Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1968
Peer-review started: November 5, 2020
First decision: December 24, 2020
Revised: December 30, 2020
Accepted: January 22, 2021
Article in press: January 22, 2021
Published online: March 16, 2021
Processing time: 120 Days and 3.6 Hours
Acquired pure red cell aplasia (aPRCA) related to human parvovirus B19 (HPV B19) is rarely reported in simultaneous pancreas-kidney transplantation (SPKT) recipients; there has yet to be a case report of early postoperative infection. In this current study, we report the case of a Chinese patient who experienced the disease in the early postoperative period.
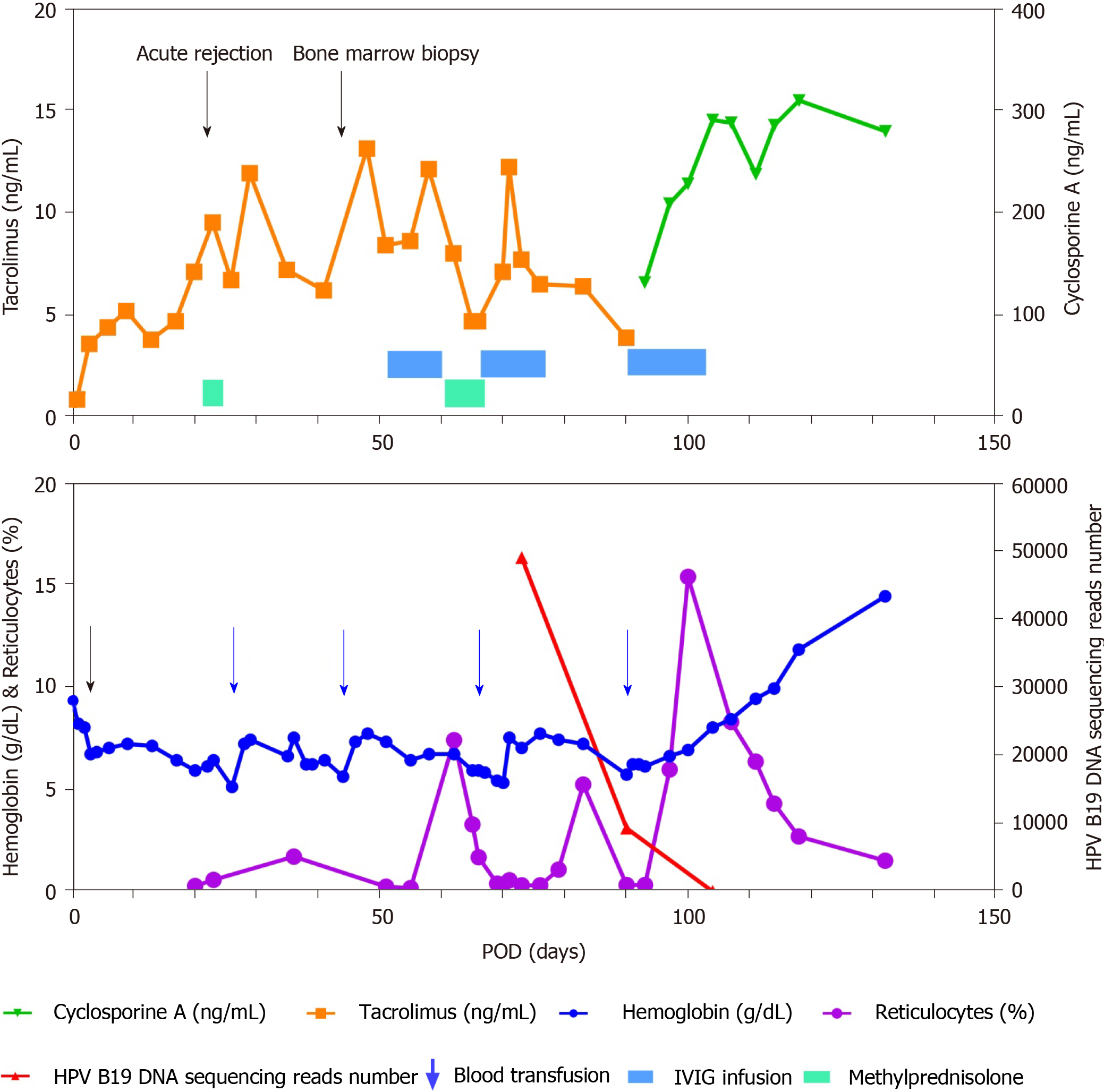
A 63-year-old man, with type 2 diabetes and end-stage renal disease, received a brain dead donor-derived SPKT. Immunosuppression treatment consisted of tacrolimus, prednisone, enteric-coated mycophenolate sodium (EC-MPS), and thymoglobulin combined with methylprednisolone as induction. The hemoglobin (Hb) level declined due to melena at postoperative day (POD) 3, erythropoietin-resistant anemia persisted, and reticulocytopenia was diagnosed at POD 20. The bone marrow aspirate showed decreased erythropoiesis and the presence of giant pronormoblasts at POD 43. Metagenomic next-generation sequencing (mNGS) of a blood sample identified HPV B19 infection at POD 66. EC-MPS was withdrawn; three cycles of intravenous immunoglobulin (IVIG) infusion therapy were administered; and tacrolimus was switched to cyclosporine. The HPV B19-associated aPRCA resolved completely and did not relapse within the 1-year follow-up period. The diminution in mNGS reads was correlated with Hb and reticulocyte count improvements.
HPV B19-associated aPRCA can occur at an early period after SPKT. An effective therapy regimen includes IVIG infusion and adjustment of the immuno-suppressive regimen. Moreover, mNGS can be used for the diagnosis and to reflect disease progression.
Core Tip: This study presents a patient who developed human parvovirus (HPV) B19-associated acquired pure red cell aplasia (aPRCA) in the early postoperative period following simultaneous pancreas-kidney transplantation. Metagenomic next-generation sequencing (mNGS) was used to diagnose HPV B19 infection and the diminution in mNGS reads was correlated with hemoglobin and reticulocyte count improvements. Effective therapy included intravenous immunoglobulin infusion and adjustment of the immunosuppressive regimen. HPV B19-associated aPRCA should be considered when a patient experiences erythropoietin-resistant anemia and reticulocytopenia in the early postoperative period. Moreover, mNGS could be used for diagnosis and to reflect disease progression.
- Citation: Wang H, Fu YX, Song WL, Wang Z, Feng G, Zhao J, Nian YQ, Cao Y. Human parvovirus B19-associated early postoperative acquired pure red cell aplasia in simultaneous pancreas-kidney transplantation: A case report. World J Clin Cases 2021; 9(8): 1968-1975
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1968.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1968
Pure red cell aplasia (PRCA) is an anemia caused by a significant reduction in the red blood cell lineage in the bone marrow. PRCA is characterized by severe normal or mild large-size red blood cell anemia, reduced or absent reticulocytes, and a significant reduction or disappearance of red blood cell precursor progenitor cells. Diamond-Blackfan anemia is a congenital PRCA in infancy. Acquired PRCA (aPRCA) can be divided into primary PRCA and secondary PRCA according to the etiology. Human parvovirus B19 (HPV B19) infection is one of the common causes of secondary aPRCA[1], but it is a relatively rare complication in organ transplantation patients. Neild et al[2] first reported a case of HPV B19 infection after organ transplantation in 1986. Simultaneous pancreas-kidney transplantation (SPKT) is the gold standard treatment for end-stage renal disease due to diabetic nephropathy. There are only a few case reports of HPV B19 infections leading to PRCA in the late postoperative period in SPKT patients[3,4]. In this study, we report a case of HPV B19-related aPRCA in the early postoperative period in our center, summarize its clinical characteristics, and review the relevant literature.
Persistent anemia in the early postoperative period after SPKT.
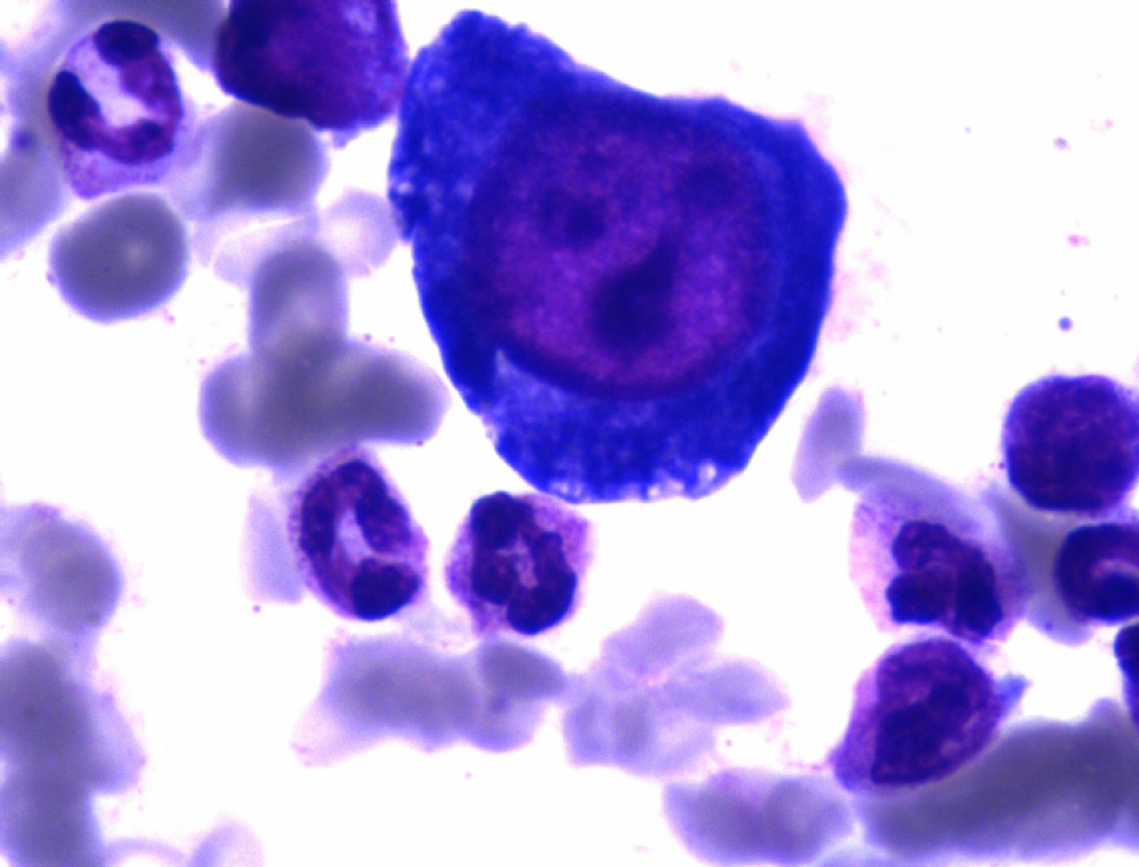
A 63-year-old man with poorly controlled type 2 diabetes mellitus and end-stage renal failure received SPKT in September 2019. The organs were recovered from a 47-year-old man who died from brain injury caused by a traffic accident. HPV B19 immunoglobulin (Ig) M antibodies in the peripheral venous blood, from both the donor and recipient, were negative. Cytomegalovirus (CMV) DNA polymerase chain reaction (PCR) and Epstein-Barr virus (EBV) DNA PCR in the serum of the patient yielded negative results. No blood transfusion was performed during the operation. The patient received induction therapy with antithymocyte globulin for a total dose of 300 mg combined with methylprednisolone for a total dose of 2.5 g. The perioperative antimicrobial prophylaxis included sulperazon and metronidazole. Maintenance immunosuppression consisted of tacrolimus, enteric-coated mycophenolate sodium (EC-MPS), and prednisone. Argatroban was infused for the first two days postoperatively and was withdrawn due to the patient experiencing melena at postoperative day (POD) 3. The hemoglobin (Hb) level declined from 82 g/L to 67 g/L, hemocoagulase was used, and the melena resolved in two days. Blood transfusions were not performed. The Hb level increased above 70 g/L (Figure 1). The dosage of EC-MPS was reduced from 1080 mg/d to 720 mg/d, and erythropoietin was administered at 10 KU twice per week. The kidney and pancreas graft function recovered well within one week, but erythropoietin therapy had no effect on correcting anemia. At POD 20, the patient reported weakness without other discomfort. The reticulocyte count was 0.27% (normal 0.8% to 2.2%), and reticulocytopenia was diagnosed. The Hb level declined to 59 g/L. We withdrew EC-MPS due to severe anemia. At POD 22, the creatinine level increased to 1.61 mg/dL and amylase level increased to 197.2 IU/L with glucostasis. The PRA was negative. Ultrasound of the kidney and pancreas graft was normal. Acute rejection was suspected, but due to lack of sufficient symptoms, kidney biopsy was not performed. Methylprednisolone was given with a total dose of 1.0 g experientially (500 mg for the first day, 250 mg for the second day and third day), and the creatinine level declined to 1.44 mg/dL and blood amylase level declined to 140.5 IU/L. At POD 26, the patient received suspended erythrocyte transfusions (2U) as the Hb level declined to 51 g/L; after the transfusion, the Hb level increased to 74 g/L. At POD 34, the Hb level declined again to 66 g/L, but the reticulocyte count increased to 1.7%. The HPV B19 IgM antibody in serum was negative. The Hb level was maintained at more than 60 g/L. At POD 44, a bone marrow aspirate and biopsy were taken, which showed decreased erythropoiesis and the presence of giant pronormoblasts (Figure 2). PRCA was diagnosed by a hematologist. Suspended erythrocytes (4U) were infused and the Hb level increased to 73 g/L. Treatment with intravenous immunoglobulin (IVIG) at a dose of 25 g/d was started at POD 51 and continued for 10 d. Additionally, methylprednisolone was infused at 80 mg/d for one week. The reticulocyte count then increased to 7.39%, but the Hb level remained low, at approximately 60 g/L. At POD 66, the Hb level declined to 59 g/L, and the reticulocyte count declined to 1.67%. IgM antibody of HPV B19 in serum was negative. Due to lack of experience, we realized that the IgM antibody might be false negative and HPV B19 infection could not be excluded. Blood specimens were sent to BGI PathoGenesis Pharmaceutical Technology (Shenzhen, China) for metagenomic next-generation sequencing (mNGS). Only HPV B19 DNA was detected and the sequencing reads number was 48857. HPV B19-related aPRCA was diagnosed. The evolution of relevant data and treatment over time in this patient are shown in Figure 1.
The patient had undergone cystolithotomy 20 yr previously, he had a history of hypertension for 14 yr and was taking regular felodipine to control his blood pressure. Before SPKT, the patient had a history of hyperglycemia for 11 yr, abnormal renal function for 1 yr, and hemodialysis for 5 mo.
The patient’s height was 179 cm and body weight, 71 kg, with a body mass index of 22.16 kg/m2. Physical examination on admission revealed pale palpebral conjunctiva and hyponychium.
No hypoleukocythemia or thrombocytopenia was detected after the operation. Additionally, the erythropoietin-resistant anemia and reticulocytopenia persisted and the evolution of Hb and reticulocytes are shown in Figure 1. At POD 20, results of direct and indirect Coombs test and laboratory examination of ferritin and transferrin and unsaturated iron-binding capacity were normal. BK virus DNA PCR, CMV DNA PCR and EBV DNA PCR all yielded negative results in the serum. At POD 66, mNGS for pathogens was performed and the sequencing reads number of HPV B19 DNA was 48857 (Figure 1).
At POD 44, a bone marrow aspirate and biopsy were obtained, which showed decreased erythropoiesis and the presence of giant pronormoblasts (Figure 2).
aPRCA and HPV B19 infection.
At POD 66, red cell infusion (4U) was repeated. IVIG at a dose of 25 g/d was infused again for two weeks. The Hb level then increased to 72 g/L and the reticulocyte count increased to 5.23%. However, at POD 90, the Hb level declined to 57 g/L and the reticulocyte count declined to 0.32%. The mNGS was performed again and the HPV B19 DNA sequencing reads number decreased from 48857 to 9269. The patient received a blood transfusion (4U red cell) at POD 90, and tacrolimus was switched to cyclosporine for immunosuppression. At the same time, IVIG at a dose of 25 g/d was infused for two weeks. The Hb level increased gradually and the reticulocyte count increased to 15.35% at POD 100. At POD 104, mNGS was performed again and the HPV B19 DNA sequencing reads number decreased to 5.
At POD 118, the Hb level increased to 118 g/L, and the reticulocyte count declined to 2.69%. EC-MPS was administered again. Thereafter, the patient was discharged and followed up regularly with normal kidney function and normoglycemia until the time of writing this report. No recurrence of anemia has been observed.
There have been only a few reports of HPV B19 infection in cases of pancreas transplants. The onset time of HPV B19-associated PRCA varied from the early to late period after pancreas transplant. In 2005, Onitilo et al[5] reported an isolated pancreas transplant recipient who experienced the disease 6 mo after transplantation; in 2012, Labbadia et al[3] reported a case in which the patient experienced the disease 8 yr after SPKT; in 2020, Nowacka-Cieciura et al[4] reported a case who experienced the disease 8 mo after SPKT. Our patient underwent bone marrow aspirate examination at POD 43, and PRCA was diagnosed. At POD 66, HPV B19 infection was diagnosed by mNGS. However, the patient had experienced erythropoietin-resistant anemia and reticulocytopenia within 20 d after SPKT. Therefore, the time of onset of HPV B19 infection was estimated to be within 20 d after SPKT.
The accurate incidence of HPV B19 infection in organ transplant recipients is unknown. A report showed that 18.75% of Chinese renal transplant patients experienced HPV B19 infection[6]. Transmission of parvovirus B19 can occur via contact with respiratory droplet secretions and rarely through blood products; transmission of parvovirus B19 infection from donor to recipient may occur at the time of transplantation[7]. The route of transmission in our patient could not be identified.
Positive results of specific IgM indicate an acute HPV B19 infection stage. In this case, HPV B19 IgM antibodies in the peripheral venous blood of the donor were negative, but HPV B19 DNA loads of the donor were not examined. Juhl et al[8] believe that the majority of blood products from donors with detectable HPV B19 DNA did not seem to be infectious to the recipients, due to the neutralizing antibodies in the blood of the donors. Transfusion of components with HPV B19 DNA at concentrations less than 106 IU/mL was safe[9]. Jia et al[10] reported that 71.91% (169/235) of Chinese plasma pools were contaminated by HPV B19, and 68.05% of contaminated plasma pools contained more than 104 IU/mL[10]. Our patient received RBC component infusion before the diagnosis of HPV B19 infection. Unfortunately, we lacked related clinical dates to identify the transmission by allografts or component infusion. Alves and Sharif reported that the incidence of HPV B19 viremia in dialysis patients was 8.3% and 10.8%, respectively[11,12]. Thus, some renal transplant recipients had experienced viremia before transplantation, and may experience HPV B19-associated aPRCA under a strong immunosuppressive state, after transplant.
The clinical manifestations of HPV B19 infection vary greatly in solid organ transplant (SOT) recipients. Eid AJ reported that 98.8% of transplant patients have anemia, especially characterized by PRCA[13]. HPV B19 infection causes anemia by inducing erythroid progenitor cell apoptosis and down-regulating erythropoietin receptor expression on the surface of erythroid progenitor cells. Aside from anemia, some recipients may experience fever, rash, arthralgia, pancytopenia, or organ invasive disease (i.e., carditis, hepatitis, pneumonitis, glomerulonephropathy, vasculitis, and neurologic disease)[7]. Due to immunosuppressive effects, these symptoms may not be typical in SOT recipients. Our patient experienced erythropoietin-resistant anemia and reticulocytopenia within 20 d after transplant-ation. However, PRCA was diagnosed by bone marrow aspirate analysis at POD 44. The patient then received IVIG infusion therapy. The reason for PRCA was not clear until the mNGS results of blood specimens indicated HPV B19 infection at POD 66. The diagnosis of HPV B19-associated aPRCA is prone to delay, as it is rare in SPKT. HPV B19 infection should be suspected in SOT recipients with erythropoietin-resistant anemia and reticulocytopenia[14].
The criteria for the diagnosis of HPV B19-associated aPRCA include a low reticulocyte count, active replication of HPV B19 in the serum, and a typical bone marrow examination[4]. If HPV B19 infection is strongly suspected and the serology and serum PCR are negative, bone marrow examination and in situ hybridization or immunohistochemical staining should be performed[7].
Typical bone marrow findings are decreased erythropoiesis with giant pronormoblasts with vacuoles, with positive in situ hybridization or immunohisto-chemical staining of HPV B19. Parvovirus B19 infection can be diagnosed by serology or direct viral detection in clinical specimens. HPV B19 IgG antibody positivity indicates remote infection and is useless for the diagnosis of acute infection. Parvovirus B19 IgM antibody was present in 75% of SOT recipients at the time of disease onset[7]. Due to inadequate or delayed antibody-mediated immune response in immunocompromised patients, serology might be falsely negative[15]. PCR assays have been used to detect HPV B19 in clinical specimens and HPV B19 viral loads can be supervised by quantitative PCR (qPCR). However, a presumed range of suspicious pathogens is required for the qPCR technique. Some PCR assays are unable to detect HPV B19 genotypes 2 and 3[16,17]. Wilson et al[18] first reported the diagnosis of infectious diseases by mNGS in 2014. Additionally, mNGS can reflect disease progression through its direct and semi-quantitative surveillance of pathogen loads[19]. mNGS testing has been used for the comprehensive diagnosis of uncommon viruses including HPV B19 in acute liver failure patients[20]. We used mNGS for both etiology diagnosis and dynamic semi-quantitative surveillance of HPV B19 loads. The results showed that the diminution in mNGS reads was correlated with Hb and reticulocyte count improvements.
IVIG infusion is an effective therapy for HPV B19-associated aPRCA. Neutralizing antibodies to parvovirus in IVIG reduces or eliminates the virus in immunocompro-mised patients[5]. The optimal dosing regimen and duration of IVIG therapy for parvovirus B19 infection have not been established. However, IVIG for five consecutive days at a dose of 0.4 g/kg/day is recommended[7]. HPV B19 infection relapsed in 27.6% of SOT recipients after receiving IVIG[13]. Repeated courses of IVIG infusion are effective in patients with recurrence[21]. The reticulocyte count in our patient increased immediately after first receiving IVIG but was not stable until the third cycle of IVIG therapy was completed. Immunosuppressive therapy could be used in PRCA; prednisone is one traditional immunosuppressive approach, at a dose of 1 mg/kg[1]. Before the diagnosis of HPV B19 infection, intravenous methylpred-nisolone at 80 mg/d was used for PRCA in our patient, but it had no effect on correcting anemia. At least 50 drugs and chemicals have been associated with PRCA[1]. Some of these drugs have been used in SPKT, such as tacrolimus, mycophenolate mofetil, and azathioprine. The adjustment of these drugs is an important part of the treatment regimen. We withdrew EC-MPS due to severe anemia at POD 20 as it was suspected that anemia in this patient may have been a side effect of EC-MPS at that time. Reduction of immunosuppression should be attempted, if possible, at the time of diagnosis[7]. Our patient had a suspected diagnosis of acute rejection at POD 22. After the diagnosis of HPV B19 infection at POD 66, the dosage of tacrolimus was not reduced due to the concern of rejection. We switched tacrolimus to cyclosporine at POD 88. It remains a challenge to balance infection with rejection.
This current case study has various limitations due to the lack of equipment and testing of other variables. With regard to the lack of equipment, IgG against HPV B19, anti-erythropoietin antibodies and HPV B19 PCR could not be tested in our hospital. HPV B19 DNA load of the donor was not examined. Additionally, immunohisto-chemical staining of the bone marrow aspirate for HPV B19 was not performed. However, the limitations of this research did not affect the patient’s diagnosis and treatment.
HPV B19-associated aPRCA can occur during the early period after SPKT and should be considered when a patient experiences erythropoietin-resistant anemia and reticulocytopenia. An effective treatment regimen includes IVIG infusion and adjustment of immunosuppressive therapy. Moreover, mNGS can be used to identify HPV B19 infection and reflect disease progression in SPKT patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bösmüller C S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Means RT Jr. Pure red cell aplasia. Blood. 2016;128:2504-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Neild G, Anderson M, Hawes S, Colvin BT. Parvovirus infection after renal transplant. Lancet. 1986;2:1226-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Labbadia F, Salido-Fierréz E, Majado-Martinez J, Cabañas-Perianes V, Moraleda JJ. Pure red cell aplasia in a simultaneous pancreas-kidney transplantation patient: inside the erythroblast. Hematol Rep. 2012;4:e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Nowacka-Cieciura E, Karakulska-Prystupiuk E, ¯uk-Wasek A, Lisik W, Basak GW, Durlik M. Pure red cell aplasia related to parvovirus B19 infection in simultaneous pancreas and kidney recipient: a case report. Transplant Proc. 2020;52:2539-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Onitilo AA, Shaw GR. Parvovirus B19 infection in an isolated pancreas transplant recipient. Transplant Proc. 2005;37:4433-4435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Xiao C, Wang CX, Liu LS, Fu Q. Clinical investigation of human parvovirus B19 infection after renal transplantation in China. Transplant Proc. 2013;45:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Eid AJ, Ardura MI; AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Juhl D, Hennig H. Parvovirus B19: What is the relevance in transfusion medicine? Front Med (Lausanne). 2018;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kleinman SH, Glynn SA, Lee TH, Tobler LH, Schlumpf KS, Todd DS, Qiao H, Yu MY, Busch MP; National Heart; Lung; and Blood Institute Retrovirus Epidemiology Donor Study-II (NHLBI REDS-II). A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood. 2009;114:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Jia J, Ma Y, Zhao X, Guo Y, Huangfu C, Fang C, Fan R, Lv M, Yin H, Zhang J. Prevalence of human parvovirus B19 in Chinese plasma pools for manufacturing plasma derivatives. Virol J. 2015;12:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Alves MT, Vilaça SS, Godoi LC, Rezende Júnior L, Carvalho Md, Silva Fde S, Guimarães FL, Fernandes AP, Dusse LM, Gomes KB. Parvovirus B19 (B19) and cytomegalovirus (CMV) infections and anti-erythropoietin (anti-EPO) antibodies in patients on dialysis hyporesponsive to erythropoietin therapy. Clin Chim Acta. 2014;431:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Sharif A, Aghakhani A, Velayati AA, Banifazl M, Sharif MR, Razeghi E, Kheirkhah D, Kazemimanesh M, Bavand A, Ramezani A. Frequency and genotype of human parvovirus B19 among Iranian hemodialysis and peritoneal dialysis patients. Intervirology. 2016;59:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrewska K, Guidi S, Salvadori M. Unusual incidence of aplastic anaemia due to B-19 parvovirus infection in renal transplant recipients. Transplant Proc. 1997;29:818-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bredl S, Plentz A, Wenzel JJ, Pfister H, Möst J, Modrow S. False-negative serology in patients with acute parvovirus B19 infection. J Clin Virol. 2011;51:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Baylis SA, Shah N, Minor PD. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods. 2004;121:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Baylis SA, Buchheit KH. A proficiency testing study to evaluate laboratory performance for the detection of different genotypes of parvovirus B19. Vox Sang. 2009;97:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, Salamat SM, Somasekar S, Federman S, Miller S, Sokolic R, Garabedian E, Candotti F, Buckley RH, Reed KD, Meyer TL, Seroogy CM, Galloway R, Henderson SL, Gern JE, DeRisi JL, Chiu CY. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 677] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 19. | Ai JW, Zhang HC, Cui P, Xu B, Gao Y, Cheng Q, Li T, Wu H, Zhang WH. Dynamic and direct pathogen load surveillance to monitor disease progression and therapeutic efficacy in central nervous system infection using a novel semi-quantitive sequencing platform. J Infect. 2018;76:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Somasekar S, Lee D, Rule J, Naccache SN, Stone M, Busch MP, Sanders C, Lee WM, Chiu CY. Viral surveillance in serum samples from patients with acute liver failure by metagenomic next-generation sequencing. Clin Infect Dis. 2017;65:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Kaya B, Paydas S. Recurrence of pure red cell aplasia in a kidney transplant recipient due to reactivation of parvovirus B19 infection despite two cycles of intravenous immunoglobulin therapy. Exp Clin Transplant. 2019;17:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |