Published online Jul 7, 2011. doi: 10.3748/wjg.v17.i25.3002
Revised: December 23, 2010
Accepted: December 30, 2010
Published online: July 7, 2011
AIM: To investigate whether cisplatin (DDP) enhances the anti-tumor activity of cytokine- induced killer (CIK) cells in a murine colon adenocarcinoma model.
METHODS: Tumor size and weight served as indicators of therapeutic response. Immunohistochemistry was performed to observe intratumoral lymphocyte infiltration and tumor microvessel density. Changes in the percentage of regulatory T (Treg) cells within the spleens of tumor-bearing mice preconditioned with DDP were monitored using flow cytometry.
RESULTS: A marked T cell-dependent, synergistic anti-tumor effect of the combined therapy was observed (1968 ± 491 mm3 vs 3872 ± 216 mm3; P = 0.003). Preconditioning chemotherapy with DDP augmented the infiltration of CD3+ T lymphocytes into the tumor mass and reduced the percentage of both intratumoral and splenic Treg cells.
CONCLUSION: Preconditioning with DDP markedly enhances the efficacy of adoptively transferred CIK cells, providing a potential clinical modality for the treatment of patients with colorectal cancer.
- Citation: Huang X, Chen YT, Song HZ, Huang GC, Chen LB. Cisplatin pretreatment enhances anti-tumor activity of cytokine-induced killer cells. World J Gastroenterol 2011; 17(25): 3002-3011
- URL: https://www.wjgnet.com/1007-9327/full/v17/i25/3002.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i25.3002
Colorectal cancer is one of the most common malignancies in the world[1]. Despite advances in surgery, chemotherapy and radiotherapy, the prognosis of the patients with advanced colorectal cancer remains poor[2,3]. Therefore, new and effective treatment modalities, such as immunotherapy, are urgently needed.
Cytokine-induced killer (CIK) cells are ex vivo-expanded T lymphocytes that share phenotypic and functional properties with both natural killer (NK) and T cells[4]. CIK therapy is a promising approach for the treatment of a broad array of malignant hematopoietic diseases and solid tumors[4-8]. However, clinical trials in CIK therapy did not show any noticeable improvement in cure rates or long-term survival[6-8], suggesting that the treatment needs to be refined to maximize its efficacy.
Recent advances in molecular immunology have unmasked the crucial mechanisms that inhibit anti-tumor immune responses in vivo. In particular, regulatory T (Treg) cells, which are a distinct lymphocyte lineage that inhibits both adaptive and innate immunity[9], have received a great deal of attention. Treg cells can also hinder the anti-tumor activity of CIK cells[10,11]. Thus, strategies aimed at depleting Treg cells may increase the efficacy of CIK cells.
A number of studies have shown that some chemotherapeutic agents, in addition to their direct cytotoxic effects on tumor cells, possess the ability to modulate anti-tumor immune responses[12,13]. Cisplatin (DDP) is one of the conventional anticancer agents endowed with immunomodulating features. It can sensitize tumor cells to lysis by NKG2D-expressing lymphocytes by up-regulating the expression of the NKG2D ligand (NKG2DL) on tumor cells[14]. It may also increase the vulnerability of tumor cells to Fas ligand (FasL)-positive immune effectors by increasing Fas expression on the targets[15]. However, there have been no studies characterizing the potential suppressive effects of DDP on Treg cells.
In this study, to investigate whether DDP can enhance the anti-tumor activity of CIK cells, we used a combined therapy consisting of pretreatment with DDP followed by adoptive CIK therapy in a murine colon adenocarcinoma model. A marked T cell-dependent synergistic anti-tumor effect was observed. Preconditioning chemotherapy with DDP also increased the infiltration of CD3+ T lymphocytes into the tumor mass and reduced the percentage of both intratumoral and splenic Treg cells, suggesting a potential mechanism underlying the immunostimulatory capacity of DDP. These results provide an immunological rationale for the combined chemoimmunotherapy and suggest a potential clinical modality for the treatment of patients with colorectal cancer.
BALB/c wild type (WT) and BALB/c nu/nu male mice were purchased from the Chinese Academy of Military Medical Sciences (Beijing, China), and those at 6-8 wk of age were used for the experiment. All mice were maintained at controlled temperature and humidity, with a 12 h light-dark cycle, and sterile food and water ad libitum. The animal studies were conducted in accordance with the Animal Experiment Guidelines of the Ethics Committee of Jingling Hospital.
Murine CT-26 colon adenocarcinoma cells were obtained from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China) and maintained in cRPMI-1640 (Hyclone, Waltham, MA, USA) supplemented with 10% fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Murine CIK cells were obtained as previously described[16]. Briefly, spleen single cell suspensions were prepared from BALB/c WT mice and enriched for lymphocytes by Ficoll-Hypaque (Beijing Chemical Reagents Company, Beijing, China) density gradient centrifugation. Cells were then resuspended in cRPMI-1640 medium supplemented with 1000 U/mL interferon γ (PEPROTECH, Rocky Hill, NJ, USA) on the first day of culture. After 24 h, interleukin-2 (PEPROTECH, Rocky Hill, NJ, USA) and an anti-CD3 antibody (eBioscience, San Diego, CA, USA) were added at 500 U/mL and 50 ng/mL, respectively. Thereafter, cRPMI-1640 supplemented with interleukin-2 (300 U/mL) was added every other day for two weeks and to generate CIK cells.
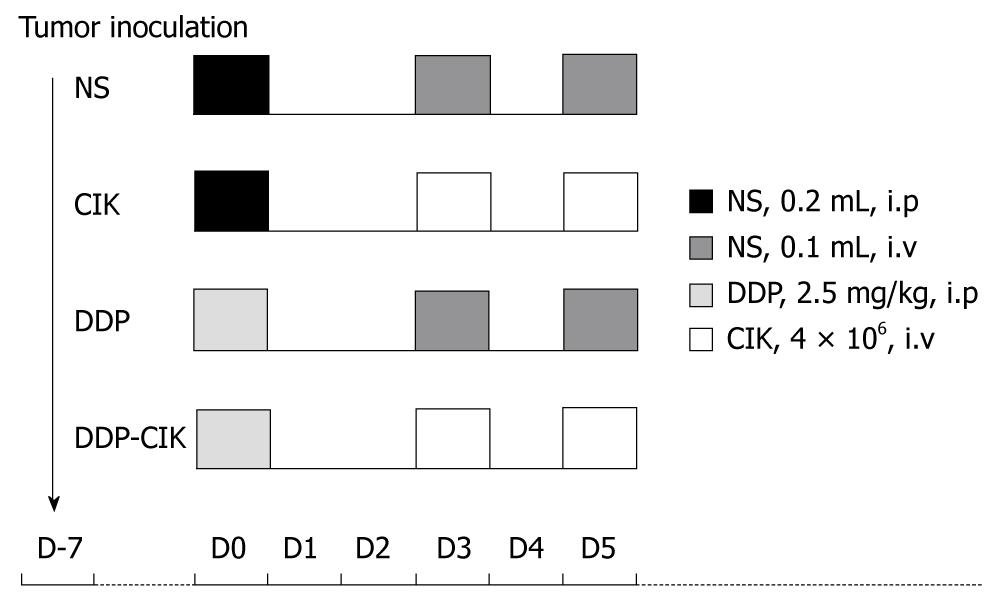
CT-26 cells (1 × 106/100 mL phosphate buffered saline) were subcutaneously inoculated into the right flank of BALB/c WT and BALB/c nu/nu mice. When tumors became approximately 5 mm in mean diameter, animals were randomly divided into four groups, five in each group, and subjected to the corresponding treatment. The preconditioning chemotherapy used in this study was a single intraperitoneal injection of 2.5 mg/kg DDP; and adoptive immunotherapy consisted of two intravenous transfusions of CIK cells at a 1-d interval (4 × 106 cells per dose in a total volume of 100 mL). The treatment scheme of each group is shown in Figure 1 and the detailed grouping was as follows: (1) Group normal saline (NS), treated with normal saline; (2) Group CIK, treated with CIK cells alone; (3) Group DDP, treated with DDP alone; and (4) Group DDP-CIK, preconditioned with DDP followed by transfusion of CIK cells. The tumor size (mm) was measured every other day using a caliper and tumor volume was calculated as: 0.5 × length × width2.
Twenty days after the treatment, the tumor mass was excised, fixed in 10% formalin, embedded in paraffin and sectioned at 3 μm for histological and immunohistochemical studies. Anti-CD3 (1:500, rat monoclonal, Abcom, Cambridge, MA, USA), anti-FoxP3 (1:1000, rabbit polyclonal, Abcom, Cambridge, MA, USA) and anti-CD31 antibodies (1:100, rat polyclonal, Abcom, Cambridge, MA, USA) were used for immunostaining. All procedures were carried out according to the manufacturer’s instructions. Images of the sections were acquired using an Olympus BX-60 microscope to determine the CD3+, FoxP3+ or CD31+ cell density. The number of positive cells was counted in 10 independent fields (0.16 mm2 at ×400 magnification) within each section by two independent observers.
Spleen single cell suspensions were prepared from untreated or DDP-pretreated tumor-bearing mice at the indicated time points and enriched for lymphocytes using Ficoll-Hypaque density gradient centrifugation. A mouse regulatory T cell staining kit (eBioscience, San Diego, CA, USA) was used to determine the percentage of Treg cells. All operations were performed according to the manufacturer’s instructions. Phenotypic analysis of splenocytes was performed using a FACSCalibur (BD Biosciences, San Jose, CA, USA). Splenic lymphocytes were gated by plotting forward vs side scatter and then by the expression of CD4 and CD25. CD4+CD25hi T cells were further analyzed for expression of FoxP3. Ten thousands gated events were collected and analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA). The following conjugated antibodies were used: PE-conjugated anti-FoxP3, FITC-conjugated anti-CD4, APC-conjugated anti-CD25, and isotype-matched controls (eBioscience, San Diego, CA, USA).
Differences between groups were compared using ANOVA, and LSD was used for multiple mean comparisons. A P value < 0.05 was considered significant. Statistical analysis was conducted using SPSS software v13.0 (SPSS Inc., Chicago, Illinois, USA).
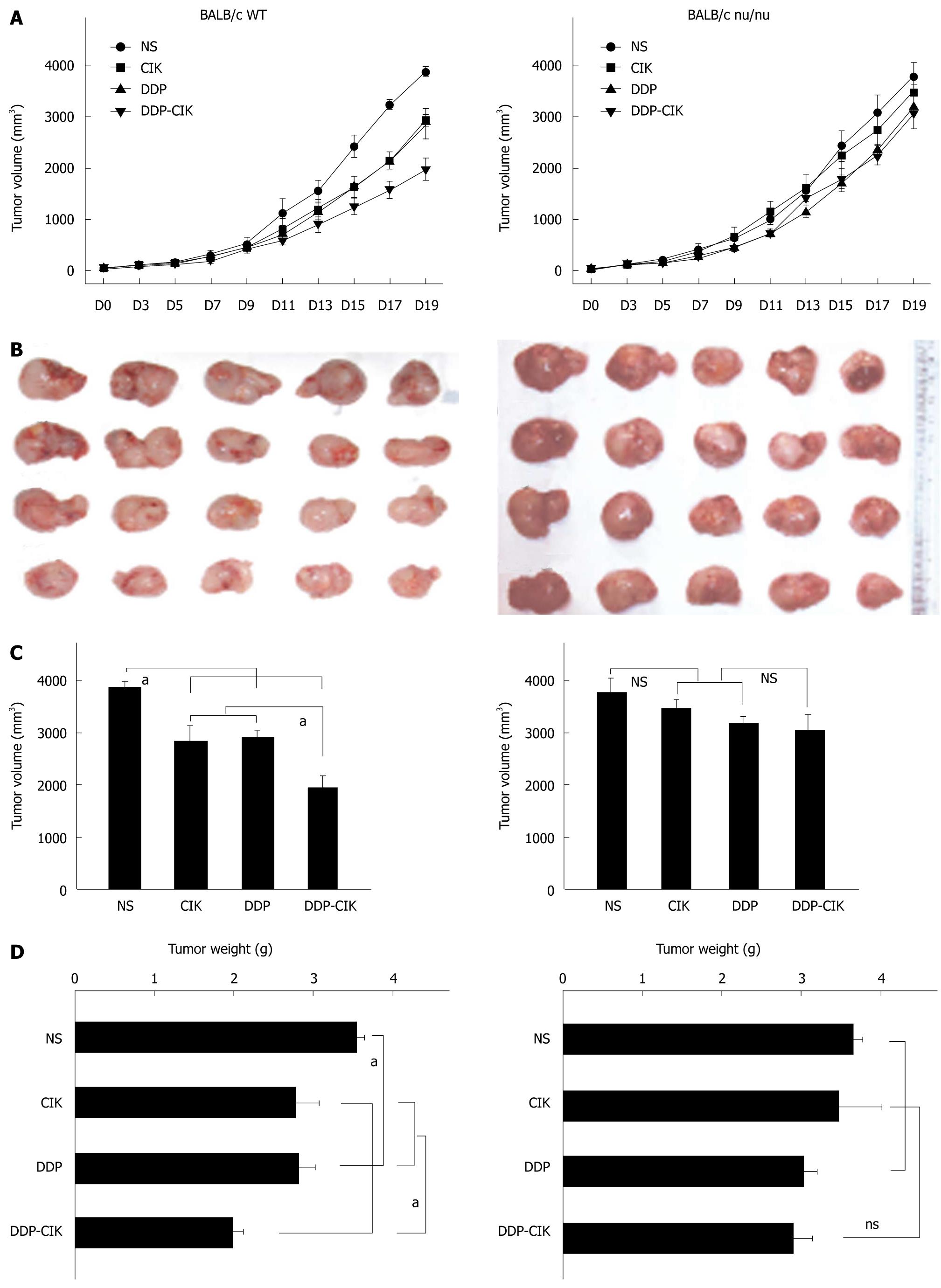
To investigate whether DDP pretreatment enhanced the anti-tumor activity of CIK therapy, CT-26 carcinoma-bearing BALB/c WT mice were injected i.p. with DDP and then i.v. with CIK cells. Tumor size change was monitored every other day throughout experiment (Figure 2A, left panel). On Day 19, the tumor mass was isolated (Figure 2B, left panel). Treatment with either DDP or CIK cells alone inhibited tumor growth compared with the NS control (Figure 2, left panel). However, a significantly greater inhibition of tumor growth was observed after the combined therapy in terms of tumor volume (Figure 2C, left panel) and tumor weight (Figure 2D, Left panel) compared with that seen in the single regimen or the NS control.
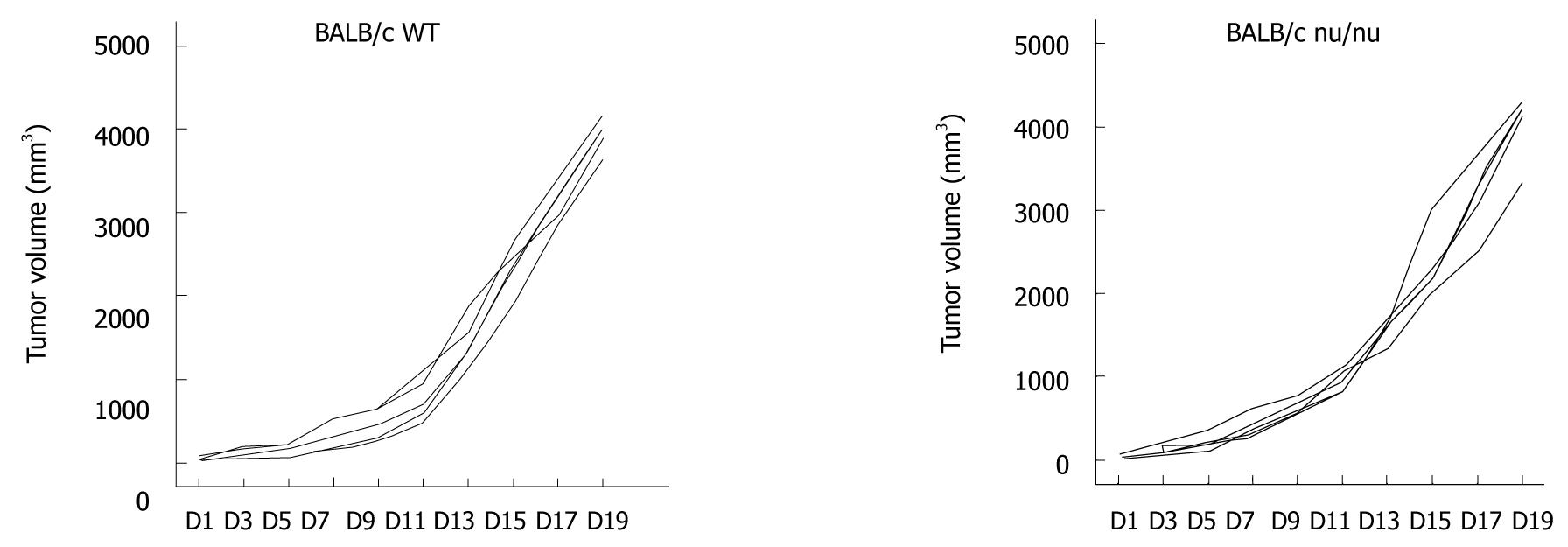
Previous studies showed that an intact immune system is essential for the immunostimulatory anti-tumor effects of chemotherapeutic agents[17,18]. To examine the mechanisms by which DDP treatment increased the efficacy of CIK therapy, the combined treatment protocol (DDP pretreatment plus CIK therapy) was also evaluated in a CT-26 carcinoma-bearing nude mouse model (Figure 2, right panel). With no treatment, the intrinsic tumor growth pattern in nude mice was similar to that in WT mice (Figure 3). In the therapeutic setting, tumor volume was monitored every other day (Figure 2A, right panel) up until Day 19, when the tumor mass was isolated (Figure 2B, right panel). DDP treatment efficiently inhibited tumor growth in WT mice (Figure 2, left panel) but showed only minor inhibitory effects on tumor growth in nude mice (Figure 2, right panel). In addition, CIK therapy alone did not inhibit tumor growth (Figure 2, right panel) when compared with the NS control. Moreover, no synergy between DDP treatment and CIK therapy was observed in the nude mice (Figure 2, right panel).
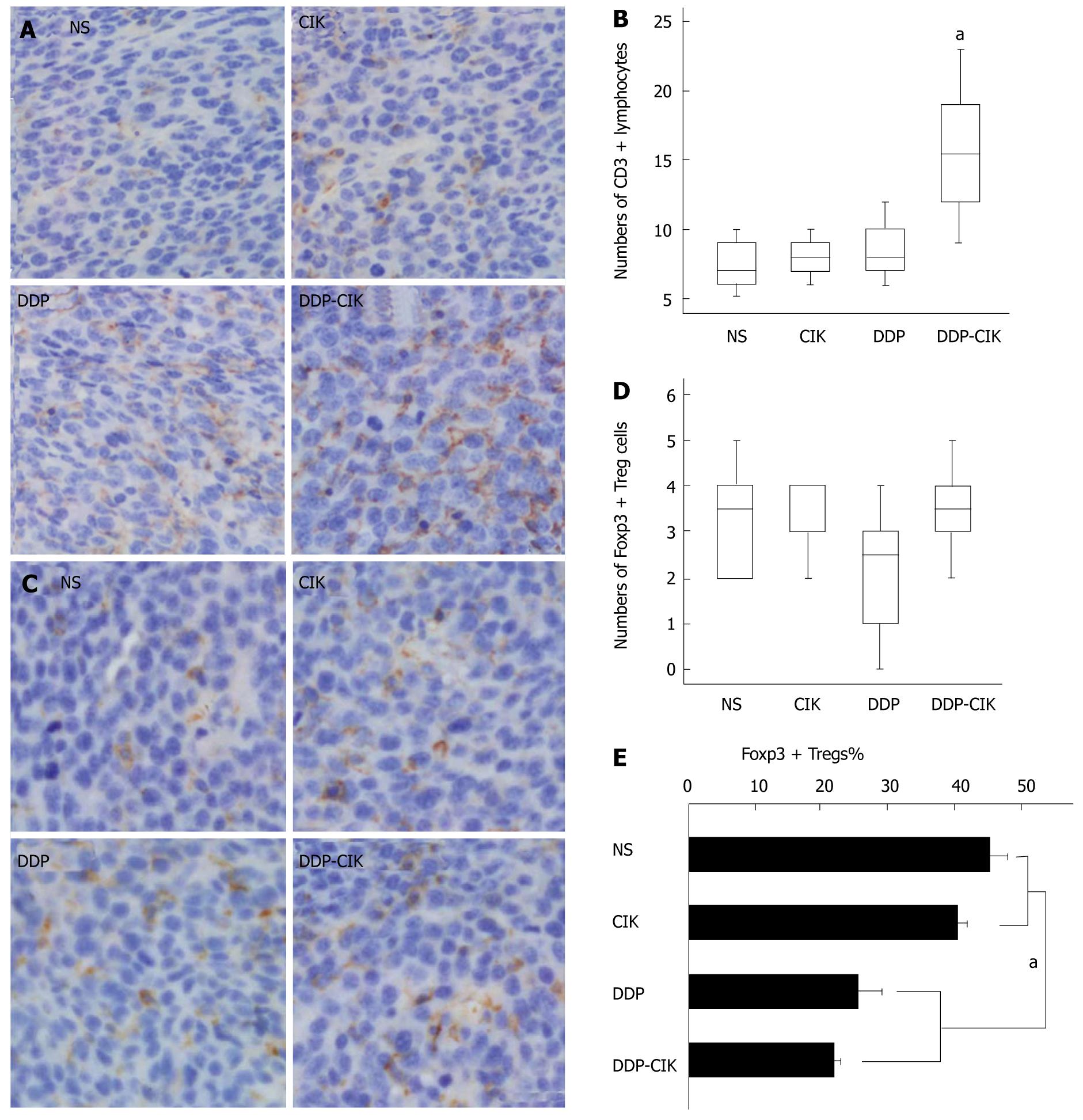
Since the synergistic anti-tumor effects of the combined therapy rely on the presence of T lymphocytes, we analyzed the intra-tumoral accumulation of lymphocytes. Tumor tissues from all the experimental groups were removed on Day 19 and CD3 was used as a specific marker for counting T lymphocytes (Figure 4A). Tumor tissues from untreated hosts were infiltrated by a small number of CD3+ T lymphocytes, and DDP or CIK treatment alone only slightly increased the density of intratumoral CD3+ T lymphocytes, but this was not significant. In contrast, DDP pretreatment combined with CIK therapy reversed this phenomenon, significantly enhancing the influx of CD3+ T lymphocytes into the tumor parenchyma (Figure 4B). This was consistent with the marked retardation of tumor growth seen in the combined treatment group.
Because some chemotherapeutic agents selectively eliminate Treg cells[19,20], we tested whether DDP possessed this Treg-reducing immunostimulatory effect. We examined the changes in intra-tumoral Treg cell numbers in mice treated with CIK, DDP or combination therapy on Day 19. Nuclear transcription factor forkhead box protein P3 (FoxP3), the most specific Treg cell marker identified to date, was used to label Treg cells infiltrating the tumor (Figure 4C). The number of Treg cells, as assessed by the density of intra-tumoral FoxP3+ cells, was not significantly different among the four groups (Figure 4D). However, due to the degree of lymphocyte infiltration into the tumor mass, it may be not accurate to determine the actual level of intratumoral Treg cells using the absolute number of FoxP3+ cells. Therefore, two consecutive sections from each tumor sample were prepared and stained for CD3 and FoxP3, respectively. The percentage of Treg cells, represented as the ratio of FoxP3+ lymphocytes to CD3+ lymphocytes, was calculated to determine the adjusted level of Treg cells within the tumor microenvironment. We found that the percentage of intratumoral Treg cells in Group DDP and Group DDP-CIK was significantly reduced compared with that in Group NS and Group CIK (Figure 4E). This suggests that the systemic administration of DDP locally reduced the percentage of Treg cells in the tumor mass.
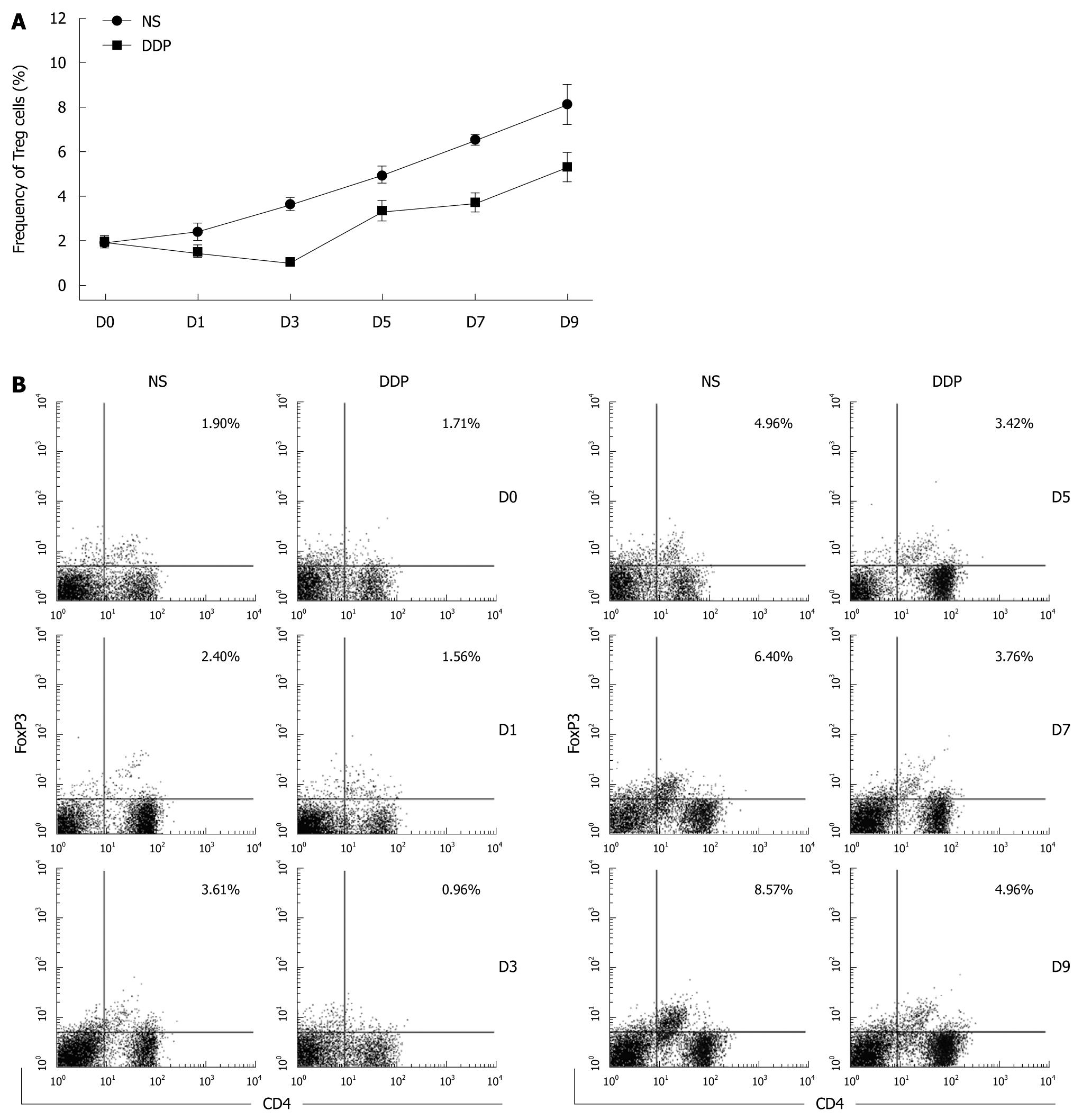
To profile the kinetics of Treg accumulation in the spleens of CT-26 carcinoma-bearing mice after DDP treatment, splenic lymphocytes were obtained at various time points and analyzed by flow cytometry. In tumor-free mice, the percentage of Treg cells in spleen was approximately 2% (data not shown). In the absence of intervention, the level of Treg cells in the tumor-bearing hosts increased in line with increasing tumor burden (Figure 5A and B). After DDP administration, the percentage of Treg cells declined at all time points compared with that in untreated mice (Figure 5A and B), suggesting that DDP significantly reduced the percentage of splenic Treg cells. We monitored the duration of Treg depletion induced by DDP, and found that the nadir was around Day 3 after treatment. Treg cell numbers then rebounded and expanded, following the pattern of tumor growth (Figure 5A and B). However, the number of Treg cells in tumor-bearing mice receiving DDP treatment was consistently lower than that in untreated mice throughout the period of observation.
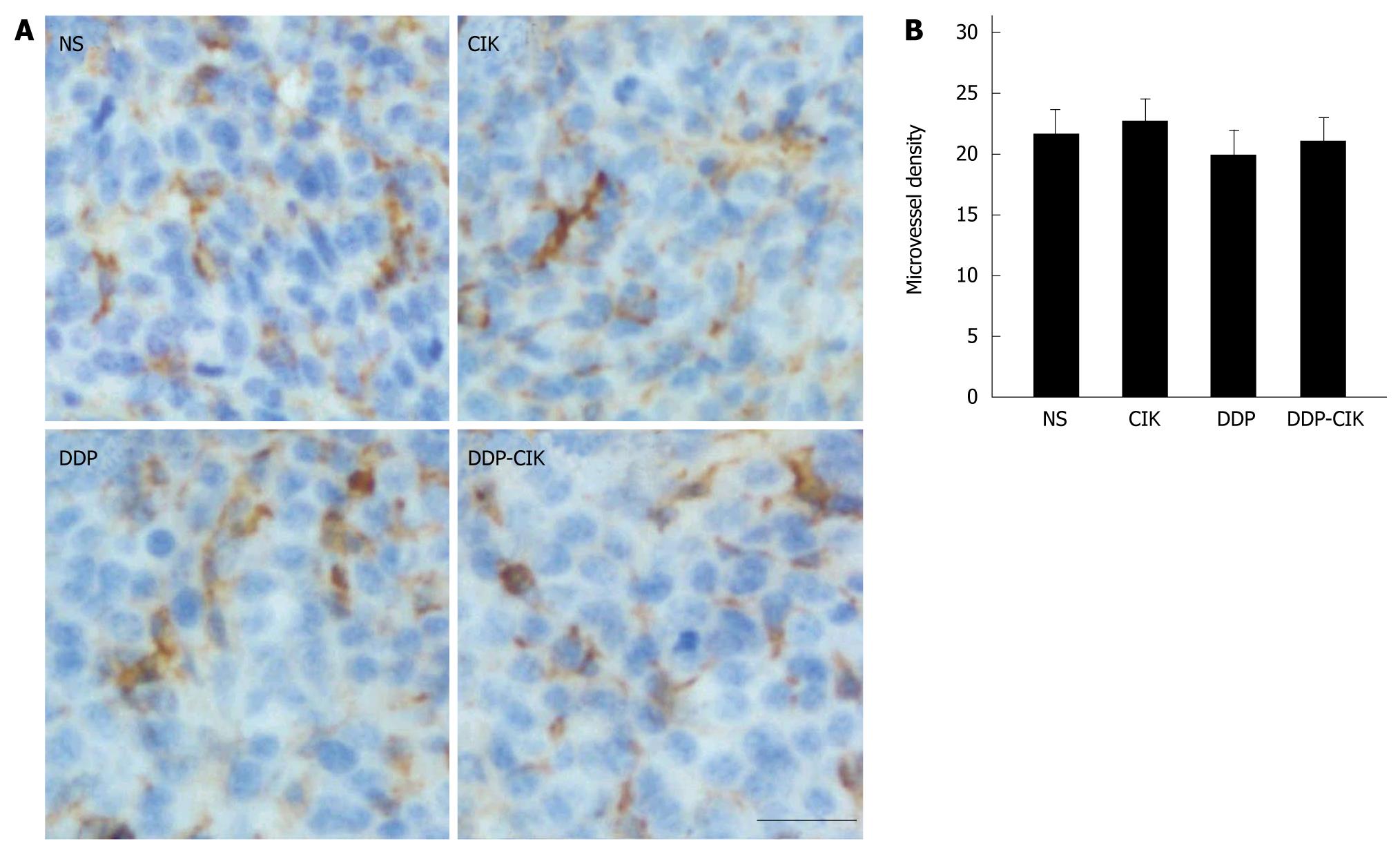
Chemotherapeutic agents have been shown to exert toxic effects on the endothelium of the growing vasculature[21]. Therefore, we tested whether, in addition to its action on the immune system, DDP possessed antiangiogenic effects. To this end, we compared the tumor microvasculature in all the experimental groups by determining microvessel density (MVD). The MVD within the tumor tissues was estimated from tumor sections stained with an antibody to CD31 (Figure 6A) and quantified as described in Materials and Methods. We found that the MVD was comparable among all four groups (Figure 6B), suggesting that DDP had no toxic effect on the tumor microvasculature in this model.
Immunosuppression is a significant obstacle to the generation of effective anti-tumor immunity. During tumor progression, tumor cells foster a tolerant and resistant microenvironment by employing various immunosuppressive mechanisms[22]. It is now clear that successful cancer immunotherapy will be achieved only after the removal of immunity-hampering barriers. Previous studies showed that preconditioning a host with immunomodulating chemotherapy can effectively augment the anti-tumor effects of adoptively transferred effectors[23]. In the present study, DDP pretreatment in combination with adoptive CIK therapy was tested in a murine model of colon adenocarcinoma. Our data showed a marked synergy between DDP chemotherapy and CIK immunotherapy in treating established CT-26 carcinomas in immunocompetent mice, suggesting that a single dose of DDP was sufficient to “groom” the immune system, eventually enhancing the efficacy of subsequent CIK therapy.
A number of murine tumor models show that eradication of tumors requires T cells[17,18,24]. In accordance with these studies, the synergic anti-tumor effect in our study was not evident in the BALB/c nu/nu mice which lack T cells, implying that T lymphocytes were the effectors in our model of anti-tumor therapeutic synergy. The natural growth pattern of CT-26 carcinomas in untreated nude mice was similar with that in immune replete WT mice, suggesting that “default” endogenous immunity was not able to prevent the growth of tumors, or to eradicate established tumors. Furthermore, DDP treatment efficiently inhibited the growth of CT-26 carcinomas in WT mice, but failed to induce tumor growth retardation in the absence of T lymphocytes. This is in line with previous findings suggesting that inhibition of tumor growth by chemotherapeutic agents is strictly dependent on T cells[17,18,24]. Based on these results, we proposed that most of the anti-tumor effect seen in DDP could be attributed to its immunostimulating capacity, rather than to any direct cytotoxicity against tumor cells. Similarly, the efficacy of CIK therapy was observed only in immune-replete hosts, implying that endogenous T lymphocytes participate in the fight against tumor cells by assisting, or co-operating with, exogenously infused CIK cells. Therefore, in T lymphocyte-deficient nude mice, neither DDP pretreatment nor CIK therapy could induce tumor shrinkage. Moreover, preconditioning with DDP lost its capacity to enhance the effect of CIK therapy in the nude mouse model, and the synergy seen with combination therapy was abrogated. We speculate that endogenous T lymphocytes comprise both pro-tumor and anti-tumor subpopulations. The anti-tumor subpopulation collaborates with CIK cells to inhibit tumor growth, and is essential for the effect of CIK therapy. Also, the protumor subgroup is suppressed by DDP, whereas the anti-tumor subgroup is stimulated by DDP, leading to DDP-induced suppression of tumor growth and increased CIK efficacy.
Because of the important effector role played by T lymphocytes in anti-tumor therapeutic synergy, the intratumoral accumulation of T lymphocytes was observed. Our data showed that, compared with the NS control and single therapy alone, the combination therapy significantly augmented the number of CD3+ T lymphocytes infiltrating into the tumor mass, which correlated well with the inhibition of tumor growth.
However, as mentioned above, T lymphocytes comprise both protumor and antitumor subpopulations. Of the protumor subpopulations, Treg cells were mainly involved in tumor-induced immunosuppression. Treg cells are CD4+CD25+ T lymphocytes with the ability to suppress anti-tumor immune response. These cells accumulate in the peripheral blood, lymph node and tumors in many human cancers and animal tumor models[25]. Elimination of Treg cells in vivo using cytotoxic agents or antibodies enhances anti-tumor responses and results in tumor regression[26]. In this study, we determined the percentage of Treg cells in the local tumor microenvironment and found that DDP decreased the percentage of intratumoral Treg cells. Based on these results, we propose that the DDP-induced reduction in the percentage of Treg cells could enable the development of an anti-tumor immune response, leading to the retardation of tumor growth.
We also examined the longitudinal changes in the percentage of splenic Treg cells in tumor-bearing mice after DDP treatment. It is noteworthy that, of the several cell types within the spleen, Treg cells were preferentially targeted by DDP. We found that the percentage of splenic Treg cells in pretreated tumor-bearing mice was reduced at all time points compared with that in untreated tumor-bearing hosts, implying that, besides its immunomodulating effect, DDP may also ameliorate systemic immunosuppressive factors by depleting splenic Treg cells. Regarding the DDP-induced dynamic changes in the number of Treg cells, the nadir was around Day 3 after treatment. Interestingly, the time points we chose for the transfer of CIK cells in the combined schedule were Days 3 and 5 after treatment, when the percentage of Treg cells was relatively low. Nevertheless, the percentage of Treg cells seemed to rebound and rise quickly on Day 5 after treatment. Therefore, CIK cells infused on Day 5 may have encountered a more “hostile” environment and were unable to exert their anti-tumor effects. Further studies are needed to determine the optimal schedule for combined therapy which triggers the maximal synergistic anti-tumor effects.
In addition to the immunosuppressive factors in vivo, angiogenesis, a pivotal process in tumor growth and metastasis, also plays a role in the failure of cellular immunotherapy. Angiogenesis suppresses the expression of the endothelial cell (EC) adhesion molecules involved in leukocyte adhesion to blood vessel walls, and that inhibition of angiogenesis may increase leukocyte-vessel wall interactions and the subsequent infiltration of lymphocytes into the tumor mass[27]. Some chemotherapeutic agents are characterized by their antiangiogenic effect when administered at small doses on a frequent schedule (sometimes referred to as metronomic chemotherapy). Metronomic administration of DDP showed toxic effects on the tumor microvasculature[28]. However, in this study, a single dose of DDP was used as the pretreatment therapy and we did not observe any antiangiogenic effect, ruling out any putative anti-angiogenic effect of DDP in this model.
In conclusion, our data showed that DDP pretreatment acted synergistically with CIK therapy to efficiently inhibit tumor growth in a murine colon adenocarcinoma model. This anti-tumor synergy was T lymphocyte-dependent. Preconditioning with DDP enhanced the infiltration of CD3+ T lymphocytes into the tumors and reduced the percentage of both intratumoral and splenic Treg cells, revealing a potential mechanism underlying the immunostimulatory effects of DDP. In summary, the results of this study provide a potential combination regimen incorporating preconditioning chemotherapy and adoptive CIK therapy for the treatment of colon cancer.
Immune suppression constitutes a large obstacle to hinder the generation of effective anti-tumor immunity. It now becomes clear that successful cancer immunotherapy can be achieved only after the removing of immunity-hampering barriers. A number of studies have shown that preconditioning a host with immunomodulating chemotherapy can effectively augment the anti-tumor efficacy of adoptively transferred effectors.
Regulatory T (Treg) cells, a distinct lymphocyte lineage inhibiting both adaptive and innate immunity, were mainly involved in tumor-induced immunosuppression. Elimination of Treg cells in vivo using agents targeting Treg cells such as cytotoxic agents or antibodies have been shown to enhance the anti-tumor responses, resulting in tumor regression.
To our knowledge, this is the first study to evaluate the potential synergy between Cisplatin (DDP) pretreatment and subsequent adoptive cytokine-induced killer cells (CIK) therapy in treatment of colon cancer. A dramatic T cell-dependent synergistic anti-tumor effect of the combination therapy was revealed in the model established in this study. Preconditioning chemotherapy with DDP could augment the infiltration of CD3+ T lymphocytes into the tumor and diminish the percentages of both intra-tumoral and splenic Treg cells, thus improving the anti-tumor effect of CIK therapy.
This is a potential combination regimen incorporating the preconditioning chemotherapy to the adoptive CIK therapy for the patients with colorectal cancer.
Preconditioning chemotherapy: Chemotherapy administered at a special dose and time point to modulate the host immune environment, thus providing a better ground for the subsequent adoptive cell therapy.
How did the authors come upon the timeline for preconditioning and timing of sacrifice? Especially with the curves demonstrated in Figure 3, are we catching this too early? In Figure 3, it still appears that the slope of all of the curves continues to go up. As such, is this an initial impediment to grow more slowly but the end result is no difference? This is especially in light of the clinical trials that demonstrate no improvement in cure rate or long-term survival.
Peer reviewer: Scott Steele, MD, FACS, FASCRS,Chief, Colon and Rectal Surgery, Deptartment of Surgery, Madigan Army Medical Center, Fort Lewis, WA 98431, United States
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177-2190. [Cited in This Article: ] |
| 2. | Chau I, Cunningham D. Treatment in advanced colorectal cancer: what, when and how? Br J Cancer. 2009;100:1704-1719. [Cited in This Article: ] |
| 3. | Shapira S, Lisiansky V, Arber N, Kraus S. Targeted immunotherapy for colorectal cancer: monoclonal antibodies and immunotoxins. Expert Opin Investig Drugs. 2010;19:S67-977. [Cited in This Article: ] |
| 4. | Nishimura R, Baker J, Beilhack A, Zeiser R, Olson JA, Sega EI, Karimi M, Negrin RS. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563-2574. [Cited in This Article: ] |
| 5. | Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, Dander E, Gaipa G, D’Amico G, Biagi E. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;927:952-959. [Cited in This Article: ] |
| 6. | Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237-2242. [Cited in This Article: ] |
| 7. | Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997-4002. [Cited in This Article: ] |
| 8. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [Cited in This Article: ] |
| 9. | Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627-629. [Cited in This Article: ] |
| 10. | Li H, Yu JP, Cao S, Wei F, Zhang P, An XM, Huang ZT, Ren XB. CD4 +CD25 + regulatory T cells decreased the antitumor activity of cytokine-induced killer (CIK) cells of lung cancer patients. J Clin Immunol. 2007;27:317-326. [Cited in This Article: ] |
| 11. | Schmidt J, Eisold S, Büchler MW, Märten A. Dendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinoma. Cancer Immunol Immunother. 2004;53:1018-1126. [Cited in This Article: ] |
| 12. | Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545-557. [Cited in This Article: ] |
| 13. | Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59-73. [Cited in This Article: ] |
| 14. | Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186-1190. [Cited in This Article: ] |
| 15. | Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997;89:783-789. [Cited in This Article: ] |
| 16. | Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923-2931. [Cited in This Article: ] |
| 17. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [Cited in This Article: ] |
| 18. | Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170-1178. [Cited in This Article: ] |
| 19. | Golovina TN, Vonderheide RH. Regulatory T cells: overcoming suppression of T-cell immunity. Cancer J. 2010;16:342-347. [Cited in This Article: ] |
| 20. | Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S. Reduced percentages and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018-2025. [Cited in This Article: ] |
| 21. | Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423-436. [Cited in This Article: ] |
| 22. | Bronte V, Mocellin S. Suppressive influences in the immune response to cancer. J Immunother. 2009;32:1-11. [Cited in This Article: ] |
| 23. | Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346-2357. [Cited in This Article: ] |
| 24. | Wang LX, Shu S, Plautz GE. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65:9547-9554. [Cited in This Article: ] |
| 25. | Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746-1752. [Cited in This Article: ] |
| 26. | Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880-887. [Cited in This Article: ] |
| 27. | Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621-630. [Cited in This Article: ] |
| 28. | Tan GH, Tian L, Wei YQ, Zhao X, Li J, Wu Y, Wen YJ, Yi T, Ding ZY, Kan B. Combination of low-dose cisplatin and recombinant xenogeneic endoglin as a vaccine induces synergistic antitumor activities. Int J Cancer. 2004;112:701-706. [Cited in This Article: ] |