Published online Mar 8, 2017. doi: 10.4254/wjh.v9.i7.368
Peer-review started: November 23, 2016
First decision: December 15, 2016
Revised: December 19, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 8, 2017
To characterize the antigen on HepG2 cell that is specifically recognized by a new monoclonal antibody raised against human liver heparan sulfate proteoglycan (HSPG), clone 1E4-1D9.
The antigen recognized by mAb 1E4-1D9 was immunoprecipitated and its amino acid sequence was analyzed LC/MS. The transmembrane domain, number of cysteine residues, and glycosylation sites were predicted from these entire sequences. Data from amino acid analysis was aligned with glypican-3 (https://www.ebi.ac.uk/Tools/msa/clustalo/). The competitive reaction of mAb 1E4-1D9 and anti-glypican-3 on HepG2 cells was demonstrated by indirect immunofluorescence and analyzed by flow cytometry. Moreover, co-immunoprecipitation of mAb 1E4-1D9 and anti-glypican-3 was performed in HepG2 cells by Western immunoblotting. The recognition by mAb 1E4-1D9 of a specific epitope on solid tumor and hematopoietic cell lines was studied using indirect immunofluorescence and analyzed by flow cytometry.
Monoclonal antibody 1E4-1D9 reacted with an HSPG isolated from human liver and a band of 67 kD was detected under both reducing and non-reducing conditions. The specific antigen pulled down by mAb 1E4-1D9, having a MW of 135 kD, was analyzed. The results showed two sequences of interest, gi30722350 (1478 amino acid) and gi60219551 (1378 amino acid). In both sequences no transmembrane regions were observed. Sequence number gi30722350 was 99.7% showed a match to FYCO1, a molecule involved in induction of autophagy. Sequence number gi60219551 contained 15 cysteines and 11 putative glycosylation sites with 6 predicted N-glycosylation sites. It was also matched with all PDZ domain proteins. Moreover, it showed an 85.7% match to glypican-3. Glypican-3 on HepG2 cells competitively reacted with both phycoerythrin-conjugated anti-glypican-3 and mAb 1E4-1C2 and resulted in an increase of double-stained cell population when higher concentration of mAb 1E4-1D9 was used. Moreover, antigens precipitated from HepG2 cell by anti-glypican-3 could be detected by mAb 1E4-1D9 and vice versa. The recognition of antigens, on other solid tumor cell lines, by mAb 1E4-1D9 was studied. The results demonstrated that mAb 1E4-1D9 reacted with Huh7, HepG2, HT29, MCF7, SW620, Caco2, B16F1, U937, K562 and Molt4 cells. It was also found to be weakly positive to SW1353 and HL60 and negative to H460 and Hela cell lines.
All findings show that mAb 1E4-1D9 specifically recognizes glypican-3. Moreover, a new partner molecule of glypican-3, FYCO1 is proposed based on the results from co-precipitation studies.
Core tip: Heparan sulfate proteoglycan (HSPG) was isolated from human liver. Preliminary results showed that it was detected by rabbit anti-glypican. Monoclonal antibody, 1E4-1D9 was raised against human liver HSPG and its specific antigen was characterized. Amino acid sequence analysis revealed that the antigen recognized by mAb 1E4-1D9 specific molecule contained no transmembrane region. It has 15 cysteines and 11 putative glycosylation sites and 6 predicted N-glycosylation sites. The sequence matched to all PDZ domain proteins with an 85.6% match to glypican-3. Studies of co-expression and co-precipitation demonstrated that mAb 1E4-1D9 could compete with anti-glypican-3. It could also react with a various tumor cell lines including solid and hematopoietic cells. The findings suggested that the antigen recognized by 1E4-1D9 was glypican-3. Moreover, findings revealed that FYCO1 co-precipitated with glypican-3 using mAb 1E4-1D9, suggesting that FYCO1 is a partner molecule of glypican-3.
- Citation: Vongchan P, Linhardt RJ. Characterization of a new monoclonal anti-glypican-3 antibody specific to the hepatocellular carcinoma cell line, HepG2. World J Hepatol 2017; 9(7): 368-384
- URL: https://www.wjgnet.com/1948-5182/full/v9/i7/368.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i7.368
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related deaths[1,2]. The majority of these cases occur in Asia and Africa. However, the incidence has also been rising in the developed world. Among liver cancer cases, 80% are HCC, which does not respond well to chemotherapy[3]. Early detection is difficult and there are poor outcomes to aggressive therapies[4,5]. Thus, early detection of HCC is a key goal in improving this poor prognosis. In addition, identification of novel molecular targets for development of diagnostic and therapeutic approaches remains of great interest. Glypican-3 is highly expressed in HCC and recently has been suggested as a good diagnostic marker for HCC[6-14]. In addition to effective early diagnosis, drugs targeting different mechanisms of action involving glypican-3 targeted antibody therapy are addressed[15]. To date, several clones of monoclonal antibodies specific to glypican-3 have been described[6,8,16-19]. These have not only been used as research tools and in diagnostic development, but some have been developed for preparing potential agents for HCC immunotherapy[17-21]. Moreover, silencing of glypican-3 was recently reported to induce apoptosis in HCC cell lines[22]. Thus, glypican-3 has great promise as an excellent molecular target for the diagnosis and therapy of HCC.
Glypican is a family of heparan sulfate proteoglycans (HSPGs) that are expressed on the extracellular membrane as a glycosylphosphatidylinositol (GPI)-anchored proteoglycan. These HSPGs regulate cellular signaling during morphogenesis, adult physiology and carcinogenesis by interaction with a multitude of extracellular matrix molecules including chemokines, growth factors or morphogens and their receptors[23-25]. Glypican is expressed in cell-, tissue- and development-specific patterns. Among the six members of the glypican family, glypican-3 has been studied most extensively[23,26,27].
Since glypican-3 is an HSPG, it typically contains a heparan sulfate glycosaminoglycan chain (GAG), but in some instances a chondroitin sulfate (GAG) can also be found on glypican-3[23]. GAG chains carry negative charge, allowing glypican-3 to interact with basic growth factors and morphogens in the extracellular space. Glypican-3 has a 70-kD core protein which can be cleaved by furin generating two fragments of 40-kD N-terminal and 30-kD C-terminal[27]. The GPI anchor linking glypican-3 to the membrane can be cleaved by lipase (notum), releasing glypican-3 to extracellular matrix[28]. The shedding of glypican-3 plays a role in regulating signaling of Wnts, hedgehogs, fibroblast growth factors, and bone morphogenetic proteins[23,26,29,30]. There has also been a report that soluble glypican-3 can inhibit HCC proliferation both in vitro and in vivo[31]. Therefore, glypican-3 can play both positive and negative role in cell growth depending on cell type[32,33]. Glypican-3 is expressed in a variety of tissues and acts as oncofetal protein. Among membrane HSPGs, glypican-3 is the only HSPG that is highly expressed on HCC tissue but it is usually not found in normal and in non-tumor liver tissues[34]. Previous findings indicate that glypican-3 stimulates in vitro and in vivo growth of HCC[26,35-39]. The mechanism in HCC growth promotion of glypican-3 is to regulate Wnt signaling as well as oncogenesis through insulin-like growth factor signaling pathway[40]. It was reported that, in primary HCC, sulfatase-2 (SULF2) enzyme with 6-O-sulfatase activity is up-regulated and associated to poor prognosis[41]. Increasing of SULF2 enhances the expression of glypican-3 in vitro and in vivo[42].
The liver is a rich source of GAGs and the liver is known to be receptor of many molecules involved in diseases and in pathogen binding[43-46]. Recently, an HSPG was isolated from human liver. The analysis of its GAG component demonstrated that it was heparan sulfate, not heparin[47]. Digestion of liver HSPG with heparin lyase I, II, III yielded a core protein product that could be detected by anti-rat glypican with a band of approximately 61 kD. These results suggested that the HSPG isolated from human liver was a glypican.
Monoclonal antibodies were raised against liver HSPG. Two of the clones obtained are 1E4-1C2 and 1E4-1D9. The clone 1E4-1C2 specifically reacts with membrane molecules of various malignant cell lines, including solid tumor and hematopoietic cells in erythromyeloid series[48]. This antibody can differentiate between acute myeloid leukemia from normal blood cells and normal blast cells in bone marrow. Moreover, mAb 1E4-1C2 strongly reacts with HepG2 cells and inhibits cell proliferation in a dose dependent manner both in vitro and in an animal model[49]. Development of HepG2 cell-targeted drug delivery based on mAb 1E4-1C2 has also been studied[50]. Intensive characterization of mAb 1E4-1C2 and its specific antigen is in progress.
Our preliminary results of mAb 1E4-1D9 showed that it could react with HepG2. Together with the previous observations that liver HSPG was a glypican and that glypican-3 is up regulated in HCC, we hypothesized that antigen recognized by mAb 1E4-1D9 was glypican-3. The present study is aimed at characterizing the specific antigen on HepG2 cells recognized by mAb 1E4-1D9.
HL60 cell line was a kind gift from Associate Professor, Dr. Songyot Anuchpreeda, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University. Huh7 was from Professor, Dr. Pa-thai Yenchitsomanus, Faculty of Medicine Siriraj Hospital, Mahidol University. The other cell lines were purchased from ATCC.
OPI supplement, fetal bovine serum, 3,3-diamino benzidine (DAB), and SuperSignal™ West Pico Chemiluminescent Substrate were purchased from Sigma-Aldrich (St. Louis, MO, United States). All culture media were from Gibco (Life Technologies, NY, United States). Mouse IgG1 and phycoerythrin (PE) conjugated mouse IgG2a were purchased from Biolegend, CA, United States and anti-glypican-3 [clone 9C2, IgG1, immunogen: Recombinant human glypican-3 (amino acid 1-580)] was from Abcam (United Kingdom). PE conjugated anti-glypican-3 [clone 307801, IgG2a, immunogen: Recombinant human glypican-3 (amino acid 25-558)] was obtained from United States Biological Life Sciences, MA, United States. Fluorescein isothiocyanate (FITC) conjugated anti-mouse Igs and horseradish peroxidase (HRP)-conjugated anti-mouse Igs were purchased from Dako (CA, United States). Protein G agarose was purchased from Pierce (Rockford, IL, United states). IsoStrip was obtained from Roche (IN, United States). Other common reagents used in these studies were purchased from local reputable companies including PCL Holdings (Thailand) and Pacific Sciences (Thailand).
The hybrid clone 1E4-1D9 was grown in OPI containing-Dulbecco’s Modified Eagle’s medium (DMEM)/high glucose supplemented with 10% fetal bovine serum to exponential phase. Cell culture supernatant was collected and mAb 1E4-1D9 was purified using protein G affinity agarose beads. Briefly, cell culture supernatant was diluted with binding buffer provided (1:1 v/v) before applying and allowed to flow completely into the resin. The column was then washed with binding buffer and eluted with the elution buffer provided. Fractions of 1 mL were collected and neutralized with neutralizing buffer (Tris-base, pH 8.0, 100 μL). Pooled purified mAb was dialyzed against phosphate buffered saline (PBS) pH 7.2, concentrated and aliquots were frozen. Isotype was determined using IsoStrip according to the manufacturer’s directions.
HSPG isolated from human liver[47] was diluted to 5 μg/mL with PBS, pH 7.2. Twenty μL of sample was mixed with 5 μL of 5 × sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 70 mmol/L sodium dodecylsulfate (SDS), 10% glycerol, 2% bromphenol blue) and non-reducing sample buffer, and boiled for 5 min. Sample was subjected to electrophoresis on 10% SDS-polyacrylamide gel electrophoresis (PAGE) at 200 V for 45 min and blotted onto polyvinyl difluoride (PVDF) membrane. Before probing with mAb 1E4-1D9, non-specific sites were blocked with 5% non-fat dried milk in tris-buffered saline (TBS) pH 7.4 (0.15 mol/L NaCl, 10 mmol/L Tris-base) for 1 h at room temperature on a rocking plate. The membrane was washed 3-times (10 min each) with TBS pH 7.4. Primary antibody (mAb 1E4-1D9, 100 μg/mL in 0.1% Tween-20 in; TBS-Tween) was added onto the membrane. The reaction was performed at room temperature for 1 h on a rocking plate. After completion, membrane was washed with TBS-Tween for 3-times (10 min each) on a rocking plate. The reaction was then detected with HRP-conjugated rabbit anti-mouse Igs for 1 h at room temperature on a rocking plate and washed. Finally, signal was then developed with DAB containing H2O2. Molecular weight (kD) was calculated from a plot of log molecular weight standard vs migration distance and a R2≥ 0.99 was obtained.
HepG2 cells cultured in DMEM high glucose supplemented with 10% fetal bovine serum (FBS) grown to exponential phase. Cells were collected, washed twice with PBS, pH 7.2. Cell viability was checked by trypan blue dye exclusion assay and adjusted to 4 × 105 cells/mL with PBS pH 7.2. Heat-inactivated normal human AB serum was added to the final concentration of 10% and incubated on ice for 30 min. An aliquot of cell suspension (50 μL) was added to an equal volume of various final concentrations of mAb 1E4-1D9. Mouse IgG1 and washing buffer [cold 1% bovine serum albumin (BSA)-PBS, 0.02% NaN3] were used as isotype and conjugated control, respectively. The reactions were incubated on ice for 30 min. After completion, cells were washed 3-times with washing buffer. Fifty microlitre of FITC-conjugated rabbit anti-mouse Igs (1:20 diluted in washing buffer) was added and reaction was incubated for another 30 min on ice. Following with 3-washes, cell pellet was suspended with 300 μL of 0.5% paraformaldehyde in PBS, pH 7.2 and analyzed by flow cytometer (Becton Dickinson, CA, United States).
HepG2 cells grown to exponential phase were harvested, washed 5-times with PBS pH 7.2 (0.137 mol/L NaCl, 2.68 mmol/L KCl, 1.88 mmol/L NaH2PO4.2H2O, 8.10 mmol/L Na2HPO4) and adjusted to 1 × 106 cells/mL. One millilitre of lysis buffer (1% Brij58, 20 mmol/L Tris–HCl pH 7.5, 0.15 mol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 5 mmol/L iodoacetamide, 1 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L pepstatin A, 10 mg/mL aprotinin) was added. The suspension was mixed thoroughly, centrifuged 12000 rpm at 4 °C for 30 min to pellet cell debris. HepG2 cell lysate was used as source of antigen precipitating by mAb immobilized protein G agarose beads.
Prior to immobilization of mAb, 50 μL of protein G was washed 3-times with binding buffer (0.2 mol/L NaH2PO4.H2O, 0.15 mol/L NaCl) followed by immunoprecipitation (IP) buffer (25 mmol/L Tris-base, 0.15 mol/L NaCl). One hundred microlitres of mAb 1E4-1D9 (100 μg/mL) was then mixed individually with protein G agarose beads for 30 min at room temperature before washing (3-times) out non-bound mAb with IP buffer. Washing buffer was discarded and 100 μL of HepG2 lysate was added. The reaction was incubated at 4 °C overnight. After completion, the reaction was centrifuged, supernatant was discarded and beads were washed 6-times with IP buffer. Fifty microlitre of elution buffer (0.1 mol/L glycine, pH 3.0) was added and mixed for 5 min. Finally beads were pelleted down and eluate, containing specific antigen, was collected. This step was repeated twice. Collected supernatants were pooled and neutralized with 10 μL of neutralizing buffer (1 mol/L Tris-base, pH 8.0).
Twenty microlitres of eluate was mixed with 5 μL of 5 × sample buffer and separated on 10% SDS-PAGE at 200 V for 45 min before blotting onto PVDF membrane. Non-specific binding sites on the membrane were blocked with 5% non-fat dried milk in TBS pH 7.4 (0.15 mol/L NaCl, 10 mmol/L Tris-base) for 1 h at room temperature on a rocking plate. PVDF membrane was then washed, with 0.1% Tween-20 in TBS (TBS-Tween), 3-times for 10 min each on a rocking plate. Primary antibody, mAb 1E4-1D9 (1 mg/mL in 1%BSA TBS-Tween) was added to each membrane. The reaction was performed at 4 °C overnight. After completion, membrane was washed (3-times for 10 min each) with 0.1% Tween-20 in TBS (TBS-Tween) on a rocking plate. The reaction was then detected with HRP-conjugated rabbit anti-mouse Igs for 1 h at room temperature on a rocking plate. After washing out the excess antibody (3-times for 10 min each) with 0.1% Tween-20 in TBS (TBS-Tween), signal was visualized by SuperSignal™ West Pico chemiluminescent substrate. Molecular weight was calculated from standard molecular weight graph as previously mentioned.
The eluate was pooled and subject to electrophoresis in 5 × non-reducing sample buffer on 10% SDS-PAGE at 200 V for 45 min. Gel was stained with Coomasie Brilliant Blue to prepare mAb 1E4-1C2 specific antigens for amino acid sequence analysis. The band of interest, selected by as comparison to result on immunoblot, was cut and sent for amino acid analysis by LC-MS (HDMS Synaptat, Waters, MA, United States) at the National Center for Genetic Engineering and Biotechnology, Bangkok, Thailand).
HepG2 cells in exponential phase were harvested and washed twice with PBS, pH 7.2. Cell viability was determined by trypan blue dye exclusion assay and adjusted to 4 × 105 cells/mL with PBS pH 7.2. After blocking with heat-inactivated normal AB serum for 30 min on ice, an aliquot of cell suspension (50 μL) was added to equal volumes of various concentrations of mAb 1E4-1D9. Mouse IgG1 was used as isotype control. The reaction was incubated on ice for 30 min follow by 3-washes with cold washing buffer. Cell pellet was re-suspended with 50 μL washing buffer and added with equal volume of FITC-conjugated rabbit anti-mouse Igs (1:20 diluted in washing buffer). After incubating on ice for another 30 min, cells were washed 3-times and PE-conjugated anti-glypican-3 (1:10 diluted with washing buffer). PE conjugated mouse IgG2a was used as isotype control. The reaction was performed on ice for 30 min and washed 3-times. Finally, cells were suspended with 300 μL of 0.5% paraformaldehyde in PBS, pH 7.2 and analyzed by flow cytometer.
HepG2 cell lysate was prepared as mentioned above and was used as source of antigen. The three different antibody immobilized protein G agarose beads, anti-glypican-3, mAb 1E4-1D9, and mouse IgG1 (isotype control) were immobilized on protein G agarose beads as mentioned.
Twenty microlitres of eluates from mAb 1E4-1D9, anti-glypican-3, or mouse IgG1 immobilized protein G agarose beads was separated on 10% SDS-PAGE at 200 V for 45 min in non-reduced condition and blotted onto PVDF membrane. Three membranes were prepared. Non-specific binding sites on the membrane were blocked with 5% non-fat dried milk in TBS, pH 7.4 for 1 h at room temperature on a rocking plate. PVDF membrane was then washed, 3-times for 10 min, with TBS-Tween each on a rocking plate. Primary antibody [mAb 1E4-1D9, anti-glypican-3, or mouse IgG1 isotype control (100 μg/mL in 1% BSA TBS-Tween)] was added to each individual membrane. The reaction was performed at 4 °C overnight. After completion, membrane was washed, 3-times for 10 min, with TBS-Tween each on a rocking plate. The reaction was then detected with HRP-conjugated rabbit anti-mouse Igs for 1 h at room temperature on a rocking plate. After washing (3-times for 10 min each) out the excess antibody with TBS-Tween, signal was then developed by SuperSignal™ West Pico chemiluminescent substrate and auto-radiographed. The molecular weight was calculated from a standard molecular weight plot as previously described.
Solid tumor cell lines (Huh7, B16F1, HT29, Caco2, MCF7, SW620, SW1353, H460 and Hela) cultured in DMEM high glucose supplemented with 10% FBS and hematopoietic cell lines (HL60, K562, U937 and Molt4) cultured in RPMI-1640 were grown to exponential phase. Cells were collected, washed twice with PBS, pH 7.2. Cell viability was checked by trypan blue dye exclusion assay and adjusted to 4 × 105 cells/mL with PBS pH 7.2. Heat-inactivated normal human AB serum was added to cell suspension to the final concentration of 10% and incubated on ice for 30 min. Aliquot of cell suspension (50 μL) was added with an equal volume of mAb 1E4-1D9 (20 μg/mL). Mouse IgG1 and washing buffer (cold 1%BSA-PBS, 0.02% NaN3) were used as isotype control and conjugated control, respectively. The reactions were incubated on ice for 30 min. After completion, cells were washed 3-times with washing buffer. Fifty microlitres of FITC-conjugated rabbit anti-mouse Igs (1:20 diluted in washing buffer) was added and reaction was incubated for another 30 min on ice. Following 3-washes with washing buffer, the cell pellet was suspended with 300 μL of 0.5% paraformaldehyde in PBS, pH 7.2 and was analyzed by flow cytometer (Becton Dickinson, CA, United States).
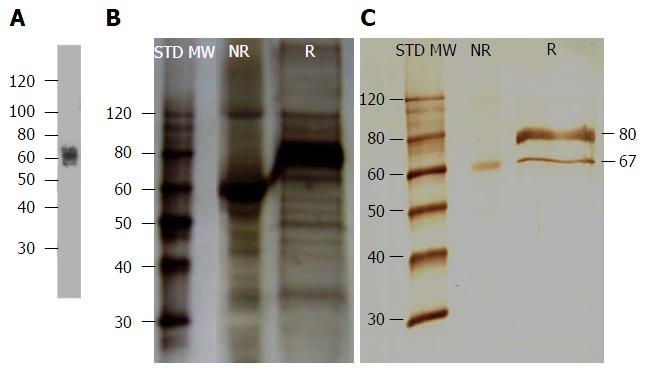
Before any assay was performed, antibody isotype was determined using a commercial isotyping kit and it was confirmed that mAb 1E4-1D9 was an IgG1. The specificity to the immunogen was also studied by Western immunoblotting of liver HSPG and probed with mAb 1E4-1D9. The results demonstrated a band was detected at approximately 67 kD under both non-reducing and reducing conditions (Figure 1C). The molecular weight was close to that reported previously where liver HSPG was probed with rabbit anti-rat glypican[47] (Figure 1A). These results suggest that the epitope of mAb 1E4-1D9 is present in both folded and linear forms.
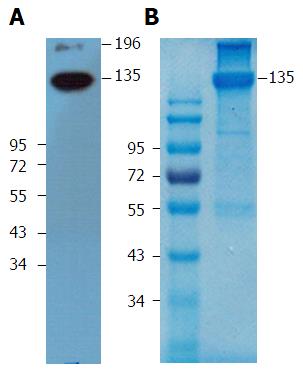
We next used indirect immunofluorescence to examine the expression of the antigen on HepG2 cells that reacts with mAb 1E4-1D9. mAb 1E4-1D9 reacted specifically to an antigen on HepG2 in concentration dependent manner (Figure 2). Moreover the highest expression of this antigen was observed during incubation (data not shown) while HepG2 was in exponential phase was at day-4 of incubation. The specific antigen was immune-precipitated by mAb 1E4-1D9 immobilized protein G agarose beads. A band at 135 kD was visualized by immunoblotting (Figure 3A) and was cut from the gel (Figure 3B) and sent for analysis.
Amino acid analysis demonstrated the presence of two hypothetical sequences, gi30722350 (1478 amino acid) and gi60219551 (1378 amino acid). Neither sequence had a transmembrane region domain based on analysis by TMHMMM software (Figure 4A). Data analysis also demonstrated the number of cysteine residues was 19 and 15 in gi30722350 and gi60219551, respectively. Moreover, gi30722350 contains two putative glycosylation sites while the latter, gi60219551 has 11 putative glycosylation sites at amino acid 139, 227, 377, 393, 577, 721, 891, 911, 1053, 1090 and 1243, respectively with 6 predicted N-glycosylation sites (amino acid 139, 227, 377, 577, 721 and 1053) (Figure 4B). Alignment of gi30722350 (https://blast.ncbi.nlm.nih.gov/Blast.cgi) demonstrated that it was matched to all FYVE containing protein with 99.7% matched to FYCO-1 (data not shown). Interestingly, gi60219551 matched to a PDZ domain protein with 85.7% match to glypican-3 (Figure 4C).
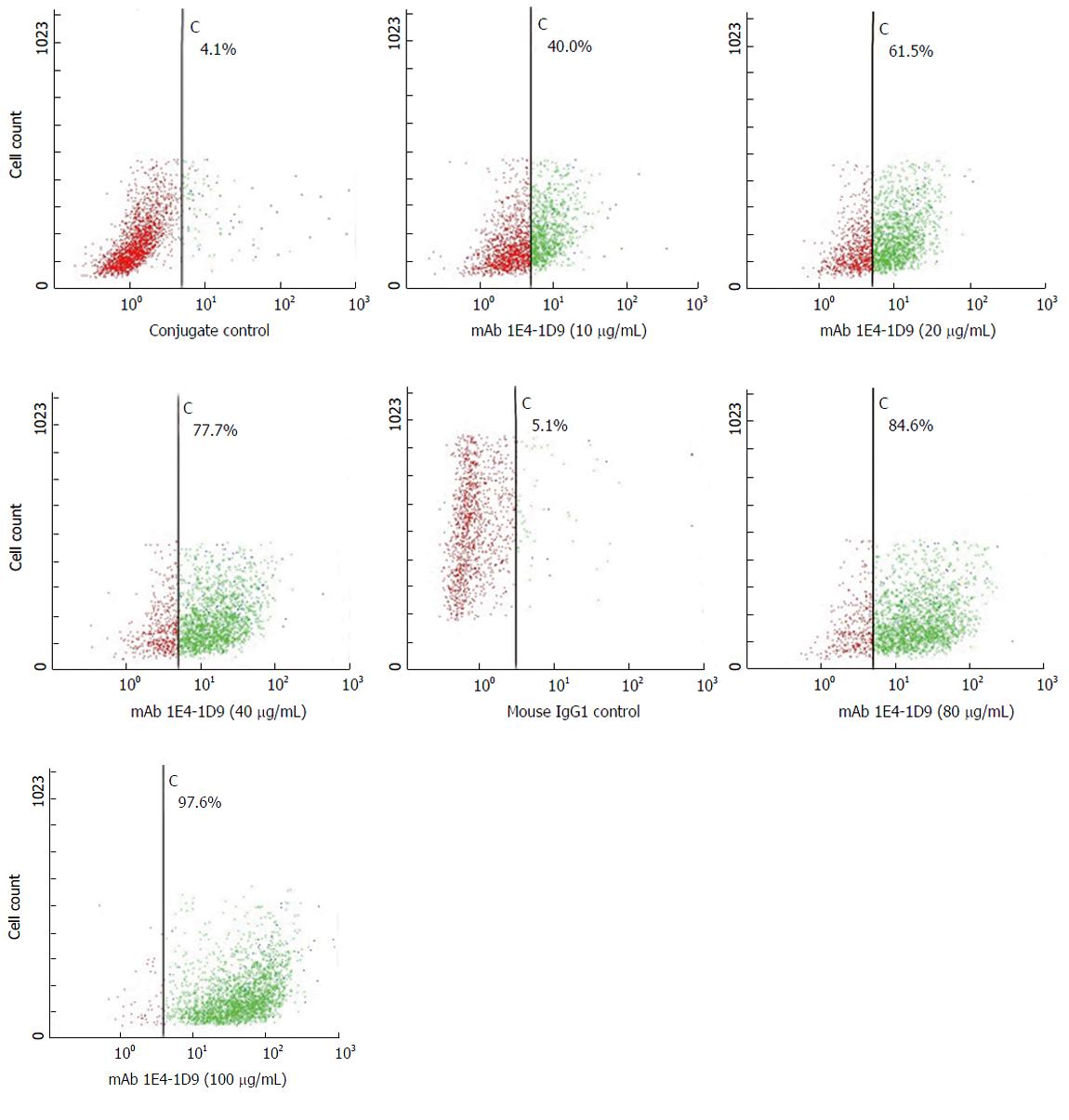
Expression of mAb 1E4-1D9 together in competition with anti-glypican-3 was undertaken to verify that antigen specific to mAb 1E4-1D9 was glypican-3. However, prior to this experiment, the concentration of PE-conjugated anti-glypican-3 was optimized for maximum intensity detection by direct immunofluorescence. The result indicated that PE-conjugated anti-glypican-3 at dilution of 1:10 could specifically react to 97.8% of antigen on HepG2 cells (data not shown). Various final concentrations of mAb 1E4-1D9 were used to react with HepG2 cells followed by fixing with PE-conjugated anti-glypican-3 and analyzed by flow cytometry. The number of cells, in the upper right quadrant (positive both FL1 and FL2), increased in dose dependent manner while FL2 signal of the PE-conjugated anti-glypican-3 decreased (Figure 5). This indicates that mAb 1E4-1D9 is specific to glypican-3 on HepG2 since mAb 1E4-1D9 could compete with PE-conjugated anti-glypican-3 used. Moreover, it suggests that antigenic site of mAb 1E4-1D9 on glypican-3 may be at or close to N-terminal region because immunogen of PE-conjugated anti-glypican-3 used was recombinant human glypican-3 (amino acid 25-558).
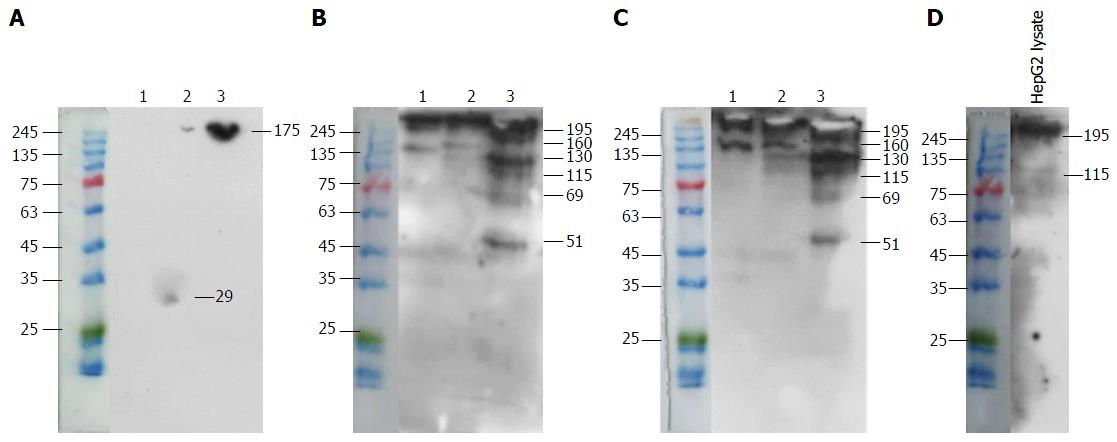
Co-immunoprecipitation of a specific antigen on HepG2 cells by mAb 1E4-1D9 and anti-glypican-3 was performed. Mouse IgG1 was used in parallel as an isotype control. Findings from experiments show that mAb 1E4-1D9 precipitated three interesting bands of 69, 115 and 130 kD (Figure 6B), which also reacted with anti-glypican-3 (Figure 6C). A protein band of 130 kD precipitated by anti-glypican-3 was clearly visualized by mAb 1E4-1D9 (Figure 6B). However, anti-glypican-3 itself showed less reaction (Figure 6C). Lysate was probed with anti-glypican-3 to verify that lysate contained glypican-3 and a band was observed at 115 kD (Figure 6D). Taken together this demonstrated that mAb 1E4-1D9 could react with antigen precipitated by anti-glypican-3 and vice versa.
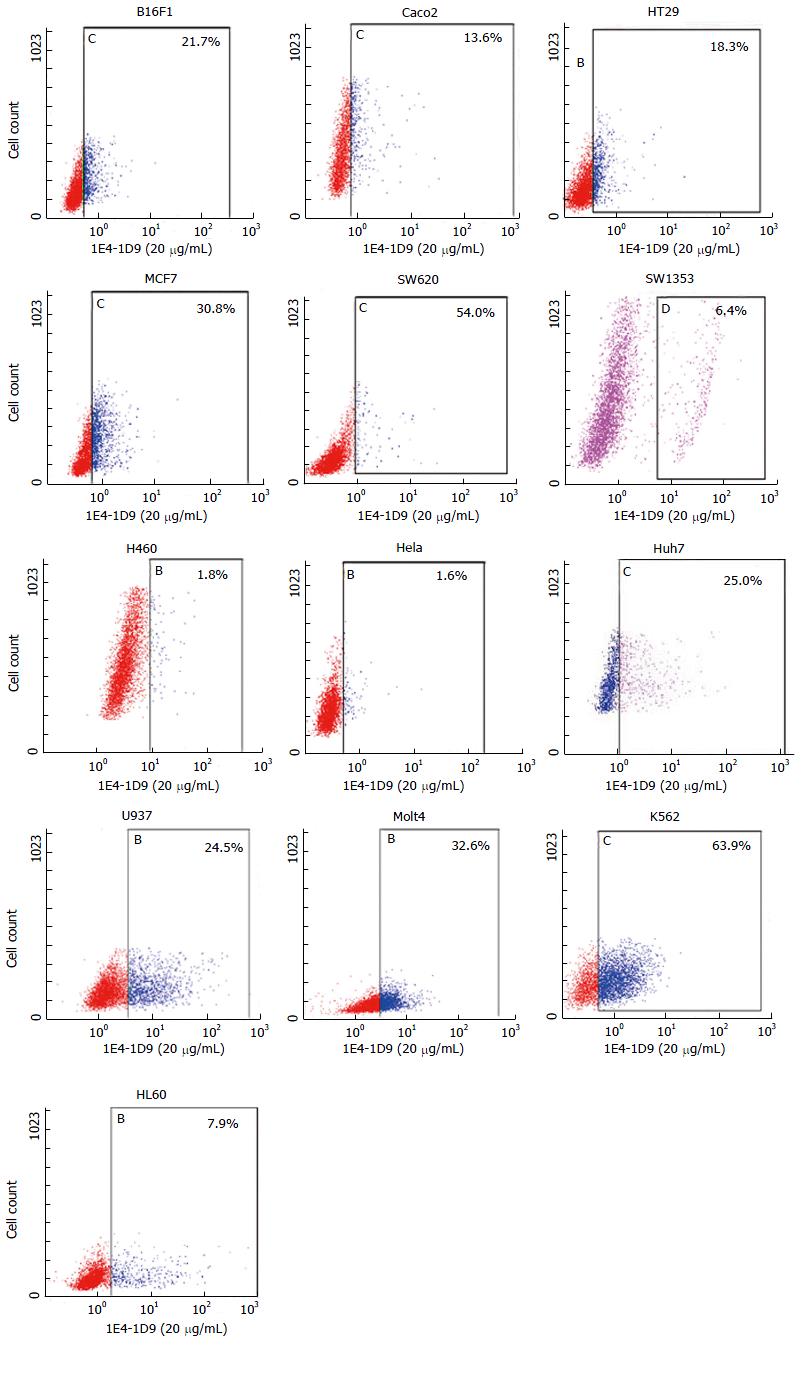
Expression of mAb 1E4-1D9 on other cells was studied by indirect immunofluorescence. We found that the antigen recognized by mAb 1E4-1D9 was expressed on a variety of cell lines tested including B16F1, Caco2, HT29, MCF7, SW620, K562, U937 and Molt4 (Figure 7). Some cells such as SW1353 and HL60 were weakly positive and some were negative (H460 and Hela cells).
HCC is one of the most common cancers worldwide with a poor prognosis and a low 5-year survival rate. Thus, specific biomarkers have become increasingly important to identify HCC. Glypican-3 is upregulated and highly expressed in HCC but not in normal or non-malignant liver tissues. Glypican-3 has important roles in cell growth, differentiation and motility[33]. As a key molecule in relation to signaling with several growth factors and growth factor receptors, glypican-3 can regulate the proliferation of malignant cells both in negative and positive ways[32]. Therefore, antibodies specific to glypican-3 are of interest and many antibody-expressing clones have been developed. Some clones are used to prepare antibodies as tools to study the glypican-3 related cellular activities and some have been applied in tumor investigation and tumor-specific drug development[6,15,20,51].
Our previous report demonstrated that HSPG isolated from human liver contained glypican[47]. Monoclonal antibody raised against human liver HSPG, mAb 1E4-1D9 was, thus, proposed to be specific to glypican-3, which is the only membrane HSPG that highly expressed by HCC[34].
Probing of liver HSPG with mAb 1E4-1D9 resulted a band of 67 kD under both reducing and non-reducing conditions indicate that epitope of mAb 1E4-1D9 can be recognized in either the folded and linear forms. Amino acid analysis of band of 135 kD precipitated from mAb 1E4-1D9 afforded two hypothetical sequences, gi30722350 (1478 amino acid) and gi60219551 (1378 amino acid). Both sequences had no transmembrane domain indicating that they might be either intracellular or external proteins. The first sequence with 19 cysteines, gi30722350 was FYVE containing protein and found 99.7% matched to FYCO1. This is very surprising since there have been no reports of a relationship between FYCO1 and glypican-3. FYCO1 is FYVE (Fab1, YOYB, Vac1, EEA1) and coiled-coil domain containing[52] FYCO1, an endogenous protein with MW of 150 kD resides on perinuclear cytosolic vesicles. However, during a starvation period, FYCO1 redistributes to the cell periphery in microtubule-dependent manner[53]. It functions as an adapter mediating autophagosome to microtubule plus-end-directed molecular motors[54]. FYCO1 can be dimerized and recruited to the phosphatidylinositol-3-phosphate, PtdIns(3)P. Findings in the study demonstrate a protein band of 160 kD co-precipitated with a band of 69 kD by anti-glypican-3 itself or mAb 1E4-1D9. This band of 160 kD was identified FYCO1. Additional studies, are required to better understand the biological function of this relationship.
Amino acid sequence analysis revealed that the second sequence, gi60219551 with 1378 amino acid was a PDZ containing protein and 85.7% matched to glypican-3. More information confirmed the structure since there are 15 cysteines and 11 putative glycosylation sites with 6 predicted N-glycosylation sites. A band of 69 kD was precipitated with mAb 1E4-1D9 as was with anti-glypican-3. However, there were two bands of 115 and 130 kD with higher MW observed which might correspond to GAG-remaining attached protein. Indirect immunofluorescence staining of various concentrations of mAb 1E4-1D9 on HepG2 cells following with the PE-conjugated anti-glypican-3 was performed to confirm the glypican-3 specificity of mAb 1E4-1D9. It was revealed that increasing amount of mAb 1E4-1D9 showed the higher number cells in upper right quadrant. This demonstrates that HepG2 can react with both antibodies through the same antigen. PE-conjugated anti-glypican-3 used in the experiment was raised against recombinant human glypican-3 at amino acid 25-558 (available information from datasheet). According to the competition experiments, we suggest that antigenic sites of mAb 1E4-1D9 are at or closed to N-terminus. This hypothesis was confirmed by co-precipitation of specific antigen from HepG2 lysate that the same protein bands were precipitated and visualized by cross-reaction between two antibodies. The protein band precipitated by mAb 1E4-1D9 was also detected by anti-glypican-3.
Moreover, since glypican-3 is expressed on a variety of malignant cell lines, indirect immunofluorescence technique was performed. We found that mAb 1E4-1D9 strongly reacted with an antigen on malignant cell lines including B16F1, Caco2, HT29, MCF7 and SW620. A strongly positive signal was observed when staining hematopoietic cell lines including K562, U937 and Molt4. In some cell lines, mAb 1E4-1D9 was weakly reacted (SW1353 and HL60) and in some no reaction was observed (H460 and Hela). These results are consistent with previous reports[55-59].
Glypican-3 is highly expressed on HCC and plays roles in cellular bioactivities, thus, it is the attractive molecule for developing a therapeutic antibody for HCC treatment. In addition, effect of anti-glypican-3 on proliferation inhibition is dependent on functional epitope of antibody[15]. Taken together, these findings support that mAb 1E4-1D9 raised against human liver HSPG is specific for glypican-3. This antibody is specific to HepG2 and glypican-3 expressing malignant cells. The effect of antibody on cell proliferation needs to be studied to understand whether it can be used as a tool for anti-cancer drug development. However, based on its specificity, it should be an excellent candidate monoclonal antibody for applications in tumor investigation as well as for tumor-targeted immunotherapy. Interestingly, the present study also discovers FYCO1 as a possible partner molecule of glypican-3. The findings merit further investigation, which may be applicable and beneficial for immuno- or gene-therapy in clinical settings for the treatment of HCC.
The authors are gratefully acknowledged Dr. Sitthiruk Roytrakul, National Center for Genetic Engineering and Biotechnology, Bangkok, for his kind analysis of amino acid sequence. Many thanks were sent to Ms. Yupanun Wutti-In for her excellent technical assistance.
Among most malignant tumors worldwide hepatocellular carcinoma (HCC) is ranked in the fifth most common malignancy and the third leading cause of death. Patients with HCC have a very poor prognosis and the 5-year survival rate of less than 5%-10%. The reasons are that clinical diagnosis usually occurs at a late stage and there are limitations in drug- and surgery-based treatment. Therefore, new strategies and effective treatment as well as early detection using tumor specific monoclonal antibodies are needed. Glypican-3, a glycosylphosphatidylinositol-linked cell surface heparan sulfate proteoglycan (HSPG) is highly expressed in HCC. In some particular conditions, glypican-3 can be cleaved and released into serum and used as a biomarker for HCC. Glypican-3 is involved in growth signalling through Wnts, hedhogs, fibroblast growth factor, and bone morphogenetic proteins. Based on its function in tumor growth regulation, an antibody specific to glypican-3, would be important for the development of tumor-targeted drug delivery and immunotherapy. Previously, HSPG was isolated from human liver. Biochemical characterization revealed that liver HSPG consisted of heparan sulfate chain with a high level of sulfation. Preliminary result showed that liver HSPG could reacted with anti-rat glypican. A monoclonal antibody against liver HSPG was raised and mAb 1E4-1D9 obtained was studied to determine whether it recognized glypican-3.
Important fields related to this study using mAb 1E4-1D9 as a tool include: (1) tumor detection and investigation such as developing of serological detection system and other clinical applications; (2) tumor-targeted drug delivery and drug design both in immunotherapy and gene therapy; and (3) understanding the role of glypican-3 in regulation of intracellular signalling in many cell types.
Glypican-3 is upregulated in HCC and many tumor cell types where it enhances cell growth in particular growth-signalling pathways. Research focusing on the production of monoclonal antibodies specific to glypican-3 are important to explore new diagnostic and therapeutic candidates. Findings from present study based on HepG2 cells indicates that specific antigen of a new monoclonal antibody, 1E4-1D9 is glypican-3. In addition, this is the first report showing FYCO1 as a potential partner molecule for glypican-3. The findings merit further investigation, which may be applicable and beneficial for immune- or gene therapy in clinical setting for the treatment of HCC.
Glypican-3 specific monoclonal antibody, 1E4-1D9, can be a tool for development of laboratory investigation for HCC and other glypican-3 expressed tumors. In addition, it will be a good candidate for tumor-targeted drug development, immunotherapy and gene therapy.
Early detection of HCC is very important to study, the glypican-3 is a good point to research, so topic of paper is novel and design of experiment is precise.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Invernizzi P, Jin B, Shen F, Yao DF S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [PubMed] [Cited in This Article: ] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 3. | Cao H, Phan H, Yang LX. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012;32:1379-1386. [PubMed] [Cited in This Article: ] |
| 4. | Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713-2722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Kern MA, Breuhahn K, Schirmacher P. Molecular pathogenesis of human hepatocellular carcinoma. Adv Cancer Res. 2002;86:67-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Kandil DH, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, Niki T, Shibahara J, Mori M, Makuuchi M. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Wang XY, Degos F, Dubois S, Tessiore S, Allegretta M, Guttmann RD, Jothy S, Belghiti J, Bedossa P, Paradis V. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Coston WM, Loera S, Lau SK, Ishizawa S, Jiang Z, Wu CL, Yen Y, Weiss LM, Chu PG. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol. 2008;32:433-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723-1728. [PubMed] [Cited in This Article: ] |
| 12. | Wang F, Jing X, Wang T, Li G, Li T, Zhang Q, Huang Y, Li J, Wang Y, Gao Y. Differential diagnostic value of GPC3-CD34 combined staining in small liver nodules with diameter less than 3 cm. Am J Clin Pathol. 2012;137:937-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wang FH, Yip YC, Zhang M, Vong HT, Chan KI, Wai KC, Wen JM. Diagnostic utility of glypican-3 for hepatocellular carcinoma on liver needle biopsy. J Clin Pathol. 2010;63:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Zhang L, Liu H, Sun L, Li N, Ding H, Zheng J. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem. 2012;114:547-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588:377-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Midorikawa Y, Ishikawa S, Iwanari H, Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M, Aburatani H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103:455-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, Furugaki K, Kinoshita Y, Ishiguro T, Hamakubo T. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832-9838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Nakano K, Ishiguro T, Konishi H, Tanaka M, Sugimoto M, Sugo I, Igawa T, Tsunoda H, Kinoshita Y, Habu K. Generation of a humanized anti-glypican 3 antibody by CDR grafting and stability optimization. Anticancer Drugs. 2010;21:907-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci USA. 2013;110:E1083-E1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Liu S, Li Y, Chen W, Zheng P, Liu T, He W, Zhang J, Zeng X. Silencing glypican-3 expression induces apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2012;419:656-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 500] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009-6021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 26. | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-6254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001;108:497-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem J. 2008;410:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Capurro MI, Shi W, Sandal S, Filmus J. Processing by convertases is not required for glypican-3-induced stimulation of hepatocellular carcinoma growth. J Biol Chem. 2005;280:41201-41206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126:1291-1301. [PubMed] [Cited in This Article: ] |
| 32. | Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008;7:2787-2790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffé E, Peters MG. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 2009;114:251-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Suzuki M, Sugimoto K, Tanaka J, Tameda M, Inagaki Y, Kusagawa S, Nojiri K, Beppu T, Yoneda K, Yamamoto N. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res. 2010;30:5055-5061. [PubMed] [Cited in This Article: ] |
| 35. | Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Büchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 37. | Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418-2423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 40. | Cheng W, Tseng CJ, Lin TT, Cheng I, Pan HW, Hsu HC, Lee YM. Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29:1319-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010;52:1680-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 42. | Chen M, Li G, Yan J, Lu X, Cui J, Ni Z, Cheng W, Qian G, Zhang J, Tu H. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma. Clin Chim Acta. 2013;423:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Bazin HG, Marques MA, Owens AP, Linhardt RJ, Crutcher KA. Inhibition of apolipoprotein E-related neurotoxicity by glycosaminoglycans and their oligosaccharides. Biochemistry. 2002;41:8203-8211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Lin YL, Lei HY, Lin YS, Yeh TM, Chen SH, Liu HS. Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antiviral Res. 2002;56:93-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Pradel G, Garapaty S, Frevert U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol Microbiol. 2002;45:637-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, Van Kuppevelt TH, Depla E. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003-41012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 368] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 47. | Vongchan P, Warda M, Toyoda H, Toida T, Marks RM, Linhardt RJ. Structural characterization of human liver heparan sulfate. Biochim Biophys Acta. 2005;1721:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Vongchan P, Linhardt RJ. Expression of human liver HSPGs on acute myeloid leukemia. Clin Immunol. 2007;122:194-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Vongchan P, Kothan S, Wutti-In Y, Linhardt RJ. Inhibition of human tumor xenograft growth in nude mice by a novel monoclonal anti-HSPG isolated from human liver. Anticancer Res. 2011;31:4067-4074. [PubMed] [Cited in This Article: ] |
| 50. | Vongchan P, Wutti-In Y, Sajomsang W, Gonil P, Kothan S, Linhardt RJ. N,N,N-Trimethyl chitosan nanoparticles for the delivery of monoclonal antibodies against hepatocellular carcinoma cells. Carbohydr Polym. 2011;85:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Zaghloul RA, Al-Gayyar MM, El-Shishtawy MM, Ebrahim MA. Cytotoxic effects of antiglypican-3 against HepG2 cell line. J App Pharm Sci. 2013;3:5. [DOI] [Cited in This Article: ] |
| 52. | Stenmark H, Aasland R, Toh BH, D’Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048-24054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 367] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjørkøy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 54. | Pankiv S, Johansen T. FYCO1: linking autophagosomes to microtubule plus end-directing molecular motors. Autophagy. 2010;6:550-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Motomura Y, Senju S, Nakatsura T, Matsuyoshi H, Hirata S, Monji M, Komori H, Fukuma D, Baba H, Nishimura Y. Embryonic stem cell-derived dendritic cells expressing glypican-3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16-F10. Cancer Res. 2006;66:2414-2422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Gonzalez AD, Kaya M, Shi W, Song H, Testa JR, Penn LZ, Filmus J. OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner. J Cell Biol. 1998;141:1407-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Huber R, Hansen RS, Strazzullo M, Pengue G, Mazzarella R, D’Urso M, Schlessinger D, Pilia G, Gartler SM, D’Esposito M. DNA methylation in transcriptional repression of two differentially expressed X-linked genes, GPC3 and SYBL1. Proc Natl Acad Sci USA. 1999;96:616-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Marquez BV, Zheleznyak A, Lapi SE. Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma: where antibody imaging dares to tread. J Nucl Med. 2014;55:708-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Khan S, Blackburn M, Mao DL, Huber R, Schlessinger D, Fant M. Glypican-3 (GPC3) expression in human placenta: localization to the differentiated syncytiotrophoblast. Histol Histopathol. 2001;16:71-78. [PubMed] [Cited in This Article: ] |