Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Dec 28, 2014; 20(48): 18284-18295
Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18284
Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18284
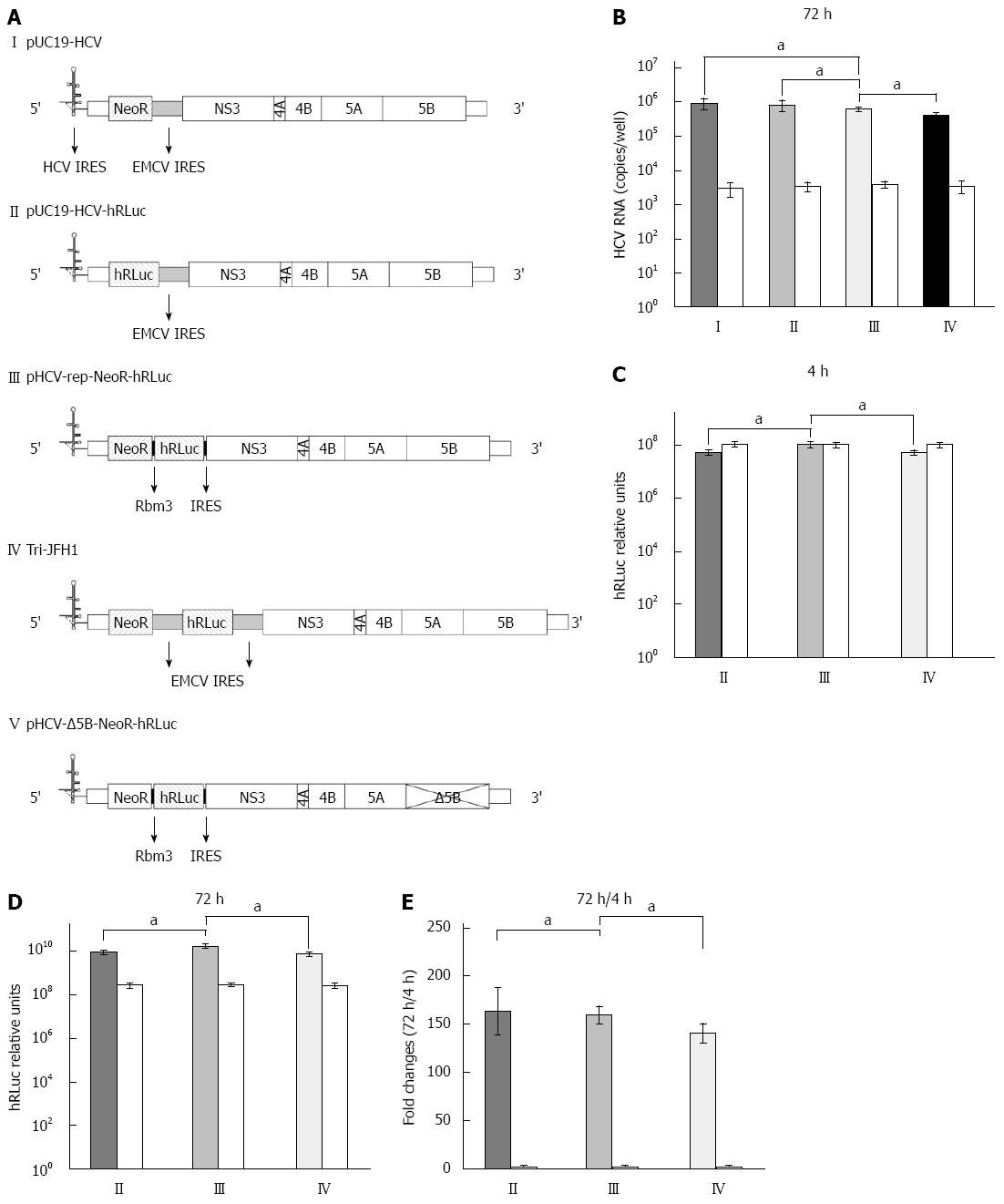
Figure 1 Replication and transgene expression of hepatitis C virus replicons.
A: Structure of hepatitis C virus (HCV) replicons. I: The parental replicon, pUC19-HCV, was a subgenomic replicon in which core, E1, E2, p7 and NS2 were replaced by neomycin phosphotransferase (NeoR) gene and encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES); II: The bicistronic replicon, pUC19-HCV-hRLuc, was derived from pUC19-HCV by replacing NeoR with humanized Renilla luciferase (hRLuc) gene; III: The novel tricistronic replicon, pHCV-rep-NeoR-hRLuc, was derived from pUC19-HCV by replacing EMCV IRES with two sequential Rbm3 IRES and the hRLuc gene for simultaneous expression of NeoR and hRLuc.; IV: The tricistronic replicon with double EMCV IRESes, Tri-JFH1, was previously reported; V: The replication defective vector, pHCV-Δ5B-NeoR- hRLuc, was generated by truncating the active site of NS5B; B: Replication of HCV RNA in Huh-7 cells transiently transfected by replicons. Huh-7 cells were co-transfected with in vitro transcribed replicon RNA (I-IV) and replication-defective RNA from pHCV-Δ5B-NeoR-hRLuc as control (white columns). The replication of tricistronic HCV replicon was comparable to that derived from pUC19-HCV and pUC19-HCV-hRLuc and higher than that derived from Tri-JFH1; C: Transgene expression 4 h after replicon RNA transfection. The transgene of hRLuc was expressed at a significantly higher level in cells transfected with Rbm3 IRES tricistronic replicon than in cells transfected with pUC19-HCV-hRLuc and Tri-JFH1; D: Transgene expression 72 h after replicon RNA transfection; E: Fold changes of hRLuc activity between the time points of 4 and 72 h after transfection. aP < 0.05 between two groups.
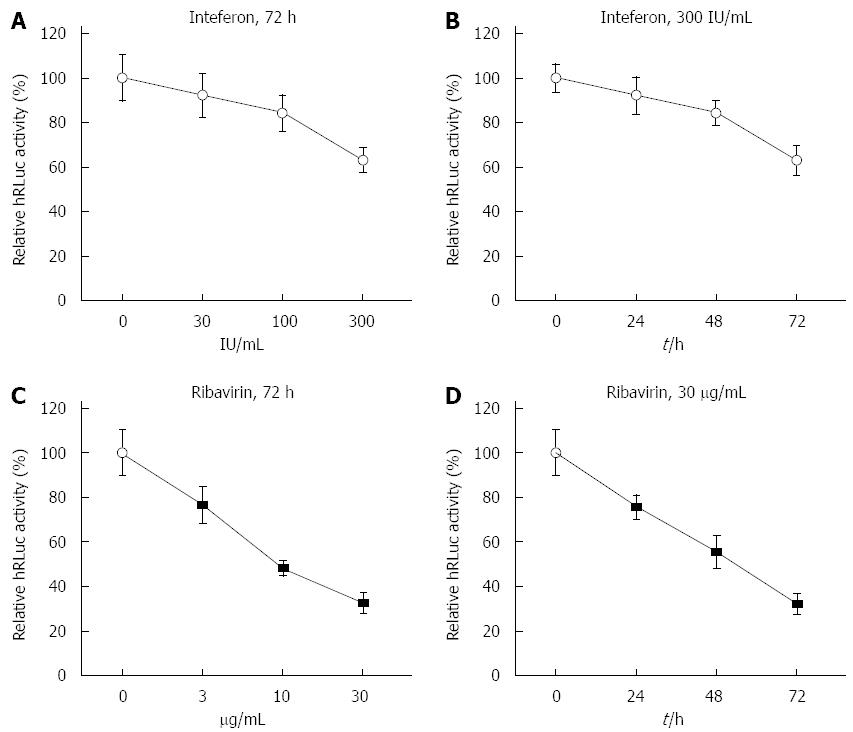
Figure 2 Response of tricistronic replicon to interferon-α2b and ribavirin.
A: Response of tricistronic replicon to increasing doses of interferon (IFN)α2b when transiently transfected. The luciferase activity was substantially reduced in Huh7 cells transiently transfected with tricistronic replicon in a dose-dependent manner. The humanized Renilla luciferase (hRLuc) activity was measured by relative light units and normalized to that of untreated cells; B: Response of tricistronic replicon to different durations of IFNα2b treatment when transiently transfected. The luciferase activity was substantially reduced in Huh7 cells transiently transfected with tricistronic replicon in a time-dependent manner; C: Response of tricistronic replicon to increasing doses of ribavirin when transiently transfected. The luciferase activity was markedly reduced in Huh7 cells transiently transfected with tricistronic replicon in a dose-dependent manner; D: Response of tricistronic replicon to different durations of ribavirin treatment when transiently transfected. The luciferase activity was markedly reduced in Huh7 cells transiently transfected with tricistronic replicon in a time-dependent manner.
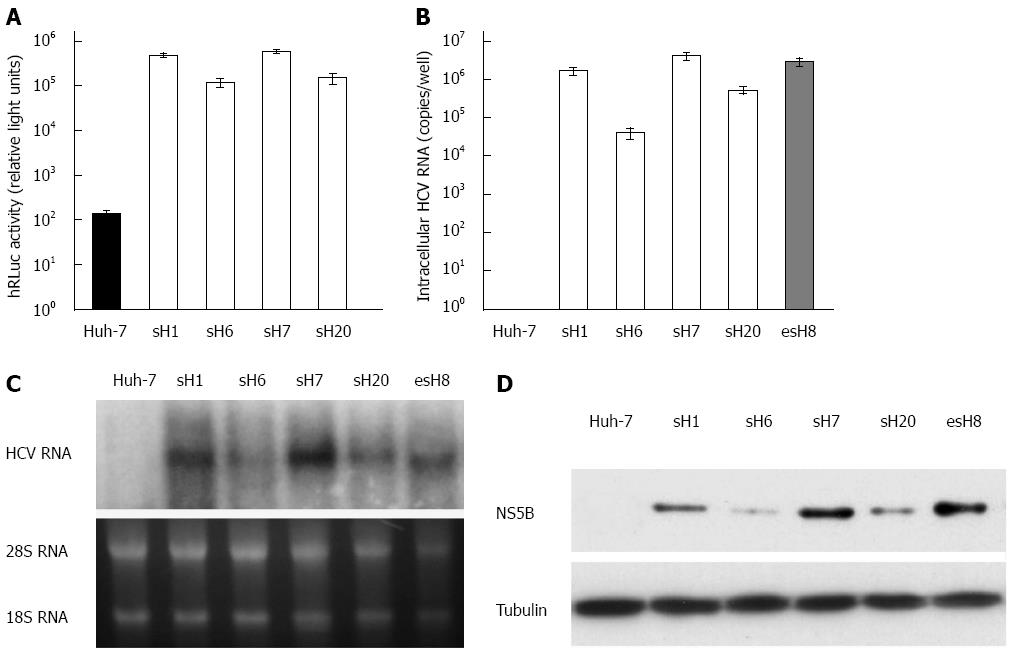
Figure 3 Characterization of the tricistronic hepatitis C virus replicon.
A: Transgene expression in cells stably transfected with tricistronic hepatitis C virus (HCV) replicon. Fifty stable cell clones were screened with G418 from Huh-7 cells transfected with the purified tricistronic HCV replicon RNA. The humanized Renilla luciferase (hRLuc) activity in sH7 cells was the highest among all of the surviving cell clones; B: HCV RNA in cells stably transfected with tricistronic HCV replicon. HCV RNA in four strains with the highest level of hRLuc activity was quantified by real-time PCR. Huh-7 cells were also transfected with pUC19-HCV and screened to form stable clones as a reference, namely esH8 after the encephalomyocarditis virus internal ribosome entry site (IRES). HCV RNA was amplified from all the tricistronic replicons strains, especially in sH7 clones, in which HCV RNA copy number was higher than any other strains and parental Huh-7 cells. HCV RNA levels in sH7 cells was even more than that in esH8, the pUC19-HCV stably transfected cells; C: Replication of HCV replicons in cells stably transfected with tricistronic HCV replicon. RNA replication was seen in all the tricistronic replicons strains, especially in sH7 clones, in which HCV RNA level was significantly higher than that of parental Huh-7 cells and even higher than that of esH8. The esH8 cells harboring pUC19-HCV replicon with NeoR gene directed by HCV IRES were also used as a reference; D: HCV non-structural gene expression in cells stably transfected with tricistronic HCV replicon. NS5B was probed in four strains with the highest level of hRLuc activity and esH8 cells by Western blot. HCV NS5B protein was found in four tricistronic replicon clones, especially in sH7 clones with the highest NS5B level. NS5B in sH7 cells was comparable to that in esH8 cells.
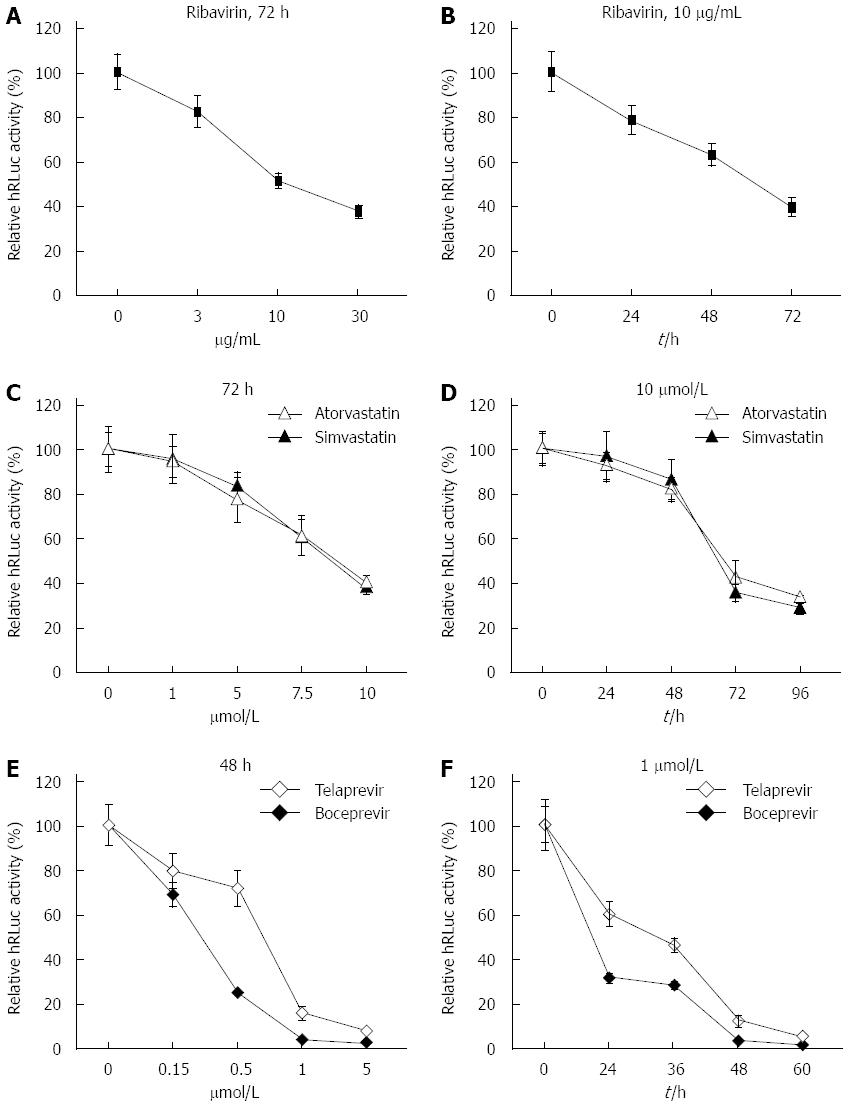
Figure 4 Response of sH7 cells to antivirals.
sH7 cells stably transfected with tricistronic hepatitis C virus (HCV) replicon were treated with ribavirin (A and B), HMG CoA reductase inhibitors (simvastatin and atorvastatin; C and D), and NS3/4A serine protease inhibitors (telaprevir and boceprevir; E and F). All three types of antivirals suppressed replication of tricistronic HCV replicons in sH7 cells in a dose- and time-dependent manner. The relative light units were normalized to those in untreated sH7 wells as a control.
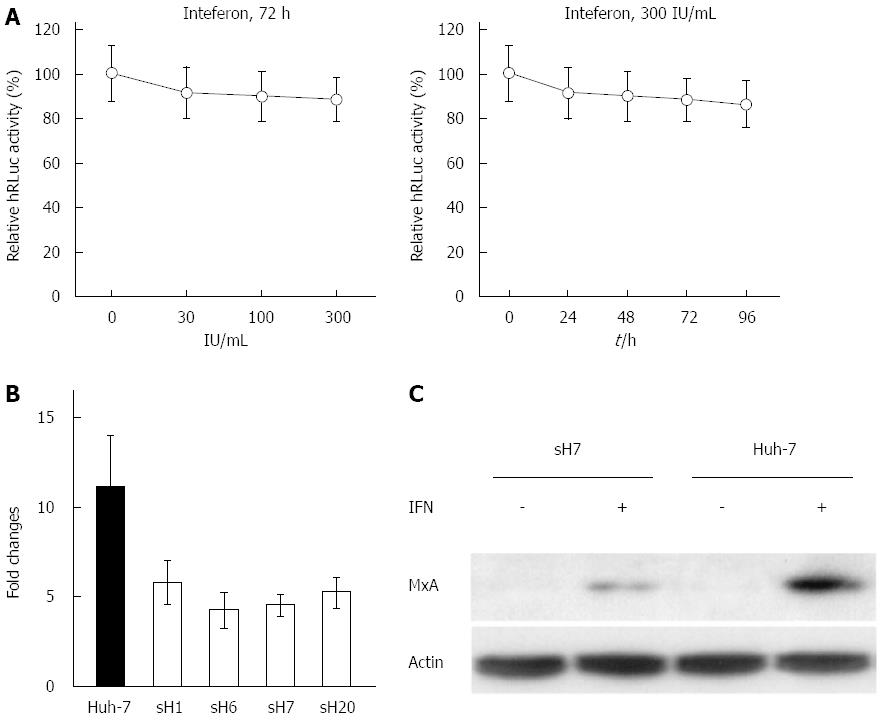
Figure 5 Resistance of hepatitis C virus replicon to interferon-α2b in sH7 cells.
A: The replication of hepatitis C virus (HCV) replicon was not blocked by interferon (IFN)α2b. The sH7 cells, in which HCV replicon was stably replicated along with humanized Renilla luciferase (hRLuc) expression, were treated with increasing doses of IFNα2b for different durations. The replication of HCV replicon was not blocked by IFNα2b up to 300 IU/mL for 96 h. The relative light units were normalized to those in untreated sH7 wells as a control; B: Real-time PCR of MxA mRNA in Huh-7 cells and HCV replicon cell lines treated with IFNα2b. The fold change of MxA mRNA in Huh-7 cells after IFN treatment was much higher than fold changes in HCV replicon stably transfected cell lines after IFN treatment; C: MxA protein in sH7 and Huh-7 cells treated with IFNα2b. MxA protein level was elevated by IFNα2b in both Huh-7 and sH7 cells, whereas no MxA was detected in these two types of cells without IFN stimulation. Additionally, the level of MxA protein was greater in parental Huh-7 cells than in sH7 cells in which HCV replicon was stably transfected.
- Citation: Cheng X, Gao XC, Wang JP, Yang XY, Wang Y, Li BS, Kang FB, Li HJ, Nan YM, Sun DX. Tricistronic hepatitis C virus subgenomic replicon expressing double transgenes. World J Gastroenterol 2014; 20(48): 18284-18295
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18284.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18284