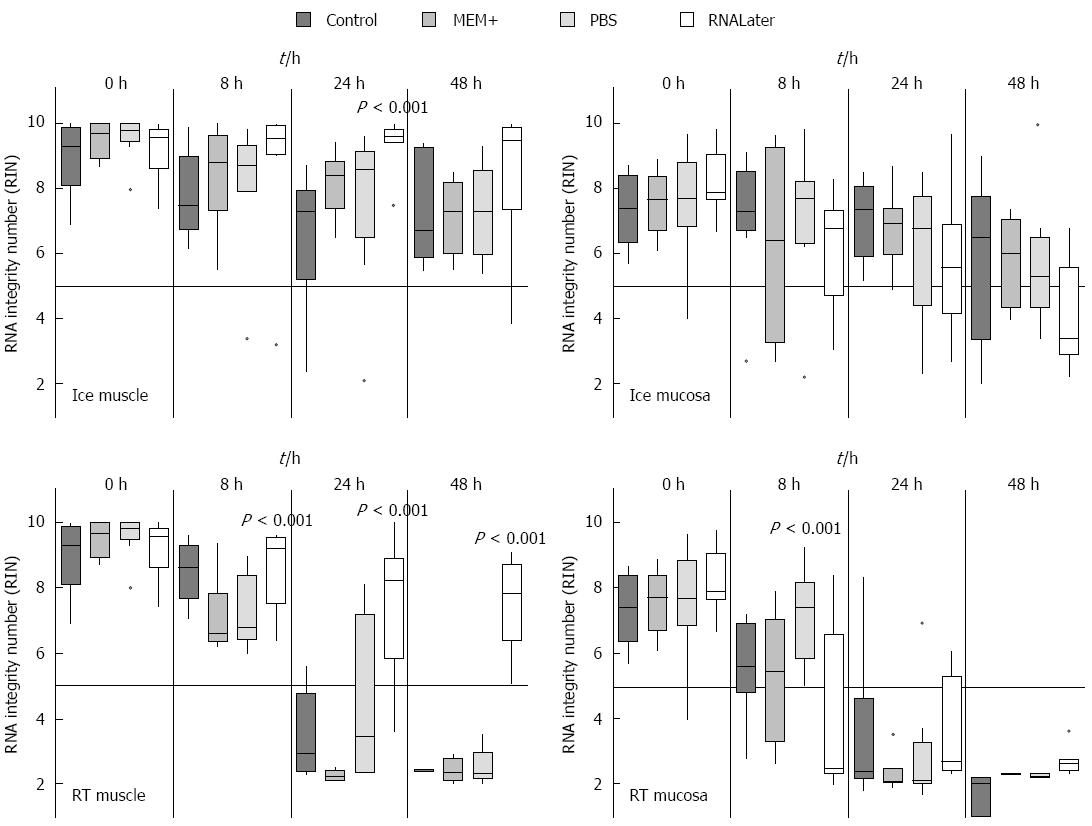
Figure 4 Time course of RNA integrity from isolated RNA from muscle and submucosal/mucosal tissue treated with different methods.
Rat gut was rinsed with different media and stored in medium on ice or at room temperature at different points in time (0 h, 8 h, 24 h and 48 h). Then the samples were separated in smooth muscle (muscle) and submucosal/mucosal layer (mucosa). The RNA Integrity number was measured and data are presented as the mean ± SE and tested for statistical significance using one way ANOVA. The results were considered significant with P < 0.001 and n = 9. RIN: RNA integrity number.
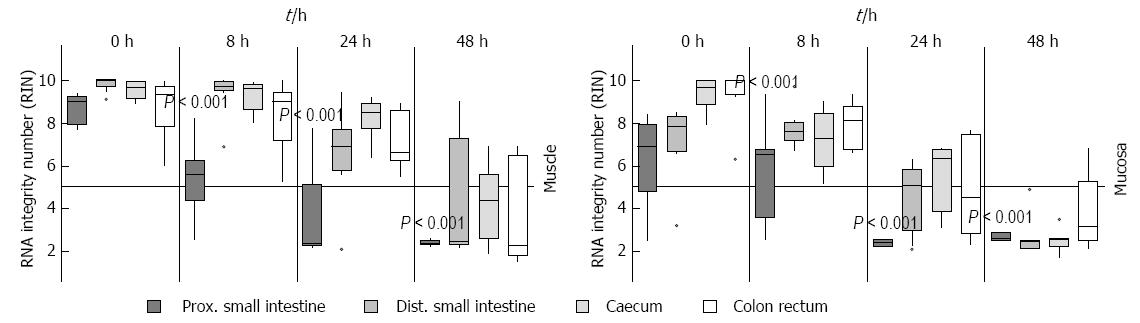
Figure 5 Measurement of RNA Integrity in different parts of the gut.
Rat gut was separated in different parts (proximal small intestine, distal small intestine, caecum and colon with rectum) and then the samples were stored in medium at room temperature and at different points in time (0 h, 8 h, 24 h and 48 h) the tissue was separated in smooth muscle (muscle) and submucosal/mucosal layer (mucosa). The RNA Integrity numbers were calculated and data presented as the mean ± SE and tested for statistical significance using ANOVA. The results were considered significant with P < 0.001 and n = 9.
- Citation: Heumüller-Klug S, Sticht C, Kaiser K, Wink E, Hagl C, Wessel L, Schäfer KH. Degradation of intestinal mRNA: A matter of treatment. World J Gastroenterol 2015; 21(12): 3499-3508
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3499