Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. May 14, 2012; 18(18): 2147-2160
Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2147
Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2147
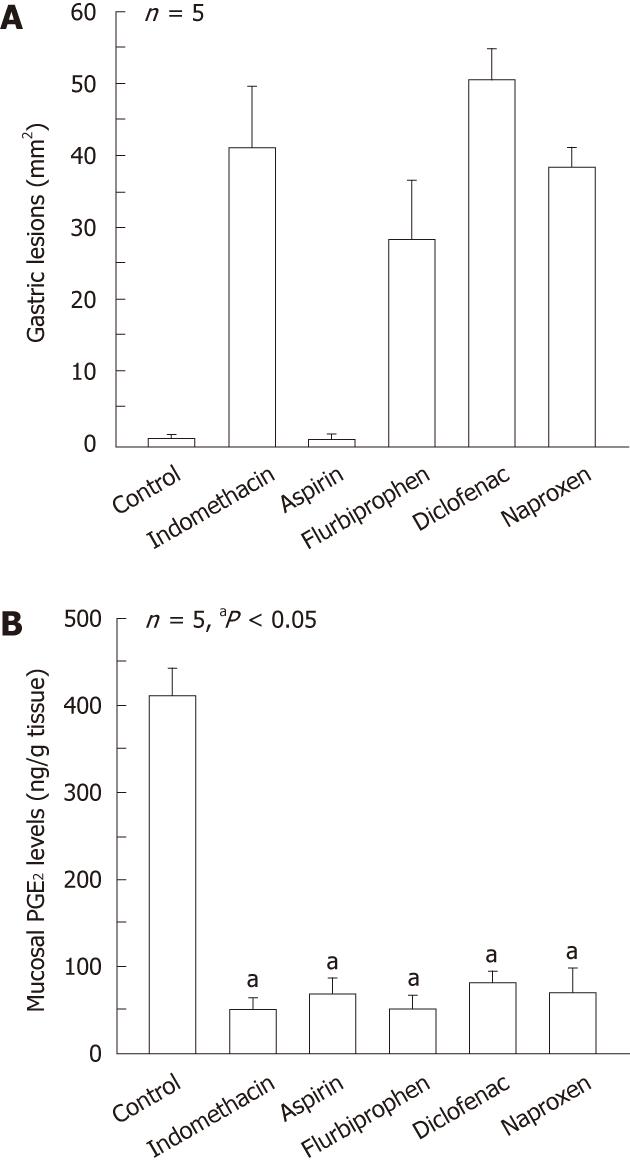
Figure 1 Gastric ulcerogenic responses (A) and changes in mucosal prostaglandin E2 content (B) induced by various non-steroidal anti-inflammatory drugs in rat stomach.
The animals were given indomethacin (30 mg/kg), aspirin (200 mg/kg), naproxen (40 mg/kg), flurbiprofen (20 mg/kg) and diclofenac (40 mg/kg) s.c., and killed 4 h later. Data are presented as the mean ± SE in 5 rats. aP < 0.05 vs control (data from ref. 18 after modification).
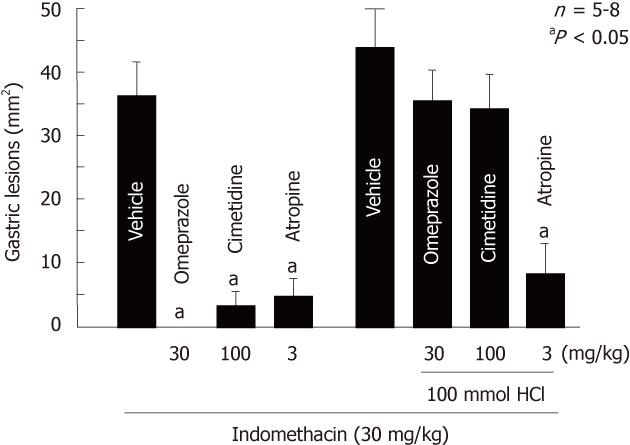
Figure 2 Effects of atropine, cimetidine and omeprazole on gastric lesions produced by indomethacin in rats.
The animals were given indomethacin (30 mg/kg, s.c.) and killed 4 h later. Omeprazole (30 mg/kg), cimetidine (100 mg/kg) and atropine (3 mg/kg, s.c.) were given 1 h before indomethacin. In some cases, the animals were given 1 mL of 100 mmol HCl p.o. immediately after the administration of indomethacin. Data are presented as the mean ± SE for 5-8 rats. aP < 0.05 vs vehicle (data from refs. 6, 26 and 27 after modification).
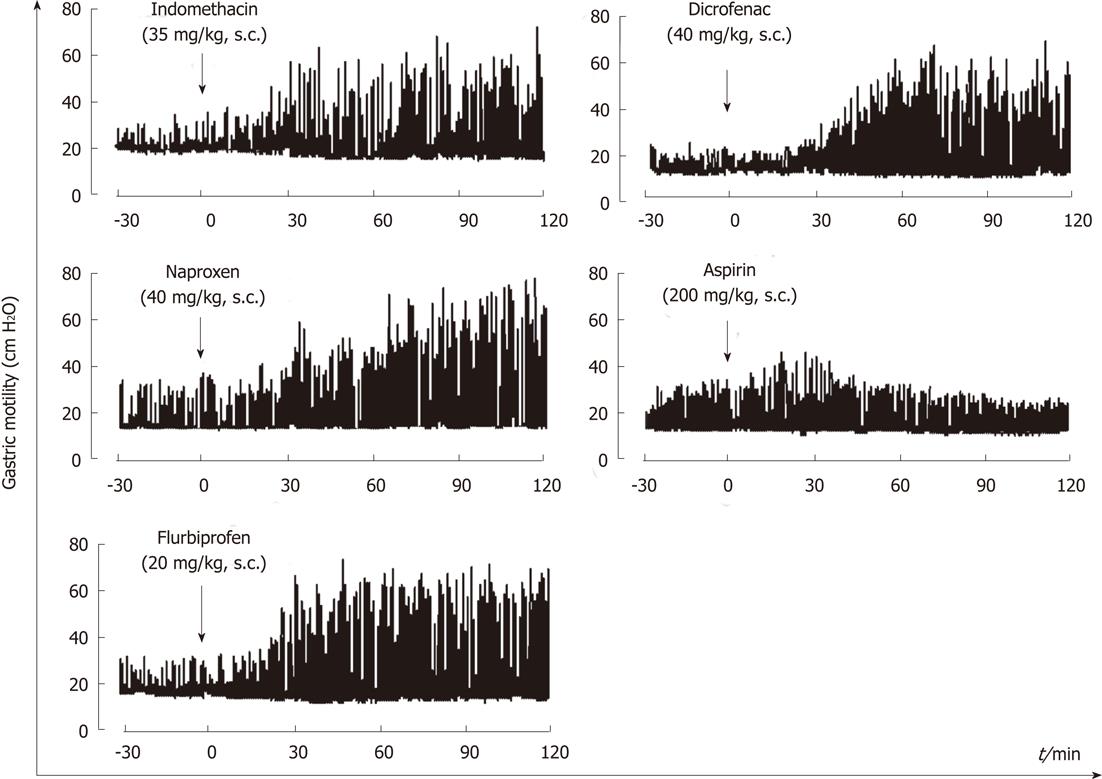
Figure 3 Representative recordings showing the effects of various non-steroidal anti-inflammatory drug on gastric motility in rats.
Indomethacin (35 g/kg), aspirin (200 mg/kg), naproxen (40 mg/kg), flurbiprofen (20 mg/kg) or diclofenac (40 mg/kg) was given s.c. after basal motility had stabilized (data from ref.18 after modification).
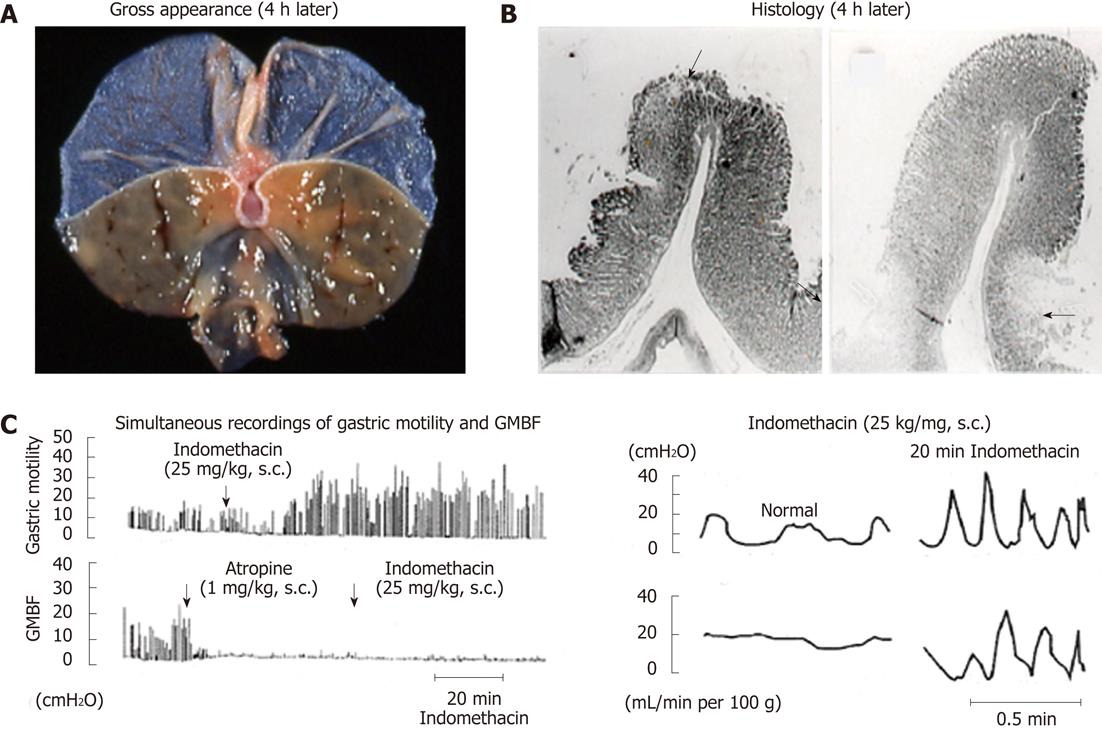
Figure 4 Macro- and microscopical observations of gastric lesions induced by indomethacin in rats (A and B) and simultaneous recordings of gastric motility and mucosal blood flow in the rat before and after administration of indomethacin (C).
A, B: The animals were given indomethacin (25 mg/kg, s.c.), and the stomachs were excised 4 h later. Note that the lesions were located, in most cases, on the upper part of the mucosal folds (arrow) and in some cases at the base of the folds (arrows); C: Indomethacin (25 mg/kg, s.c.) was given, while atropine (1 mg/kg, s.c.) was given 1 h after indomethacin treatment. Note that during hypermotility states the mucosal blood flow repeated a decrease and an increase, respectively, corresponding to contraction and relaxation of the stomach wall (data from refs. 8 and 33 after modification). GMBF: Gastric mucosal blood flow.
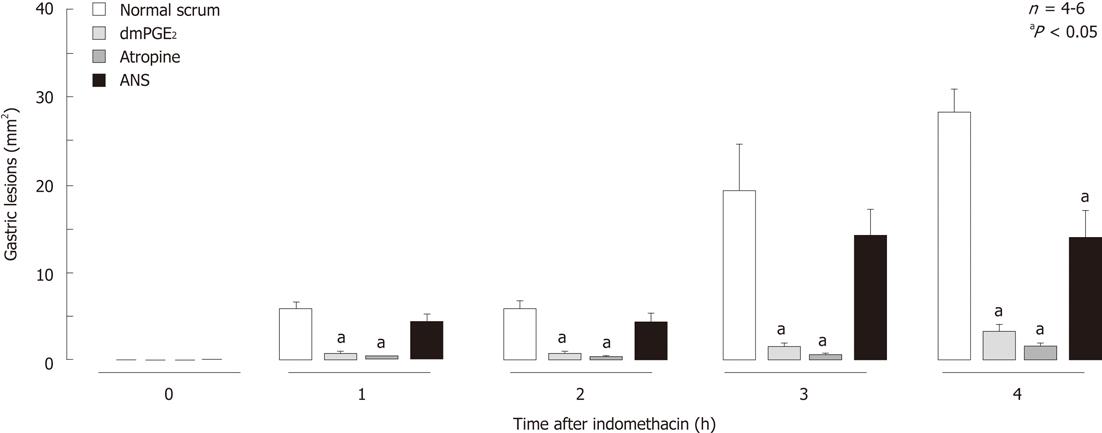
Figure 5 Time-course of changes in gastric lesions following administration of indomethacin (30 mg/kg, s.
c.) in rats, with or without pretreatment. Atropine (1 mg/kg) was given s.c. 30 min before indomethacin, while dmPGE2 (10 μg/kg) or anti-neutrophil antiserum (ANS, 0.2 mL/rat) was given i.v. 10 min and 1 h, respectively, before indomethacin. Data are presented as the mean ± SE in 4-6 rats. aP < 0.05 vs control group given normal serum (data from ref. 28 after modification).
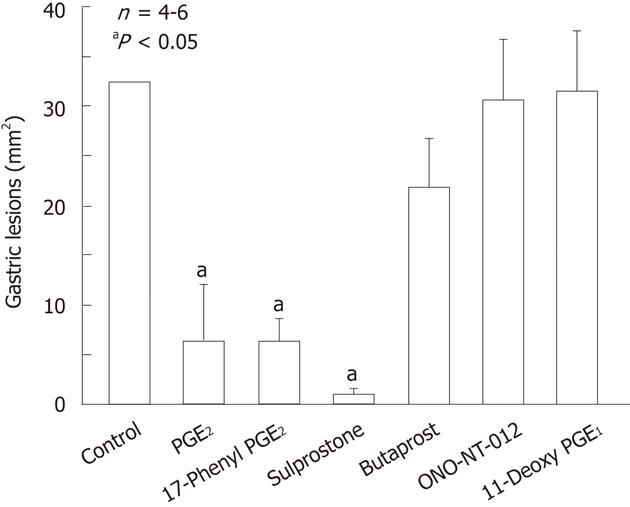
Figure 6 Effects of various prostaglandin E agonists on gastric lesions generated by indomethacin in rats.
The animals were given indomethacin (30 mg/kg) s.c. and killed 4 h later. Prostaglandin E2 (PGE2, 0.3 mg/kg), 17-phenyl PGE2 (0.3 mg/kg; EP1 agonist), sulprostone (0.3 mg/kg; EP1/EP3 agonist), butaprost (10 mg/kg; EP2 agonist), ONO-NT-012 (10 mg/kg; EP3 agonist) and 11-deoxy PGE1 (3 mg/kg; EP3/EP4 agonist) were given i.v. 10 min before indomethacin. Data are presented as the mean ± SE in 4-6 rats. aP < 0.05 vs control (data from ref. 39 after modification).
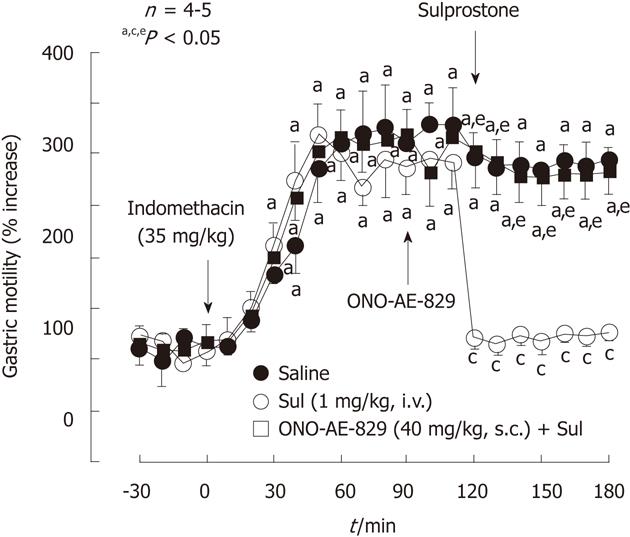
Figure 7 Effect of sulprostone on the increased gastric motility caused by indomethacin in rats.
Indomethacin (35 mg/kg) was given s.c.. Sulprostone (Sul, 1 mg/kg) was given i.v. as a single injection 2 h after indomethacin, while ONO-AE-829 (40 mg/kg) was given s.c. 30 min before the administration of sulprostone. Data are presented as the mean ± SE of values determined every 10 min in 4-5 rats. Significant difference at P < 0.05; afrom basal values in the corresponding group; cfrom saline group; efrom indomethacin plus sulprostone (data from ref. 39 after modification).
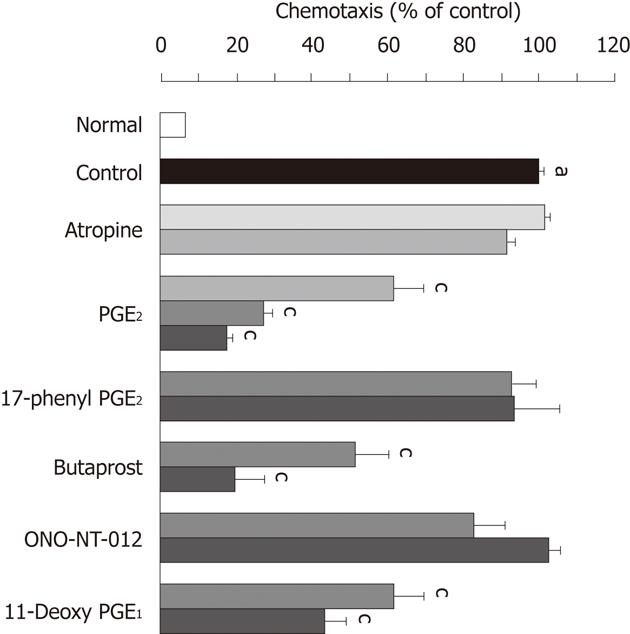
Figure 8 Effects of atropine and various prostaglandin E agonists on the neutrophil chemotaxis stimulated by formyl-methionyl-leucyl-phenylalanine.
Neutrophils were pretreated for 45 min with atropine and various prostaglandin E agonists such as PGE2, 17-phenyl PGE2, butaprost, ONO-NT-012 and 11-deoxy PGE1 at the indicated concentrations, and then the cells were stimulated by incubation with formyl-methionyl-leucyl-phenylalanine (fMLP, 1x10-7 mol) for another 45 min. Data are expressed as a percentage of the stimulated values (control) observed in the presence of fMLP and represent the mean ± SE from 4 experiments. Significant difference at P < 0.05; afrom normal; cfrom control (data from ref. 39 after modification).
Figure 9 Gastric ulcerogenic responses to various cyclooxygenase inhibitors and their effects on prostaglandin E2 content in rat stomachs.
A: Gastric ulcerogenic responses induced by various cyclooxygenase (COX) inhibitors in rat stomach. The animals were given indomethacin (nonselective COX inhibitor; 10 and 30 mg/kg), SC-560 (selective COX-1 inhibitor; 10 and 30 mg/kg), or rofecoxib (selective COX-2 inhibitor; 10 and 30 mg/kg) p.o. and killed 8 h later; B: Effects of various COX inhibitors on gastric mucosal prostaglandin E2 (PGE2) content in rats. The animals were given indomethacin (10 mg/kg), SC-560 (10 mg/kg), or rofecoxib (10 mg/kg) p.o. and killed 2 h later. Data are presented as the mean ± SE in 5-6 rats. aP < 0.05 vs control (data from refs. 18 and 19 after modification).
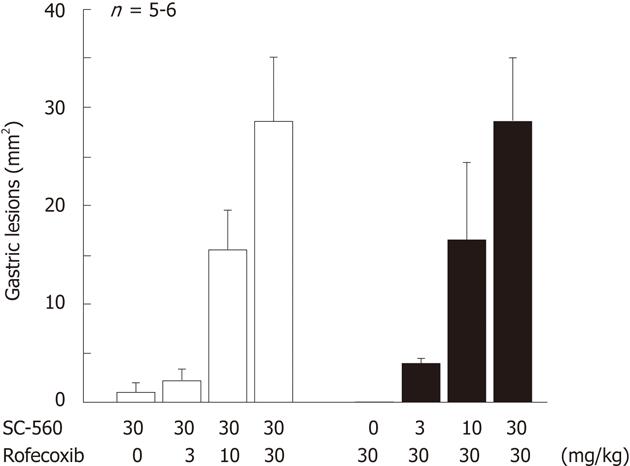
Figure 10 Gastric ulcerogenic response induced by combined administration of SC-560 and rofecoxib in rats.
The animals were administered SC-560 (3-30 mg/kg) and rofecoxib (3-30 mg/kg) p.o. either alone or in combination, and killed 8 h later. Data are presented as the means ± SE in 5-6 rats (data from ref. 18 after modification).
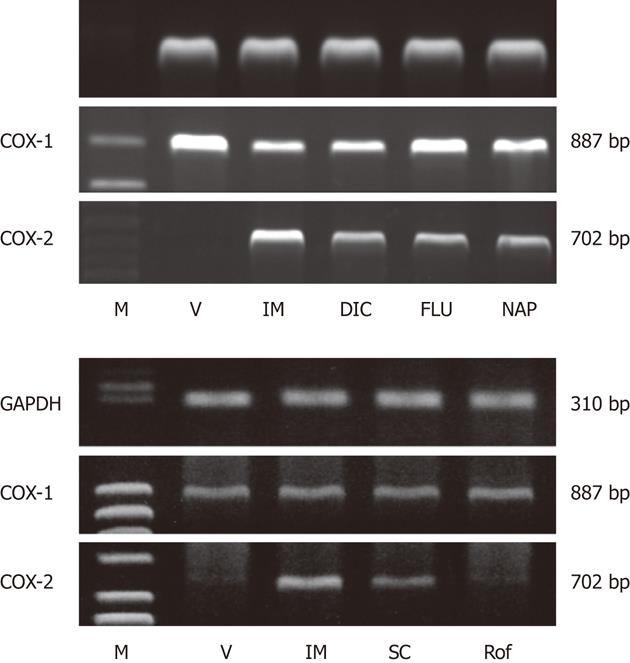
Figure 11 Gene expression of cyclooxygenase-1 and cyclooxygenase-2 in rat gastric mucosa after administration of various non-steroidal anti-inflammatory drugs (A) or various cyclooxygenase inhibitors (B).
The animals were given indomethacin (IM, 30 mg/kg), naproxen (NAP, 40 mg/kg), flurbiprofen (FLU, 20 mg/kg), dicrofenac (DIC, 40 mg/kg), SC-560 (SC, 30 mg/kg), or rofecoxib (Rof, 30 mg/kg) p.o., and the expression of cyclooxygenase (COX)-1 and COX-2 mRNA was examined by reverse transcription polymerase chain reaction 4 h later. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; M: Marker, V: Vehicle (data from refs. 18 and 19 after modification).
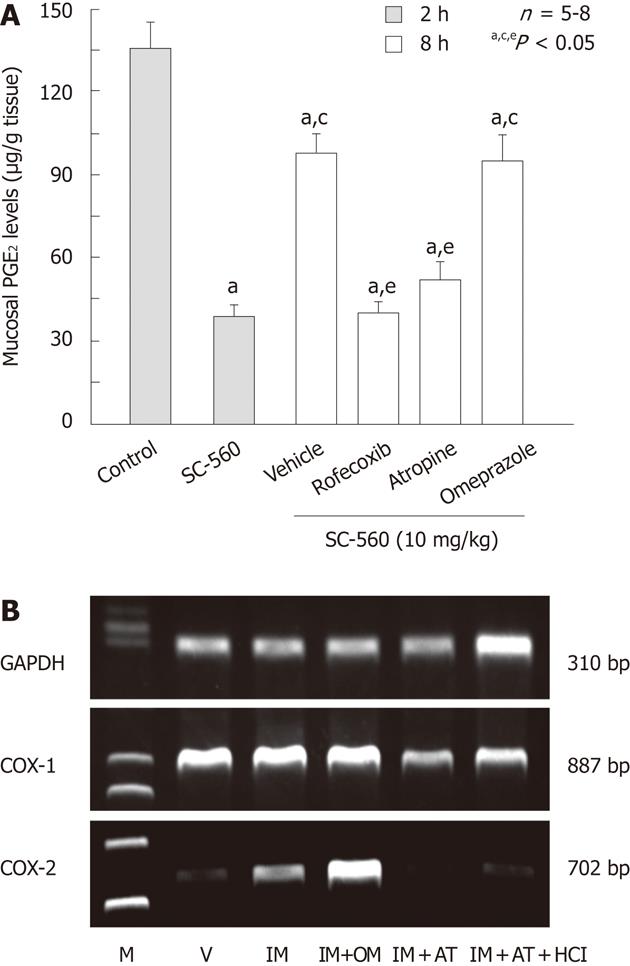
Figure 12 Effects of various drugs on prostaglandin E2 production and cyclooxygenase-2 expression in rat gastric mucosa.
A: Effects of various drugs on prostaglandin E2 (PGE2) levels in the rat gastric mucosa at 8 h after administration of SC-560. The animals were administered SC-560 (10 mg/kg) p.o., and killed 2 or 8 h later. Rofecoxib (30 mg/kg) was given p.o. together with SC-560 while omeprazole (OM, 30 mg/kg) or atropine (AT, 3 mg/kg) was given s.c. 1 h before the administration of SC-560. Data are presented as the mean ± SE in 6 rats. Significant difference at P < 0.05; afrom control; cfrom SC-560 (2 h); efrom vehicle; B: Effect of OM and AT on cyclooxygenase (COX)-2 expression after administration of indomethacin (IM) in rat stomach. The animals were administered IM (30 mg/kg) p.o., and killed 4 h later. OM (30 mg/kg) or AT (3 mg/kg) was given s.c. 1 h before indomethacin. Some animals were given 1 mL of 100 mmol HCl immediately after administration of IM. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; M: Marker, V: Vehicle (data from ref. 48 after modification).
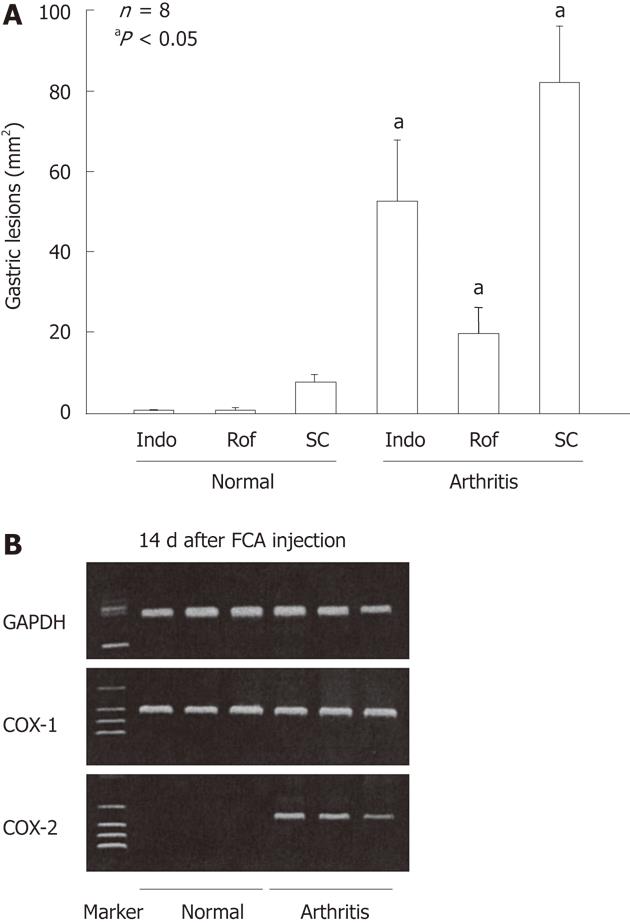
Figure 13 Gastric ulcerogenic effect of indomethacin, rofecoxib and SC-560 and the expression of cyclooxygenase-1 and cyclooxygenase-2 mRNA in the gastric mucosa of normal and arthritic rats.
A: Arthritis was induced by injecting Freund’s complete adjuvant (FCA) into the plantar region of the right hindfoot, and the experiments were performed 14 d after the injection. Indomethacin (Indo) (3 mg/kg), rofecoxib (Rof) (30 mg/kg), or SC-560 (SC) (30 mg/kg) were administered p.o., and the animals were killed 4 h later. Data are presented as the mean ± SE in 4-8 animals, aP < 0.05 vs the corresponding group in normal rats; B: COX-2 mRNA was not detected in the normal rats, but clearly observed in the arthritic rats on day 14 after the FCA injection, whereas COX-1 mRNA was observed in the stomach of both normal and arthritic rats. Lane 1, marker; lanes 2-4, normal rats; lanes 5-7, arthritic rats (data from ref. 23 after modification). GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
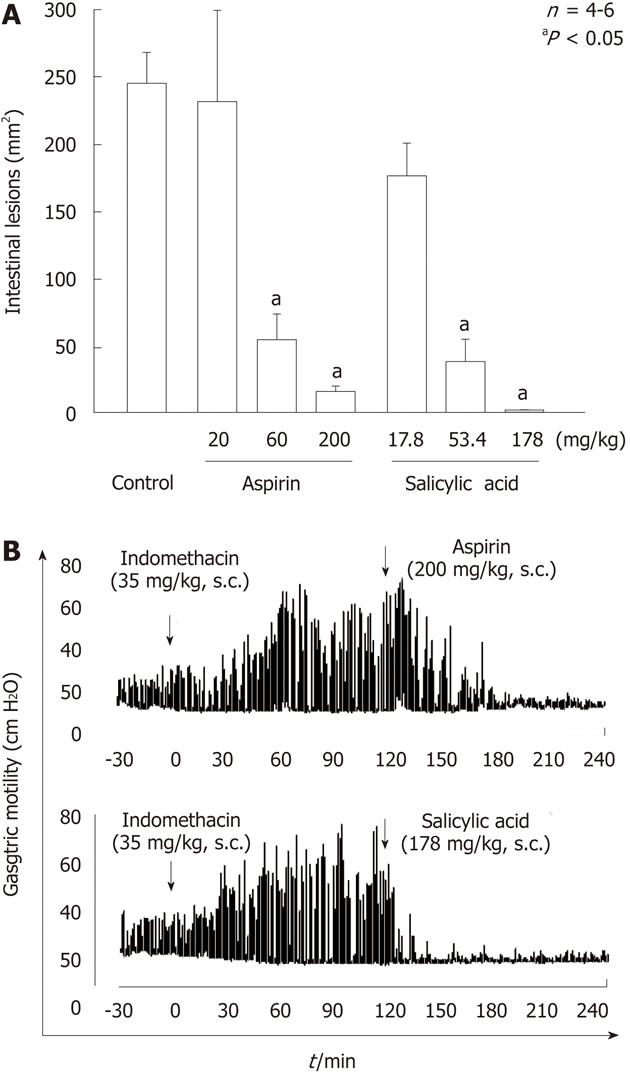
Figure 14 Effects of aspirin and salicylic acid on gastric lesions (A) and gastric hypermotility (B) caused by indomethacin in rats.
A: The animals were given indomethacin (35 mg/kg) s.c., and killed 24 h later. Aspirin (20-200 mg/kg) or salicylic acid (17.8-178 mg/kg) was given s.c. 30 min before indomethacin. Data are presented as the mean ± SE in 4-6 rats. aP < 0.05 vs control; B: Animals were given indomethacin (30 mg/kg) s.c. and subsequently aspirin (200 mg/kg) or salicylic acid (178 mg/kg) s.c. 2 h later. Note that both aspirin and salicylic acid markedly inhibited the intestinal hypermotility induced by indomethacin, with the effect of salicylic acid appearing much earlier than that of aspirin (data from ref. 25 after modification).
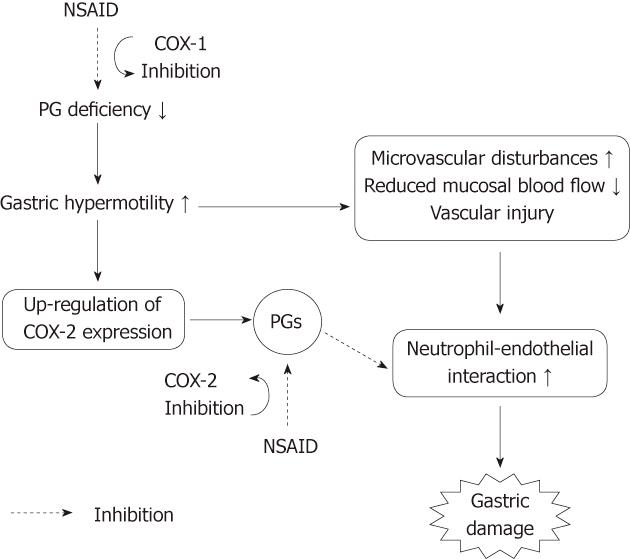
Figure 15 Working hypothesis on the roles of COX-1 and COX-2 in the pathogenic mechanism of non-steroidal anti-inflammatory drug-induced gastric damage.
Non-steroidal anti-inflammatory drugs (NSAIDs) cause gastric hypermotility, followed by microvascular disturbances and neutrophil activation, leading to gastric damage. Gastric hypermotility and subsequent vascular disturbances are associated with a prostaglandin (PG) deficiency caused by COX-1 inhibition. The inhibition of COX-1 up-regulates COX-2 expression, and PGs produced by COX-2 may suppress the neutrophil-endothelial interaction caused by microvascular disturbances due to COX-1 inhibition.
- Citation: Takeuchi K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol 2012; 18(18): 2147-2160
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2147.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2147