Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1953
Peer-review started: November 4, 2020
First decision: November 23, 2020
Revised: December 23, 2020
Accepted: January 20, 2021
Article in press: January 20, 2021
Published online: March 16, 2021
Processing time: 116 Days and 0.5 Hours
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2, is a worldwide pandemic. Some COVID-19 patients develop severe acute respiratory distress syndrome and progress to respiratory failure. In such cases, extracorporeal membrane oxygenation (ECMO) treatment is a necessary life-saving procedure.
Two special COVID-19 cases—one full-term pregnant woman and one elderly (72-year-old) man—were treated by veno-venous (VV)-ECMO in the Second People’s Hospital of Zhongshan, Zhongshan City, Guangdong Province, China. Both patients had developed refractory hypoxemia shortly after hospital admission, despite conventional support, and were therefore managed by VV-ECMO. Although both experienced multiple ECMO-related complications on top of the COVID-19 disease, their conditions improved gradually. Both patients were weaned successfully from the ECMO therapy. At the time of writing of this report, the woman has recovered completely and been discharged from hospital to home; the man remains on mechanical ventilation, due to respiratory muscle weakness and suspected lung fibrosis. As ECMO itself is associated with various complications, it is very important to understand and treat these complications to achieve optimal outcome.
VV-ECMO can provide sufficient gas exchange for COVID-19 patients with acute respiratory distress syndrome. However, it is crucial to understand and treat ECMO-related complications.
Core Tip: We present two cases of severe coronavirus disease 2019 (COVID-19) treated by extracorporeal membrane oxygenation (ECMO). The first, a full-term pregnant woman, was discharged with child loss but in good health, and the second, an elderly man, remains on ventilation support and may require lung transplantation. ECMO provided sufficient support for these COVID-19 patients with severe respiratory failure; however, both experienced several complications during the ECMO therapy, including right ventricular dysfunction, bleeding, thrombosis, acute renal failure, respiratory muscle weakness, and suspected pulmonary fibrosis. More case studies are needed to determine the optimal ECMO management strategy for COVID-19 patients.
- Citation: Wen JL, Sun QZ, Cheng Z, Liao XZ, Wang LQ, Yuan Y, Li JW, Hou LS, Gao WJ, Wang WJ, Soh WY, Li BF, Ma DQ. Extracorporeal membrane oxygenation for coronavirus disease 2019-associated acute respiratory distress syndrome: Report of two cases and review of the literature. World J Clin Cases 2021; 9(8): 1953-1967
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1953.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1953
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has rapidly evolved into a worldwide pandemic. To date, there are more than 33 million confirmed cases and 1 million deaths globally[1]. It has been shown that the lungs are the most affected organ, and many COVID-19 patients developed acute respiratory distress syndrome (ARDS) during their illness[2,3].
Extracorporeal membrane oxygenation (ECMO) is a mechanical pump-assisted device that provides circulatory and respiratory support for patients experiencing cardiopulmonary failure. As a life-supporting intervention, ECMO is recommended by the World Health Organisation’s interim guidance as a treatment for patients with refractory hypoxemia despite conventional ventilation support[4].
Here, we report two special COVID-19 patients—one full-term pregnant woman and one elderly (72-year-old) man—whose lives were saved by veno-venous (VV-)ECMO support in the Second People’s Hospital of Zhongshan, Guangdong Province, China.
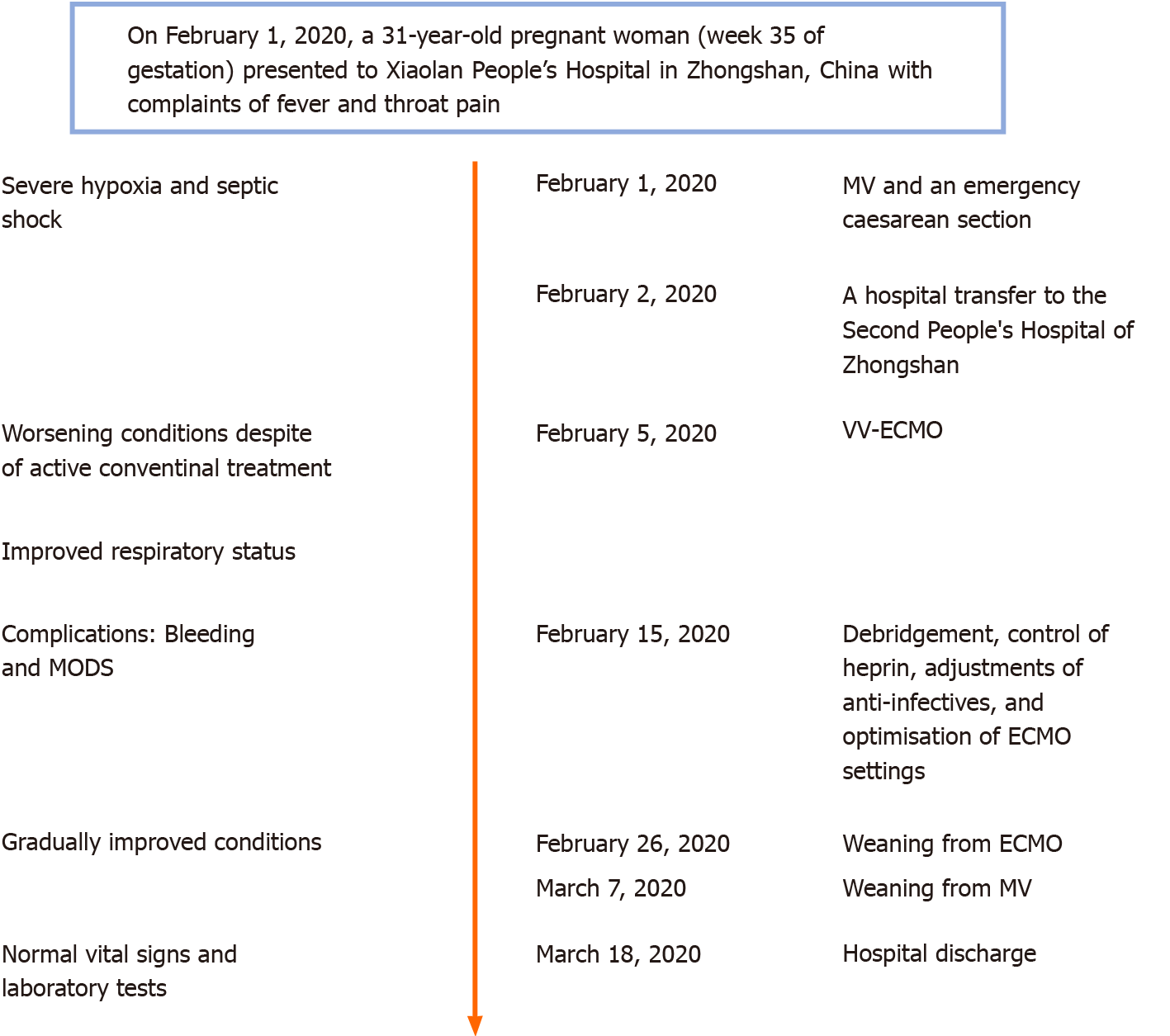
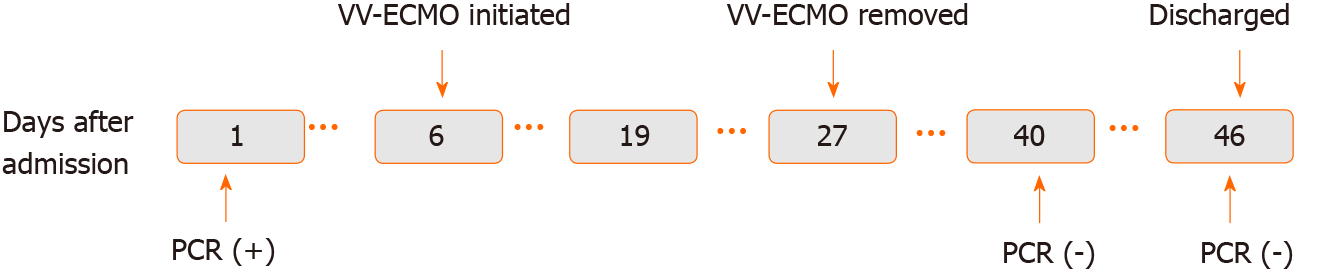
Case 1: A timeline of the main events is provided in Figure 1. On February 1, 2020, a 31-year-old pregnant woman (week 35 of gestation) presented to Xiaolan People’s Hospital in Zhongshan, China with complaints of fever and throat pain.
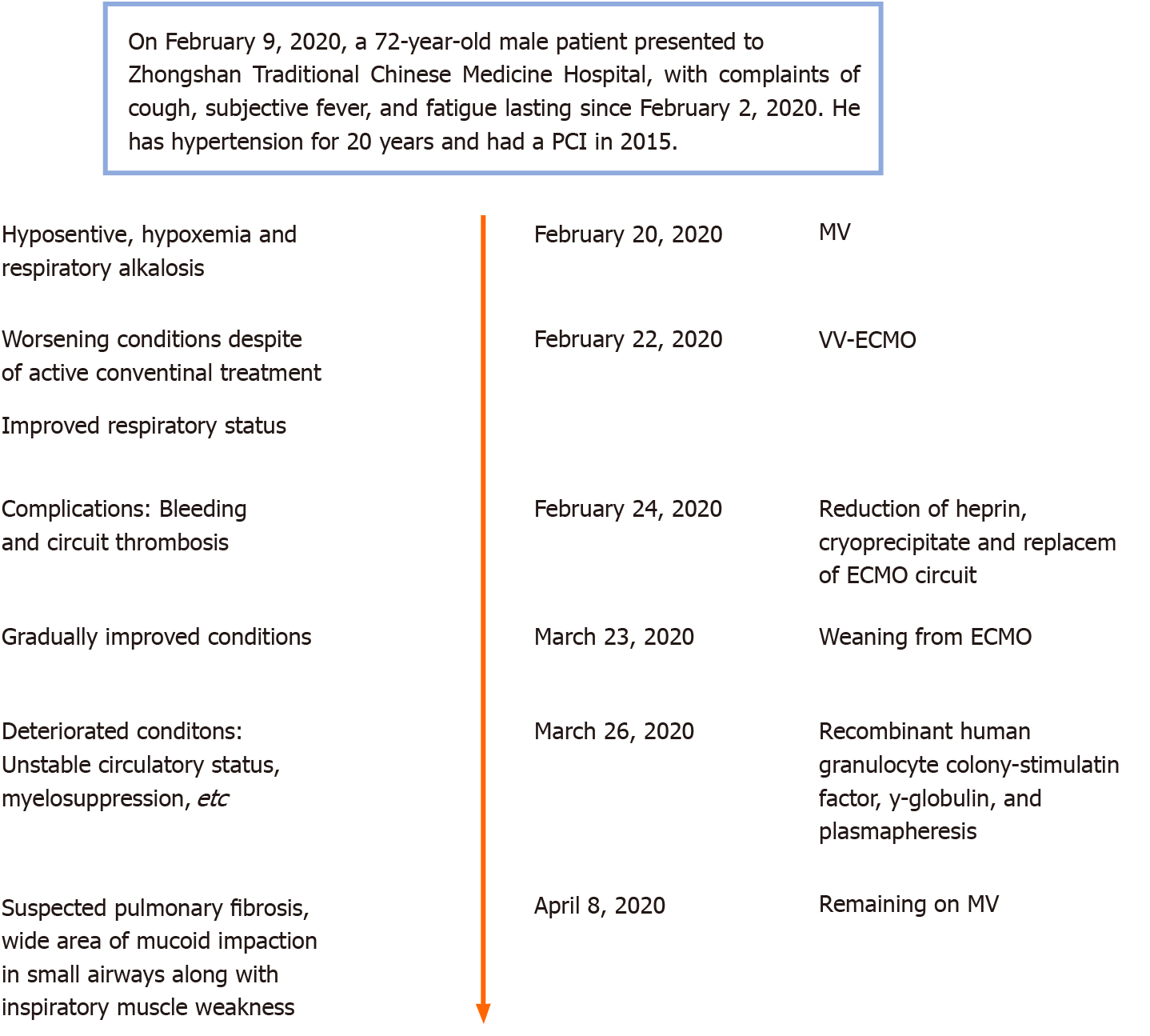
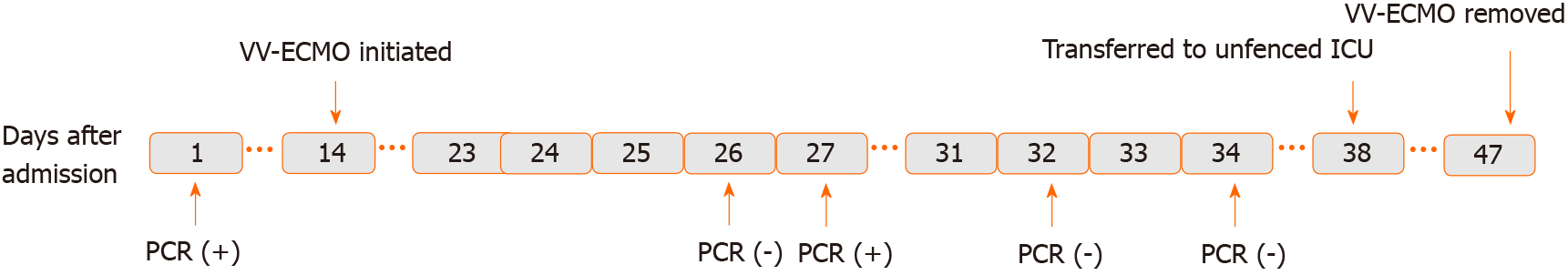
Case 2: A timeline of the main events is provided in Figure 2. On February 9, 2020, a 72-year-old male patient presented to Zhongshan Traditional Chinese Medicine Hospital, with complaints of cough, subjective fever, and fatigue lasting since February 2, 2020.
Case 1: The patient reported symptoms having started 4 d prior, coinciding with a travel back to Zhongshan from Wuhan on January 25, 2020. She reported having attended regular antenatal checks throughout her pregnancy, with all examination results having been normal. Within 6 h of admission to the Xiaolan People’s Hospital, she developed severe hypoxia and septic shock. She was intubated and sedated for administration of mechanical ventilation (MV). An emergency caesarean delivery was carried out at bedside, and a live male was delivered. The infant weighed 2700 g and had an Apgar score of 1 at 1 min immediately after delivery. Cord blood tests indicated severe acidosis, hypoxemia, and potential cardiac damage. The infant died on the same day, despite receipt of active care. The patient’s family refused a post-mortem examination, precluding determination of a definitive cause of death (i.e., viral infection and/or hypoxia before birth).
As the mother’s condition was extremely unstable, she was transferred to the Second People’s Hospital of Zhongshan the day after delivery, in the early morning.
Case 2: The patient’s symptoms started 1 wk before presenting to hospital.
Case 1: The patient had previous good health.
Case 2: The patient had a long-term history of hypertension (20 years) and had undergone percutaneous coronary intervention (PCI) for left main and three-vessel coronary artery disease 5 years prior.
Case 1: The patient declared no remarkable family medical history.
Case 1: Upon admission to the Second People’s Hospital of Zhongshan, the patient was placed on assisted MV [positive end-expiratory pressure (PEEP) of 15 cmH2O] under mild sedation. Intravenous norepinephrine was administered at 0.8 μg/kg/min. The patient’s vital signs at admission were: Body temperature, 36.3 °C; pulse, 150 beats/min; and respiratory rate, 25 breaths/min. Her blood pressure was 122/79 mmHg and peripheral capillary oxygen saturation was 96.4%. Chest auscultation revealed coarse crackles in the left inferior lobe. No pathologic heart sound was detected by cardiac auscultation.
Case 2: The patient’s vital signs at admission were body temperature of 37.5 °C, pulse of 98 beats/min, and respiratory rate of 20 breaths/min. His blood pressure was 129/71 mmHg. Chest auscultation detected crackles in both lungs. There was no pathologic sound detected from cardiac auscultation.
Case 1: Routine blood tests administered at admission gave the following results: White blood cell count, 6.8 × 109/L (normal range: 4-10 × 109/L); lymphocytes, 14% (normal range: 17%-48%); neutrophils, 83.2% (normal range: 43%-76%); haemoglobin, 110 g/L (normal range: 110-150 g/L); red blood cell count, 3.48 × 1012/L (normal range: 3.5-5.5 × 1012/L); and platelet count (PLT), 160 × 109/L (normal range: 100-300 × 109/L). Biochemical tests showed high-level aspartate aminotransferase (AST) (190.6 U/L; normal range: 10-40 U/L), alanine aminotransferase (ALT) (137 U/L; normal range: 7-56 U/L), lactate dehydrogenase (LDH) (528.76 U/L; normal range: 140-280 U/L), C-reactive protein (CRP) (44.3 mg/L; normal range: < 8 mg/L), and creatine kinase (CK)-MB (33.4 U/L; normal range: < 24 U/L). Her blood electrolyte levels were normal. SARS-CoV-2 test, from nasopharyngeal swab specimen, was positive.
Case 2: Blood measures yielded the following results: White blood cell count, 6.8 × 109/L (normal range: 4-10 × 109/L); lymphocyte count, 0.93 × 109/L (normal range: 0.8-4.0 × 109/L); haemoglobin, 121 g/L (normal range: 120-160 g/L); and PLT, 102 × 109/L (normal range: 100-300 × 109/L). Biochemical tests yielded the following results: AST, 59.3 U/L (normal range: 10-40 U/L); CK, 478 U/L (normal range: 22-198 U/L); LDH, 366.98 U/L (normal range: 140-280 U/L); CRP, 44.3 mg/L (normal range: < 8 mg/L); creatinine, 189.00 μmoL/L (normal range: 74-107 μmoL/L); blood urea nitrogen, 15.40 mmoL/L (normal range: 2.9-7.5 mmoL/L); and potassium, 5.60 mmoL/L (normal range: 3.5-5.0 mmoL/L). His SARS-CoV-2 nucleic acid test was positive.
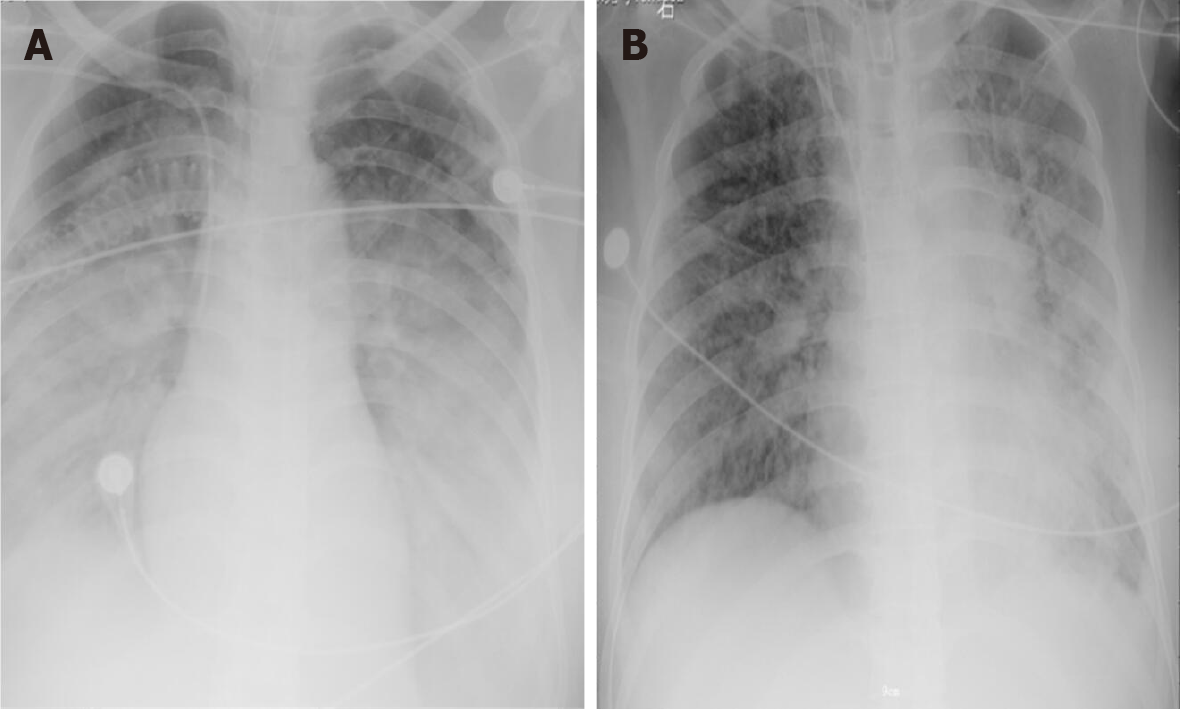
Case 1: Chest X-ray showed large patchy shadows in bilateral lungs (Figure 3A). Fibreoptic bronchoscopy detected massive frothy sputum.
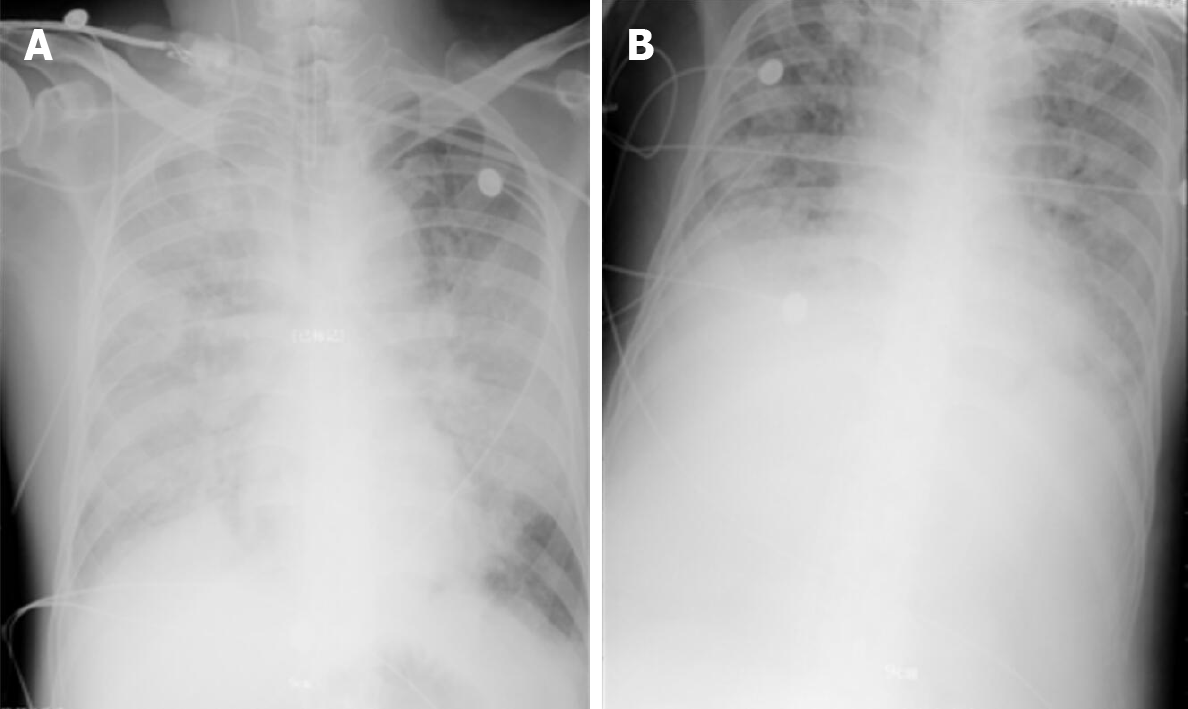
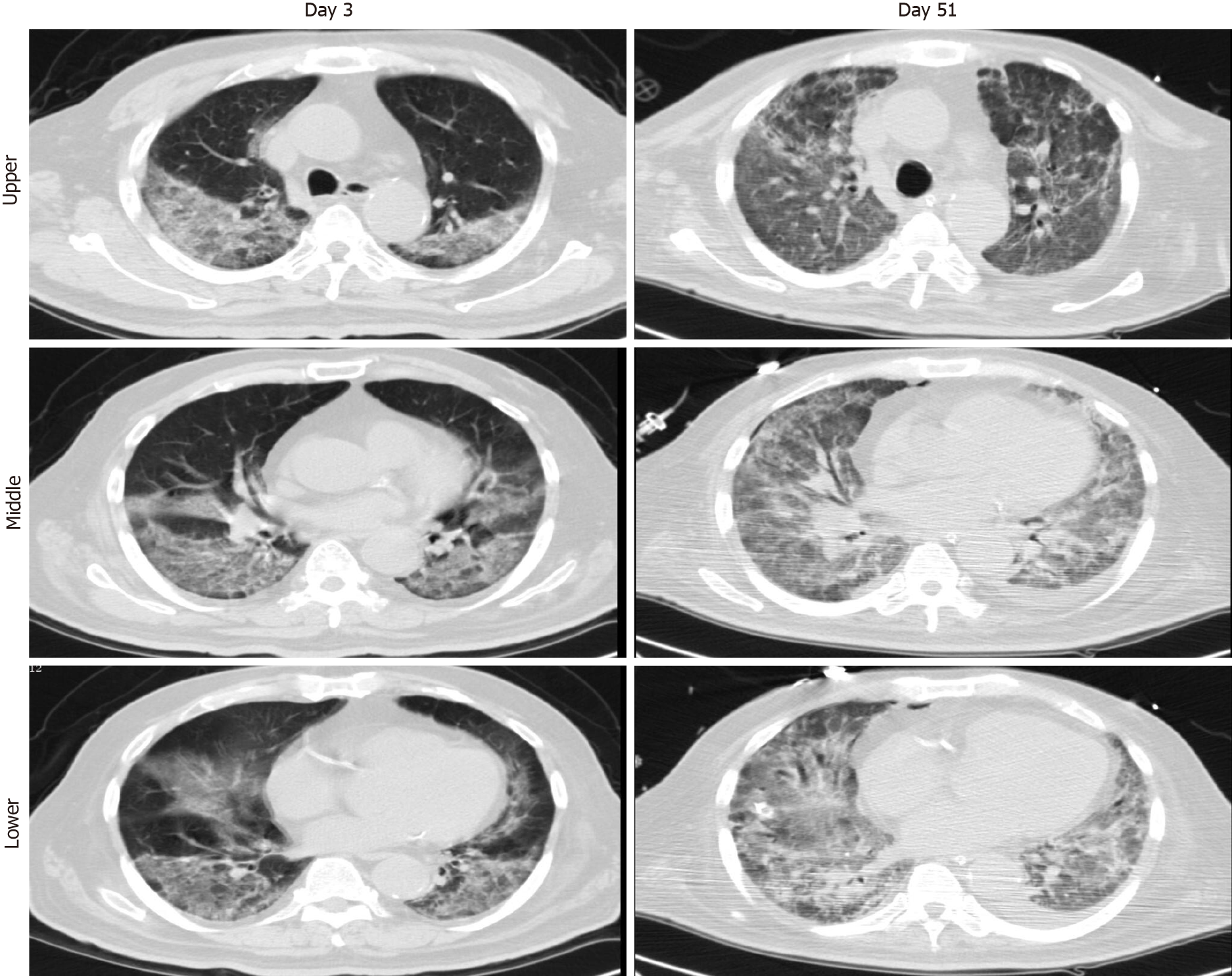
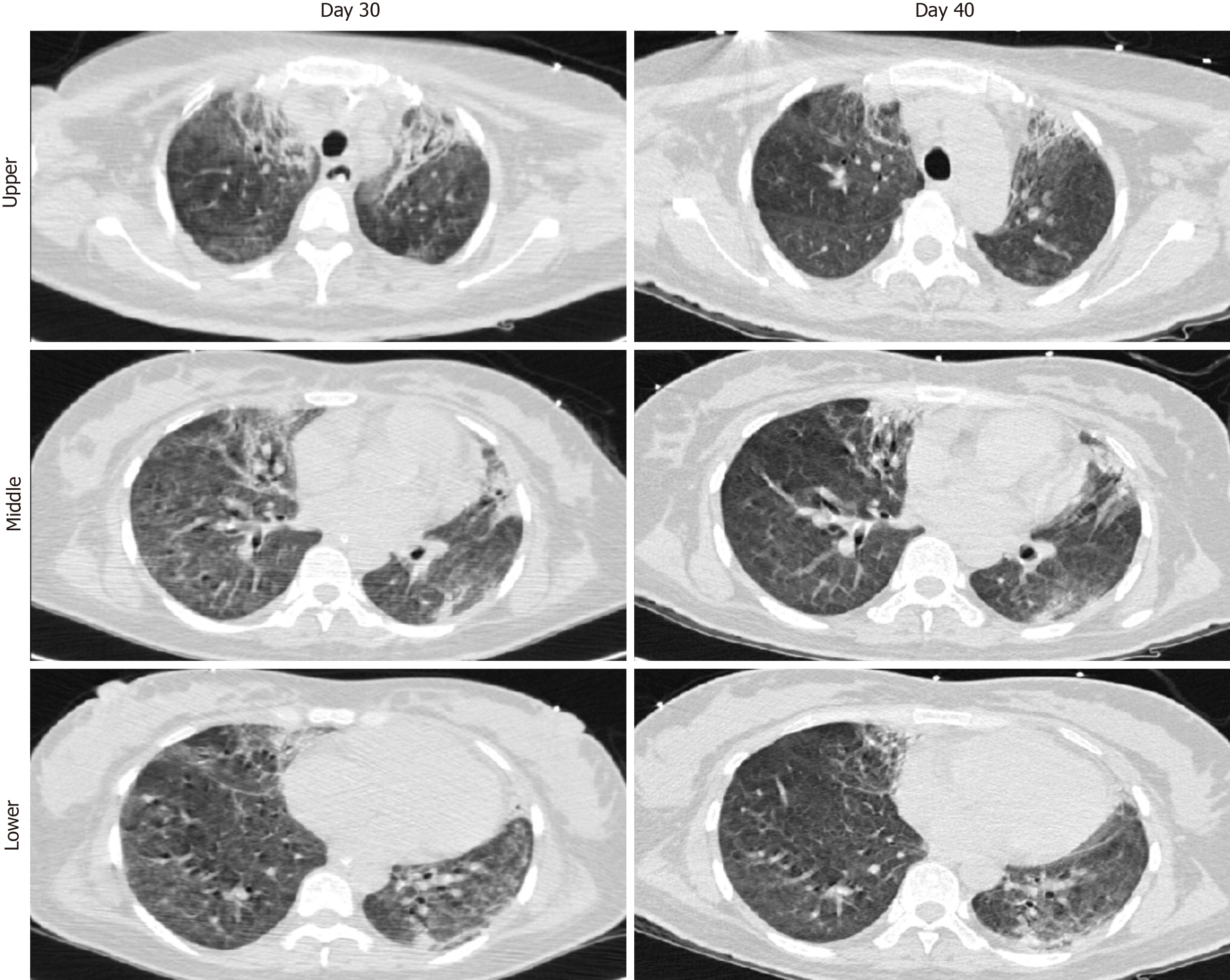
Case 2: Chest X-ray showed infiltrates in bilateral lungs (Figure 4A). A chest computed tomography (CT) scan demonstrated ground-glass opacities and consolidation in bilateral lungs. The representative upper, middle, and lower transverse chest CT images showed multiple ground glass shadows in both lungs (day 3; Figure 5).
Case 1: COVID-19 pneumonia, ARDS, septic shock, septic cardiomyopathy, multiple organ dysfunction syndrome, and post-caesarean condition.
Case 2: COVID-19 pneumonia, post-PCI condition, and hypertension.
Case 1: To improve oxygenation, achieve better fluid balance, and remove excessive inflammatory factors, continuous renal replacement therapy (CRRT) was applied. Simultaneously, the patient received antiviral therapy, antibiotic therapy, steroid therapy, and other standard therapies (Figure 6). However, her condition deteriorated rapidly, over the next few hours. Arterial blood gas analysis demonstrated respiratory acidosis [pH, 7.34; partial pressure of carbon dioxide (PaCO2), 52.3 mmHg; partial pressure of oxygen (PaO2), 91.4 mmHg; HCO3-, 27.9 mmoL/L; fraction of inspired oxygen (FiO2), 1 L/L]. Consequently, she was placed on controlled MV, at a rate of 18 [bilevel positive airway pressure (BIPAP), FiO2, 1 L/L; inspiratory pressure, 35 cmH2O; PEEP, 15 cmH2O], with deep sedation (via morphine, midazolam and propofol combination).
Despite the use of various anti-infective and supportive treatments, the patient’s condition continued to deteriorate. She developed mild jaundice. Her blood test revealed leucocytosis (white blood cell count of 23.86 × 109/L; normal range: 4-10 × 109/L), neutrophilia (neutrophil percentage of 89.10%; normal range: 43%-76%), thrombocytopenia (PLT of 68.00 × 109/L), high-level LDH (326.53 U/L; normal range: 140-280 U/L), hyperbilirubinemia [total bilirubin (TBIL) of 117.80 μmoL/L; normal range: 1.71-20.5 μmoL/L, with direct bilirubin (DBIL) of 68.30 μmoL/L; normal range: 0-5.1 μmoL/L and indirect bilirubin (IBIL) of 49.50 μmoL/L; normal range: 0-13.7 μmoL/L], high total bile acid [(TBA) of 14.2 mmoL/L; normal range: 0.1-10.0 μmoL/L)], low prealbumin (45 mg/L; normal range: 280-360 mg/L), and high inflammatory biomarkers [high-sensitivity (hs-) CRP of 181.92 mg/L; normal range: < 1 mg/L; procalcitonin of 3.29 ng/mL; normal range: < 0.05 ng/mL]. Her arterial blood gas analysis showed severe hypoxemia and respiratory acidosis (pH, 7.27; PaCO2, 61.9 mmHg; PaO2, 62.3 mmHg; HCO3-, 28.2 mmoL/L; FiO2, 1 L/L). In consideration of her worsening hypoxemia despite maximum conventional support, VV-ECMO support was applied by the Zhongshan People’s Hospital ECMO team.
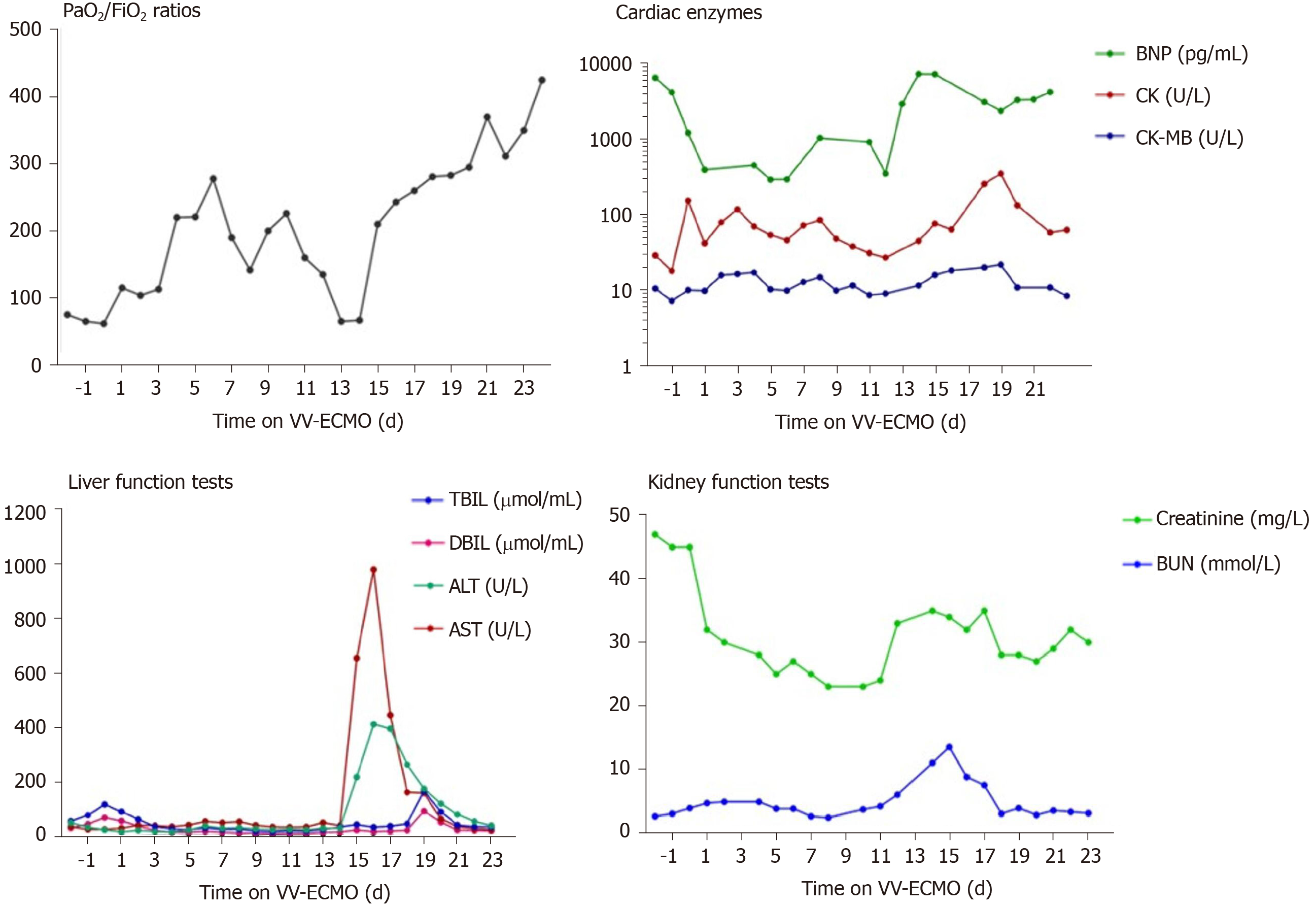
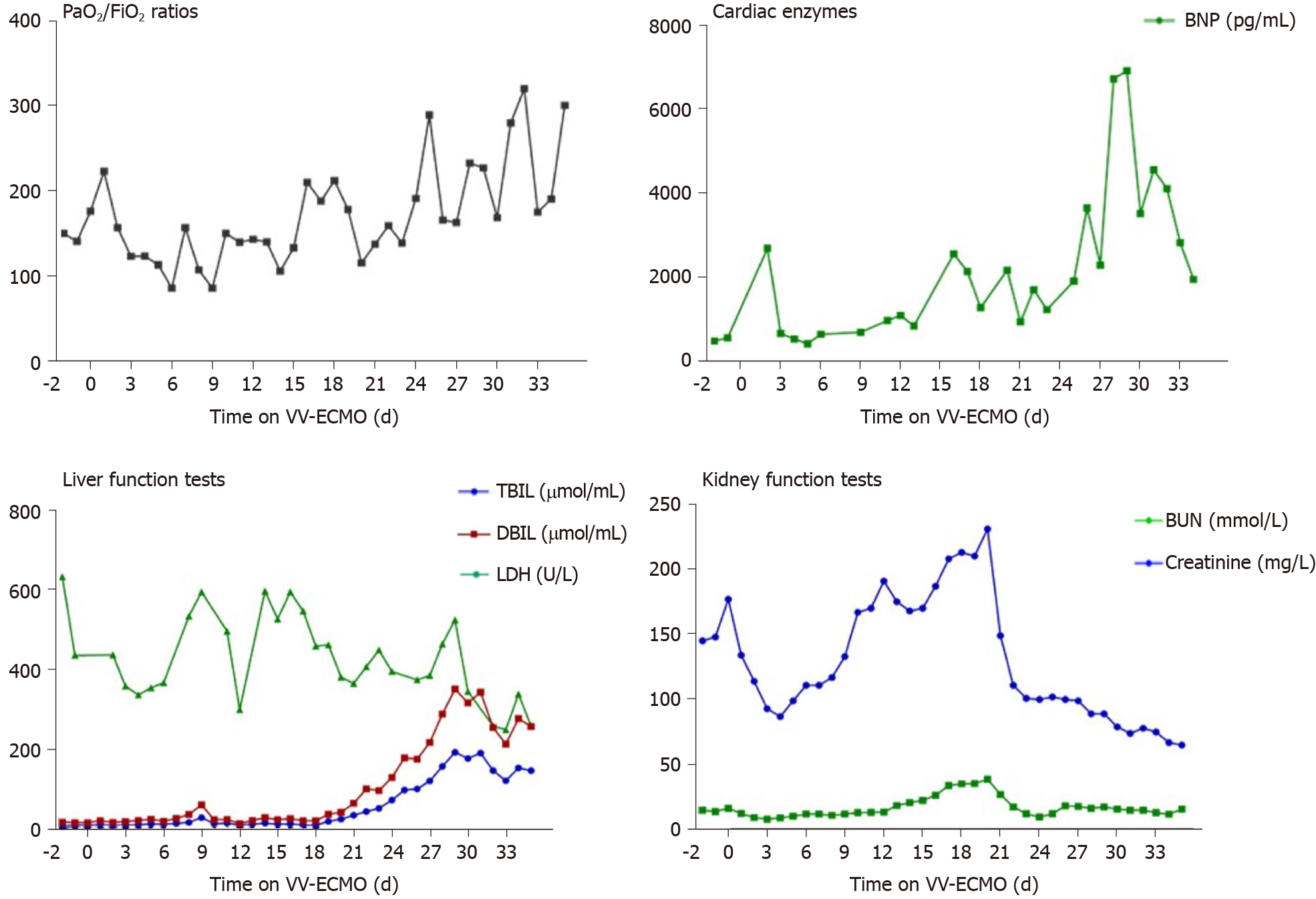
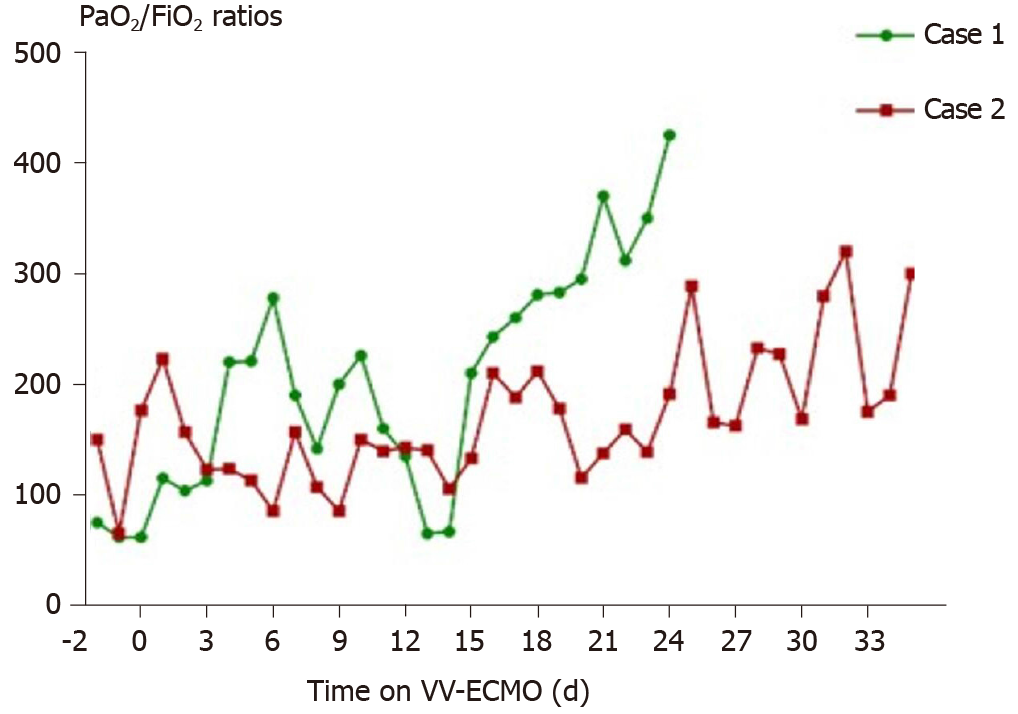
The ECMO circuit was established through the internal jugular vein and femoral vein percutaneously. The blood flow setting ranged from 2.5-3.5 L/min during the period of ECMO support. The patient’s respiratory status improved in the first 10 d, with PaO2/FiO2 ratios raising from 62 to 226. Considering her needs of prolonged MV after ECMO, a tracheostomy was performed on the day 10 of ECMO support. Surgical bleeding occurred during the operation, and the use of heparin was temporarily halted while a debridement was performed, which successfully controlled the bleeding. Between days 10 and 16 of the ECMO support, her condition worsened again, with PaO2/FiO2 falling to 65. Blood measurements showed a marked increase of NT-proB-type natriuretic peptide (NT-proBNP) (reaching a maximum of 7267 pg/mL on day 14; normal range: < 125 pg/mL), CK (reaching a maximum of 350 U/L on day 19; normal range: 22-198 U/L), LDH (reaching a maximum of 1943 U/L on day 16; normal range: 140-280 U/L), TBIL (reaching a maximum of 167 μmoL/L on day 19; normal range: 1.71-20.5 μmoL/L), DBIL (reaching a maximum of 92.5 μmoL/L on day 19; normal range: 0-5.1 μmoL/L), ALT (reaching a maximum of 411 U/L on day 16; normal range: 10-40 U/L), AST (reaching a maximum of 980.7 U/L on day 16; normal range: 7-56 U/L), creatinine (reaching a maximum of 35 mg/L on day 14; normal range: 5-11 mg/L), and blood urea nitrogen (reaching a maximum of 13.5 mmoL/L on day 15; normal range: 2.9-7.5 mmoL/L), indicating damage of the heart, kidney, and liver (Figure 7). Echocardiography revealed enlargement of the right ventricle (right ventricular diameter of 28 mm), indicating development of right ventricular dysfunction (RVD).
To support her right ventricle function, the fluid management, ECMO perfusion, and PEEP were optimised. Meanwhile, to avoid potential hepatotoxicity, the use of imipenem cilastatin sodium was discontinued and replaced with piperacillin/ tazobactam. The use of vancomycin and voriconazole was also paused.
Over the next week, the patient’s condition improved gradually. She demonstrated sufficient oxygenation (PaO2/FiO2 raised to 370), partial recovery of lung function (tidal volume > 6 mL/kg in the condition of PEEP < 10 cmH2O and Ppeak < 28 cmH2O), and improved chest radiography findings (Figure 3B). Blood tests showed that the levels of organ damage biomarkers were decreased remarkably.
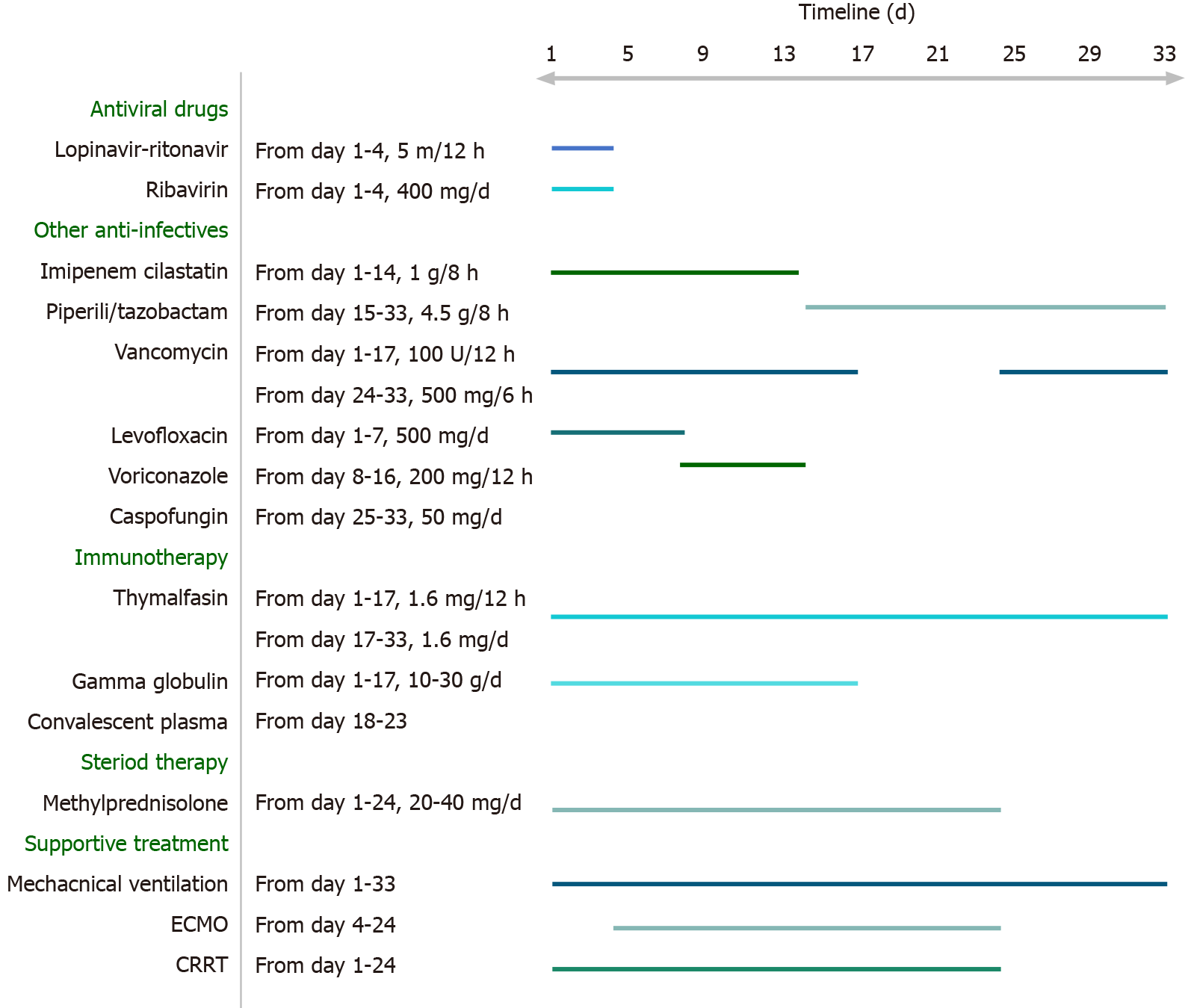
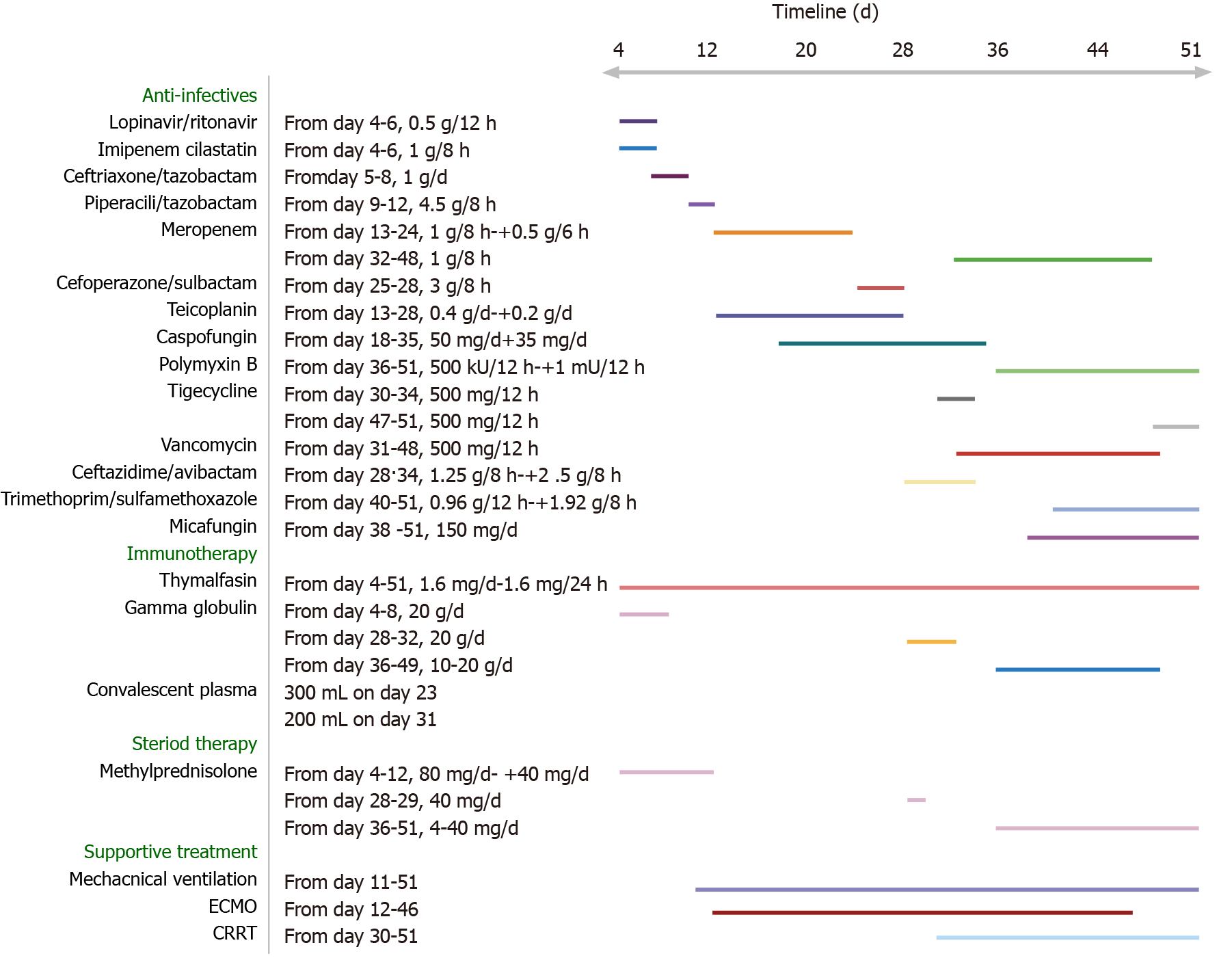
Case 2: Treatments applied since admission were mainly supportive, concomitant with antiviral treatment (Figure 8). However, after 11 d, on February 20, 2020, the patient’s condition worsened. He became severely hypotensive, requiring vasoactive support (norepinephrine at 0.15 mg/kg/min). Arterial blood gas analysis revealed severe hypoxemia and respiratory alkalosis (pH, 7.49; PaO2, 52 mmHg; PaCO2, 31 mmHg). The next day, intermittent non-invasive ventilation was initiated, but the patient complained of a sense of suffocation. His arterial blood gas parameters were pH of 7.50, PaO2 of 71 mmHg, and PaCO2 of 32 mmHg. Since his respiratory status did not improve and due to his intolerance of the intermittent non-invasive ventilation, MV was applied (BIPAP; FiO2, 1 L/L; PEEP, 15 cmH2O). On February 22, 2020, the patient’s condition deteriorated, with worsening of arterial blood gas parameters (pH, 7.29; PaCO2, 62 mmHg; PaO2, 65 mmHg). Failure of the conventional therapies to improve the patient’s condition led to a decision to apply VV-ECMO support.
During the first 2 d of ECMO support, the patient’s haemodynamics were stable and oxygenation was improved (Figure 9). Over the next 10 d, however, he developed bleeding and thrombosis. The posterior pharyngeal wall was accidentally injured during sputum suctioning, which caused repeated bleeding. A chest drain was inserted to address right-sided pleural effusion (approximately 7 cm on B-scan ultrasonography); the next day yielded a total drainage volume of 1500 mL blood. As thrombi were found in the ECMO canula, the ECMO circuit was replaced. The combination of bleeding and thrombi caused difficulty in regulating anticoagulation. On day 18 of ECMO support, the decision was made to perform a tracheostomy, in recognition of the need for prolonged ventilation support after weaning-off of ECMO, with the condition of reducing heparin and applying normal plasma transfusion and cryoprecipitate. No significant bleeding occurred during the surgery. The continuation of antiviral therapy, vasoactive drugs, CRRT, anti-inflammation therapy, steroid therapy, and plasmapheresis facilitated a gradual improvement in the patient’s status. Cardiac function stabilised, with decreased level of BNP (from 6925 to 1957 pg/mL; normal range: < 125 pg/mL). Chest X-ray showed decreased infiltrates in bilateral lungs (Figure 4B).
On March 26, 2020, the patient was successfully weaned from VV-ECMO, after 33 d on ECMO. Unfortunately, following the weaning-off, the circulatory status became extremely unstable and increased levels of vasoactive drugs, sedatives, analgesics, and muscle relaxants were required. Over the next 10 d, the patient’s condition continued to deteriorate further. Myelosuppression developed and blood tests revealed leukopenia, thrombocytopenia, and increased levels of inflammatory biomarkers. Therapy with recombinant human granulocyte colony-stimulating factor, γ-globulin, and plasmapheresis was initiated.
Case 1: The VV-ECMO therapy was stopped 21 d after ECMO application. The patient’s chest X-ray was much improved, prompting weaning from MV after 36 d of such. A chest CT scan on day 40 showed absorption of inflammatory lesions compared to day 30 (Figure 10). Her SARS-CoV-2 nucleic acid test result became negative after 40 d of admission (Figure 11). The patient was discharged from hospital to home, with normal vital signs and laboratory tests, after 46 d of admission.
Case 2: From April 8 to April 29, 2020, the patient’s condition stabilised, with his pulmonary compliance and circulatory status gradually improving. The result of SARS-CoV-2 nucleic acid test became negative (Figure 12). However, he had developed pulmonary fibrosis and a wide area of mucoid impaction in small airways, along with inspiratory muscle weakness. A CT scan showed diffuse ground glass opacities and honeycombing in both lungs, indicating the development of pulmonary fibrosis (day 51; Figure 5). As such, he remained on ventilatory support and (of the time of writing this report) may require a lung transplantation.
ECMO is a life-supporting intervention, whereby an external mechanical pump collects venous blood and performs gas-exchange through an oxygenator. ECMO has been used in patients with severe ARDS for decades[5]. Since the outbreak of the COVID-19 pandemic, there has been increased usage of ECMO in supporting COVID-19 patients as an ultimate treatment to sustain their lives[6]. The advantage of ECMO in COVID-19 patients is that it maintains the oxygen supply while providing a time window for the lungs to rest and recover. However, as a highly invasive treatment, ECMO itself is associated with many life-threatening complications, such as cardiac failure, renal failure, bleeding, thrombosis, and infection. Therefore, efforts to and experience in preventing and treating ECMO-related complications are very important.
In this report, we present two COVID-19 patients who were supported by ECMO. After admission, both patients experienced a rapid deterioration of their respiratory function, with a decrease of PaO2 from 91 mmHg to 62 mmHg in the female patient and 150 mmHg to 65 mmHg in the male patient within 24 h under ventilation with FiO2 more than 600 mL/L. Of interest, the man (case 2) experienced various fluctuations of PaO2/FiO2 ratios throughout the course of ECMO, much more so than those experienced by the woman (case 1), indicating that the outcome may not be optimal (Figure 13). As both patients developed severe hypoxemia despite maximum conventional ventilation support, VV-ECMO support was applied. There was an immediate increase in PaO2/FiO2 ratios after the initiation of ECMO, indicating the advantage of VV-ECMO for improving oxygenation in severe COVID-19 cases. In fact, the rapid deterioration experienced, also known as the “second week crash”, has been presented in multiple previous studies as a feature of COVID-19[7-10]. It is characterized as a phenomenon that occurs 7-8 d after the initial symptom onset, with some COVID-19 patients’ symptoms progressing suddenly from mild to severe. A recent immunology study revealed that this unique pattern of clinical course is associated with over-production of inflammatory factors[11]. Overall experience suggests that early application of anti-inflammatory therapies may relieve this life-threatening course, making it worthwhile to investigate its mechanism and optimal strategies in the future.
In our case series, the first patient was discharged from hospital with normal vital signs and laboratory tests, while the other remained in-hospital on ventilation support and may require a lung transplantation due to pulmonary fibrosis. Several factors may explain the outcome difference between these two patients. One of those factors is age. It has been shown that older people are at an increased risk of developing pulmonary fibrosis following COVID-19-induced ARDS (45.4 ± 16.9 vs 33.8 ± 10.2 years of age)[12,13]. Similarly, retrospective studies have revealed that older patients were more prone to developing pulmonary fibrosis following SARS- or Middle East respiratory syndrome (commonly known as MERS)-induced ARDS[14]. This should be taken into consideration since ECMO is such a resource-intensive procedure and involves expensive equipment. Another possible factor is the timing of MV. For our case 1, the MV was initiated immediately after oxygen therapy failure, while for our case 2, high-concentration oxygen therapy was applied for 10 d and shortness of breath occurring over 1 d prompted MV support. Although oxygen therapy is a life-saving technique for patients with hypoxia, it has been demonstrated that continuous high-concentration oxygen therapy can cause lung injury[15]. Besides, studies have shown that the high transpulmonary pressure caused by shortness of breath can also damage the lungs[16], which may have happened in our patient (case 2). There is probably a survival benefit to the application of MV at an earlier time for COVID-19 patients in whom oxygen therapy cannot provide a satisfactory level of oxygenation, and this needs to be investigated in the future. In addition, hospital-acquired infection may have contributed to the opposite outcomes of our patients as well. In case 1, no bacteria were detected in blood culture; in case 2, Streptomonas maltophilia was detected in blood culture after removing ECMO. This pathogen may have further aggravated the COVID-19-related inflammation in his lungs and deterioration of his pulmonary function.
Acute cardiac failure is one complication of ECMO. On day 10 of ECMO support, our female patient (case 1) developed acute RVD. RVD is defined as a clinical syndrome meeting at least one of the following criteria: Acute presence of right ventricle systolic dysfunction, confirmed by echocardiography; and increase of cardiac enzymes in the absence of left ventricle and renal dysfunction and moderate to severe right ventricular strain patterns detected by electrocardiogram[17,18]. RVD is a common complication of ARDS, with a reported incidence ranging from 30% to 56%[19,20]; of note, a consecutive study of 74 ARDS patients referred to ECMO found an RVD incidence of 28.6%[21]. The main direct aetiology of RVD in ARDS is right ventricle overload[22]. The most common cause of excessive right ventricle preload is fluid overload. VV-ECMO application over a long duration and high flow can also be a cause of increased right ventricle preload. As VV-ECMO provides non-pulsatile flow, it may cause a right ventricle volume overload over time[23]. Optimisations of fluid management and ECMO flow, therefore, may serve as effective ways to treat excessive right ventricle preload-related right ventricle dysfunction. On the other hand, right ventricle overloading has been associated with hypoxic/hypercarbic vasoconstriction and high PEEP setting in MV[18,23]. Improving gas exchange and reducing PEEP may, therefore, help decrease right ventricle afterload. To prevent RVD, early diagnosis and intervention are important. There are four predictors of RVD in ARDS that have been identified recently, namely, pneumonia-caused ARDS, driving pressure of ≥ 18 cmH2O (calculated as the difference between plateau pressure and total PEEP), PaCO2 of ≥ 48 mmHg, and PaO2/FiO2 of < 150 mmHg[20]. As severe COVID-19 patients who require VV-ECMO are always associated with at least one of these four predictors[24], routine electrocardiogram and echocardiographic monitoring, and early application of right ventricle protective strategies should be applied in advance.
Bleeding and thrombosis are common complications of ECMO and are associated with mortality[25-29]. Since there is not a standard anticoagulation protocol currently available for ECMO[30], anticoagulation management during ECMO is a substantial challenge facing clinical care providers. In our case series, case 1 experienced bleeding at the surgical site after receiving a tracheostomy. The event was well-controlled by temporarily halting the use of heparin and performance of a debridement by otolaryngologists. Case 2 developed a combination of bleeding and thrombosis in the ECMO tube system, both of which were alleviated by adjustments of the unfractionated heparin level, administration of plasma transfusion and cryoprecipitate, and replacement of the ECMO circuit. Our anticoagulation strategy involved heparin-coated circuits with standard-level unfractionated heparin infusion, titrated to an activated coagulation time (ACT) of 150-180 s. Evidence shows that COVID-19 has an association with reduced platelet count and elevations in D-dimers and fibrin-fibrinogen degradation products, indicating that this novel disease may have an impact on coagulation status. Thus, to achieve the best outcome for these patients, a more individualised anticoagulation regimen can be generated upon close monitoring of each patient’s coagulation status. On the other hand, although ACT is a gold standard for monitoring anticoagulation during ECMO, multiple studies have shown that inadequate anticoagulation or bleeding can occur despite ACT monitoring[31-33]. Since bleeding and thrombosis represent such common life-threatening complications during ECMO, introducing alternative coagulation tests, such as the anti-Xa assay and thromboelastography, can be beneficial for COVID-19 patients.
Our case series showed that ECMO provided sufficient support of gas exchange for COVID-19 patients with severe respiratory failure. Nevertheless, many complications occurred during the ECMO support application, including RVD, bleeding, thrombosis, acute renal failure, respiratory muscle weakness, and suspected pulmonary fibrosis. Our experience also supports the World Health Organization’s interim guidance for ECMO to be considered only in patients with refractory respiratory failure after conventional support. The disease complexity (e.g., cytokine storm, coagulation changes, and multiple organ failure) of COVID-19 may have an extra-impact on ECMO management, and more knowledge needs to be gained through focused case studies.
We would like to express our sincere gratitude to those clinicians who made tremendous efforts towards saving lives during the COVID-19 pandemic. We want to thank the patients presented herein for giving permission to present their cases and to the unnamed patients who donated convalescent plasma.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: He YF, Merkely B S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, Ng LPW, Wong YKE, Pei XM, Li MJW, Wong SC. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2020;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (1)] |
| 2. | Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we "Notch" the inflammatory storm? Basic Res Cardiol. 2020;115:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 3. | Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 882] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. [13 March 2020]. World Health Organization, 2020. Available from: https://apps.who.int/iris/handle/10665/331446;jsessionid=4C3E879A9883ED0A8241C0609DED0322. |
| 5. | Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 670] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 7. | Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health? Lancet. 2020;395:542-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 812] [Cited by in RCA: 861] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 9. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30124] [Article Influence: 6024.8] [Reference Citation Analysis (3)] |
| 10. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12979] [Article Influence: 2595.8] [Reference Citation Analysis (1)] |
| 11. | Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020; 27: 992-1000. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1556] [Article Influence: 311.2] [Reference Citation Analysis (0)] |
| 12. | Wong KT, Antonio GE, Hui DS, Ho C, Chan PN, Ng WH, Shing KK, Wu A, Lee N, Yap F, Joynt GM, Sung JJ, Ahuja AT. Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J Comput Assist Tomogr. 2004;28:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, Larsson SG, Langer RD. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 14. | Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, Sverzellati N, Maher TM. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 15. | Budinger GRS, Mutlu GM. Balancing the risks and benefits of oxygen therapy in critically III adults. Chest. 2013;143:1151-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J; PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine). Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 17. | Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033-3069, 3069a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1883] [Cited by in RCA: 1897] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 18. | Grignola JC, Domingo E. Acute Right Ventricular Dysfunction in Intensive Care Unit. Biomed Res Int. 2017;2017:8217105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, Brun-Buisson C, Vignon P, Vieillard-Baron A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 21. | Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Terenzi P, Bernardo P, Valente S, Gensini GF, Peris A. Right ventricle dilation as a prognostic factor in refractory acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation. Minerva Anestesiol. 2016;82:1043-1049. [PubMed] |
| 22. | Bunge JJH, Caliskan K, Gommers D, Reis Miranda D. Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J Thorac Dis. 2018;10:S674-S682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Lee SH, Jung JS, Chung JH, Lee KH, Kim HJ, Son HS, Sun K. Right Heart Failure during Veno-Venous Extracorporeal Membrane Oxygenation for H1N1 Induced Acute Respiratory Distress Syndrome: Case Report and Literature Review. Korean J Thorac Cardiovasc Surg. 2015;48:289-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, Rycus PT, Hyer SJ, Anders MM, Agerstrand CL, Hryniewicz K, Diaz R, Lorusso R, Combes A, Brodie D; Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 650] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 25. | Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, Murphy D, Scheinkestel C, Cooper DJ, Capellier G, Pellegrino V, Pilcher D, McQuilten Z. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Lotz C, Streiber N, Roewer N, Lepper PM, Muellenbach RM, Kredel M. Therapeutic Interventions and Risk Factors of Bleeding During Extracorporeal Membrane Oxygenation. ASAIO J. 2017;63:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. 2013;17:R73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 28. | Rastan AJ, Lachmann N, Walther T, Doll N, Gradistanac T, Gommert JF, Lehmann S, Wittekind C, Mohr FW. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO). Int J Artif Organs. 2006;29:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, Davis AK. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 30. | Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14:e77-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 31. | Owings JT, Pollock ME, Gosselin RC, Ireland K, Jahr JS, Larkin EC. Anticoagulation of children undergoing cardiopulmonary bypass is overestimated by current monitoring techniques. Arch Surg. 2000;135:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Kitchens CS. To bleed or not to bleed? J Thromb Haemost. 2005;3:2607-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Sievert A, Uber W, Laws S, Cochran J. Improvement in long-term ECMO by detailed monitoring of anticoagulation: a case report. Perfusion. 2011;26:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |