Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1844
Peer-review started: July 18, 2020
First decision: November 30, 2020
Revised: December 12, 2020
Accepted: January 20, 2021
Article in press: January 20, 2021
Published online: March 16, 2021
Processing time: 229 Days and 21 Hours
Maple syrup urine disease (MSUD) is a rare autosomal-recessive disorder that affects branched-chain amino acid (BCAA) metabolism and is named after the distinctive sweet odor of affected infants’ urine. This disease is characterized by the accumulation of BCAAs and corresponding branched-chain ketoacids of leucine, isoleucine, and valine in the plasma, urine, and cerebrospinal fluid. However, the mechanisms of MSUD-induced brain damage remain poorly defined. The accumulation of BCAAs in the brain inhibits the activity of pyruvate dehydrogenase and α-ketoglutarate, disrupting the citric acid cycle and consequently impacting the synthesis of amino acids, causing cerebral edema and abnormal myelination.
We report three neonates admitted to our hospital with the classic subtype of MSUD. All three patients, with a transient normal period, presented with poor feeding, vomiting, poor weight gain, and increasing lethargy after birth. Laboratory testing revealed metabolic acidosis. The serum tandem mass spectrometry amino acid profile showed elevated plasma levels of BCAAs (leucine, isoleucine, and valine). Brain magnetic resonance imaging (MRI) presented abnormal signals mainly involving the globus pallidus, thalamus, internal capsule, brainstem, and cerebellar white matter, which represent the typical myelinated areas in normal full-term neonates.
In our patients, MRI showed typical features, in concordance with the available literature. Early detection and timely treatment are very helpful for the prognosis of MSUD patients. Therefore, we discuss the neuroimaging features of MSUD to enhance the knowledge of pediatricians about this disease.
Core Tip: This article reports three patients with maple syrup urine disease with typical clinical manifestations and magnetic resonance imaging features and reviews the related literature. The pathogenesis, pathophysiological characteristics, and typical imaging findings of this rare genetic metabolic disease are discussed.
- Citation: Li Y, Liu X, Duan CF, Song XF, Zhuang XH. Brain magnetic resonance imaging findings and radiologic review of maple syrup urine disease: Report of three cases. World J Clin Cases 2021; 9(8): 1844-1852
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1844
Maple syrup urine disease (MSUD), also known as branched-chain ketoaciduria (BCKA), is a clinically rare autosomal-recessive disorder of branched-chain amino acid (BCAA) metabolism that is caused by deficiency of the branched-chain α-ketoacid dehydrogenase (BCKD) complex[1]. MSUD was first reported by Menkes in 1954 because of the α-ketoacid excreted in urine, which smells like maple syrup, leading to the name of the disease. The annual incidence rate of MSUD is 1 in 180000 live births worldwide[2]. The initial symptoms of affected infants are complex and lack specific characteristics; therefore, the disease is often misdiagnosed. However, the disease progresses to mental retardation, spastic paralysis, cortical blindness, and eventually death[3]. The classic form of MSUD involves early onset, most commonly with severe symptoms. Due to the characteristic magnetic resonance imaging (MRI) findings of MSUD encephalopathy, medical imaging provides a useful tool for early diagnosis.
We report three neonatal patients with the classic subtype of MSUD with neuroimaging findings. MRI examination was performed in all cases with a 1.5T MRI scanner (Signa HDe, GE Healthcare, United States). A propeller T2 [repetition time (TR) 5000 ms, echo time (TE) 130 ms] sequence was performed in the axial plane, and a turbo spin echo (TSE) T2 (TR 3040 ms, TE 100 ms) sequence was performed in the coronal plane. TSE T1 (TR 500 ms, TE 20 ms) sequence was obtained in both the axial and sagittal planes. Fluid-attenuated inversion recovery (FLAIR) (TR 9000 ms, TE 120 ms) and diffusion-weighted imaging (DWI) (TR 4000 ms, TE 100 ms, b = 0 s/mm2 and b = 1000 s/mm2) sequences were acquired in the axial plane. The section thickness was 4 mm in the axial images and 6 mm in both the coronal and sagittal images. The interlamellar spacing was 1 mm in the axial images and 2 mm in both the coronal and sagittal images.
Case 1: An 11-day-old male infant was born at term after an uneventful pregnancy and a normal vaginal delivery in our hospital. The patient presented with poor feeding and milk choking before 5 d of life.
Case 2: A 16-day-old female infant was born after a full-term normal delivery with a birth weight of 3.55 kg. She cried immediately at birth, and there was no history of respiratory distress or neonatal jaundice. One week after birth, her milk intake decreased. She was lethargic and had a poor response.
Case 3: A full-term male infant born after a normal vaginal delivery who cried immediately after birth presented on the 4th postnatal day with approximately 1-min paroxysmal seizures in which his complexion became violet, both eyes squinted, and he exhibited limb jitter, followed by relief.
Case 1: The patient was admitted to the neonatal intensive care unit, where he exhibited sleepiness, neck stiffness, lethargy, shrill cries with low volume, and irritability.
Case 2: The infant experienced repeated seizures after admission. She also presented sleepiness, lethargy, and irritability.
Case 3: The patient was hospitalized, where he presented poor feeding, vomiting, increasing lethargy, and poor weight gain.
Case 1: The patient had an insignificant medical history.
Case 2: The patient had an insignificant medical history.
Case 3: The patient had an insignificant medical history.
These three patients had insignificant personal and family history.
Case 1: On physical examination, both lower limbs were in the cross position and hypothermia was detected, but other vital signs were stable.
Case 2: Physical examination showed no obvious abnormalities.
Case 3: Cardiovascular, abdominal, and respiratory examinations were normal.
Case 1: The patient's blood metabolism was abnormal, showing elevated blood levels of ammonia (99.1 mmol/L; normal range: 18-72 mmol/L), lactic acid (5.80 mmol/L; normal range: 0.5-1.7 mmol/L), and β-hydroxybutyric acid (1.59 mmol/L; normal range: 0.031-0.263 mmol/L). Confirmatory testing of plasma amino acid levels performed with samples collected at 7 d of life showed elevated levels of BCAAs. Laboratory testing revealed metabolic acidosis (blood pH = 7.25, serum CHCO3 = 10.0 mmol/L).
Case 2: Laboratory testing revealed metabolic acidosis. The serum tandem mass spectrometry amino acid profile showed elevations of leucine, isoleucine, hydroxyproline (3392 µmol/L in total; normal value: < 450 µmol/L), and valine (1080 µmol/L; normal range: 80-300 µmol/L), suggesting MSUD.
Case 3: Biochemistry examinations revealed an elevated serum ammonia level, hypoglycemia, and ketonuria. The serum tandem mass spectrometry showed elevated plasma levels of BCAAs (leucine, isoleucine, and valine).
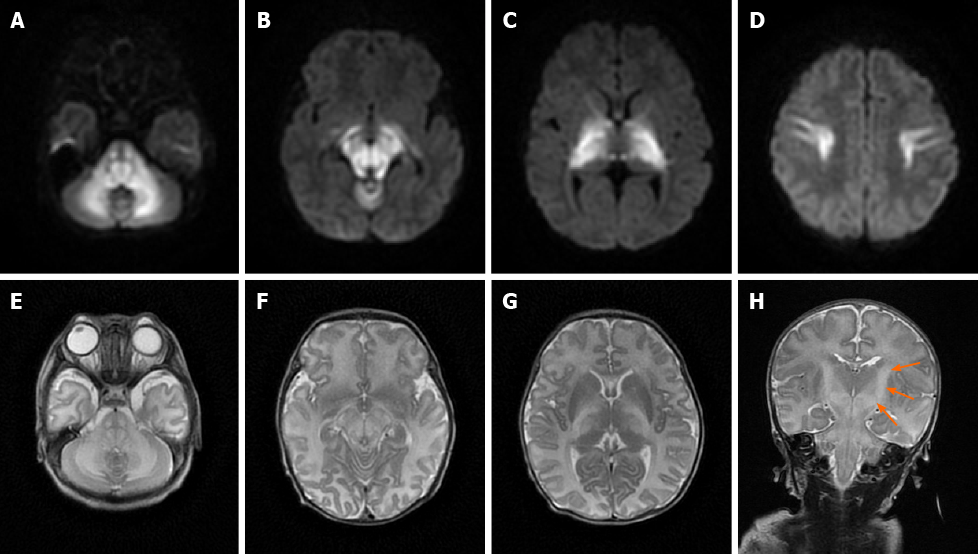
Case 1: MRI showed bilateral symmetrical lesions involving the corticospinal tracts, thalami, globus pallidus, midbrain, dorsal brain stem, and cerebellar white matter (Figure 1).
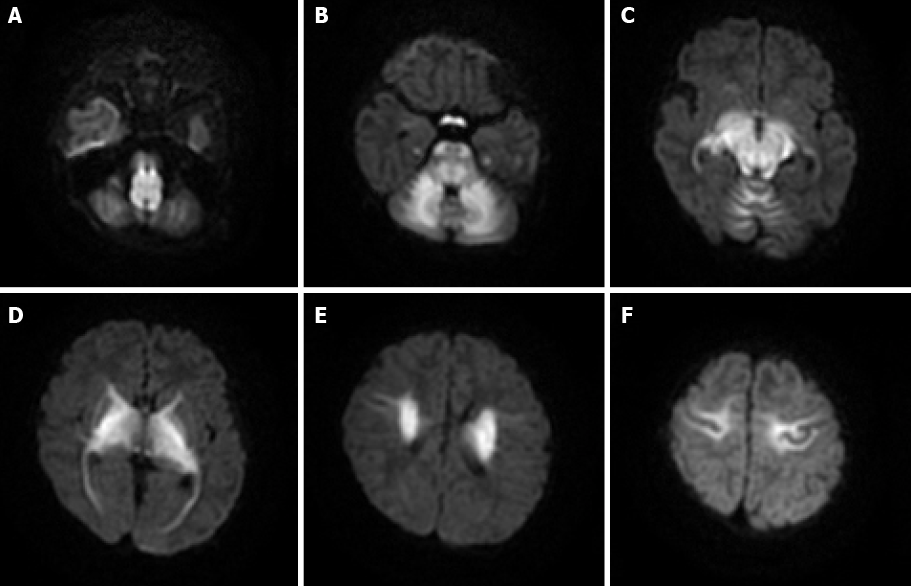
Case 2: MRI showed restricted diffusion involving the corticospinal tracts, internal capsule, thalami, globus palladi, optic tracts, brainstem, and cerebellar white matter (Figure 2).
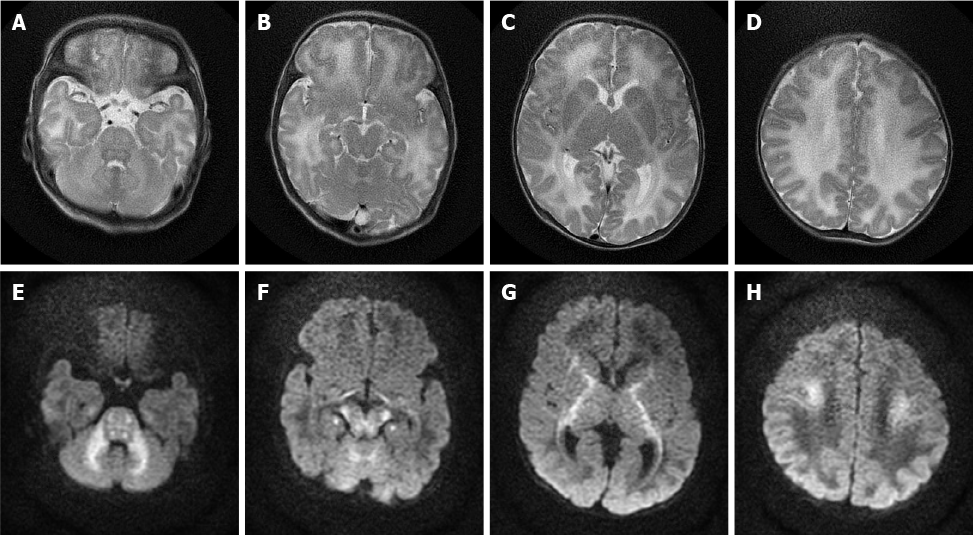
Case 3: MRI of the brain was performed, which revealed bilateral symmetrical areas of hyperintensity on T2-weighted sequences (Figure 3A-D) and restricted diffusion on DWI sequences (Figure 3E-H) involving the white matter of the cerebellum, pons, dorsal aspect of the mid brain, cerebral peduncles, corticospinal tract, internal capsule, and centrum semiovale.
Based on this pattern of restricted diffusion on MRI, a diagnosis of MSUD was made for the three patients, which was confirmed by the elevated plasma levels of BCAAs (leucine, isoleucine, and valine).
All the patients were given supportive treatment such as oxygen and intravenous fluids, and were fed special milk powder without BCAAs. Case 2 and case 3 were given phenobarbital sedation during convulsions. Case 2 underwent peritoneal dialysis to reduce BCAAs and their metabolites.
After effective treatment, all the three patients get through the acute phase and survived. After discharge, the patients continued to be fed special milk powder without BCAAs, and also avoid leucine and other essential amino acids and nutrient deficiencies. No acute attack occurred in the follow-up, but the mental development of patients lags behind that of normal children of the same age.
The BCKD complex consists of four subunits, E1α, E1β, E2, and E3[4]. The E1α subunit is synthesized by the BCKDHA gene on chromosome 19; the E1β subunit is synthesized by the BCKDHB gene on chromosome 6, the E2 subunit is synthesized by the DBT gene on chromosome 1, and the E3 subunit is synthesized by the DLD gene on chromosome 7. Gene mutations are an essential element of MSUD severity but not the sole determinant factor. The mechanisms of brain injury in patients with MSUD remain unclear, but some studies[3,5] suggest that three converging mechanisms may exist: (1) Neurotransmitter deficiencies and growth restriction associated with BCAA accumulation; (2) Competitive replacement of essential amino acids in the brain; and (3) Energy deprivation through Krebs cycle disruption associated with branched-chain ketoacid accumulation, which causes cerebral edema and abnormal myelination.
MSUD is classified into: (1) Classic; (2) Intermediate; (3) Intermittent; (4) Thiamine-responsive; and (5) E3-deficient forms based on age of onset of the disease and response to thiamine, which carry differing severities of clinical presentation and prognostic factors. The classic, intermediate, and intermittent forms of MSUD can be caused by gene mutations in the E1α, E1β, E2, and E3 subunits, but the BCKD activity of the latter two forms is higher than that of the classic form. The classic form is the most common; this form always harbors BCKDHB mutations and produces more diffuse and severe alterations in the central nervous system[6]. Neonates with the classic type of MSUD are usually normal at birth, but symptoms, which typically include poor feeding, vomiting, poor weight gain, and increasing lethargy, will manifest after a disease-free interval (usually 4-7 d), which suggests that the metabolic crisis has already begun to manifest[7,8]. In this metabolic crisis, because of the large accumulation of isoleucine, the patient's urine ordinarily smells like maple syrup, but the maple syrup odor may be difficult to identify in the first few days of life.
Many early imaging reports have mentioned that both diffuse cerebral edema and severe local edema have been observed in the acute phase[9]; these reports referred to this distinct edema pattern as MSUD edema. Because of the DWI hyperintensity on MRI, some previous studies have suggested that local edema is cytotoxic, but follow-up studies have found that this cytotoxic edema-like change did not progress to tissue infarct. Therefore, this phenomenon was considered to be the result of reversible intramyelinic edema[9-11]. This idea was supported by an animal experiment showing that in MSUD, intramyelinic vacuole formation occurs through water accumulation between the myelinic lamellae; additionally, intramyelinic edema was demonstrated by electron microscopy[12]. This theory has explained why MSUD edema mainly involves the globus pallidus, thalamus, internal capsule, brainstem, and cerebellar white matter; these represent the typical myelinated areas in normal full-term neonates at birth[13,14]. The diffuse edema found in MSUD edema is vasogenic-interstitial edema, which is usually observed in unmyelinated areas due to alterations in the blood-brain barrier[12]. Diffuse edema is relatively rare in the classic form of MSUD, but related reports are available[15].
Early studies have shown that computed tomography (CT) scans can detect brain edema due to severe metabolic disorder, but the lack of specificity and the observed CT changes are helpful only for the differential diagnosis of the disease[16].
Most recent studies have shown that MRI, especially the DWI sequence, is the preferred and optimum imaging examination for diagnosis of MSUD[10,17]. Imaging features are diagnostic in the early weeks of life; the hyperintensity on DWI mainly involves the globus pallidus, thalamus, internal capsule, brainstem, and cerebellar white matter, which represent the typical myelinated areas in normal full-term neonates[13,14]. MRI results can be obtained in few minutes, while several days are required to obtain the results of other diagnostic examinations; therefore, MRI should be conducted in all neonates who are suspected of having MSUD before mass spectrometry analysis or genetic examinations are performed. We searched PubMed, ScienceDirect, SpringerLink, and other literature databases for currently available reports and found a total of 23 cases of classical type MSUD in neonates, infants, and children with MRI manifestations. All patients showed distinctive and wide-ranging abnormal brain parenchyma signals, including abnormalities in the basal ganglia in 21 cases; the cerebellum, mesencephalon, pons, and corticospinal tract area in 20 cases; and the thalamus in 18 cases (Table 1)[8,11,18-27]. This result indicates that the classic form of MSUD has typical imaging manifestations, namely, a hyperintense signal on DWI and FLAIR sequences involving the globus pallidus, thalamus, internal capsule, brainstem, and cerebellar white matter. The three cases that we reported had similar features, which strongly supports the above-mentioned theory. The alterations in brain tissue were more pronounced on DWI images than on FLAIR images, but some studies[18] have also mentioned an increased signal in the medulla area on FLAIR images with no apparent diffusion coefficient value reduction in the corresponding white matter. We suggest that this finding represents demyelination and disrupted water content in the white matter. This manifestation in the white matter was reported in 12 of the 23 cases that we found in the literature databases.
| Ref. | Total patients | Corticospinal tract | Central portion of the centrum semiovale | Basal ganglia | Thalamus | Pons | Mesencephalon | Cerebellum |
| Cheng et al[18] 2017 | 6 | 5 | 5 | 6 | 4 | 5 | 5 | 5 |
| Hou et al[19] 2016 | 7 | 5 | 3 | 5 | 4 | 5 | 5 | 5 |
| Sener et al[20] 2007 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Cavalleri et al[21] 2002 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Shah et al[22] 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Jain et al[23] 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ha et al[11] 2004 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Cornelius et al[24] 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Kilicarslan et al[25] 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Indiran et al[26] 2013 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Kathait et al[27] 2018 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Jain et al[8] 2013 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
At present, there are few imaging studies on MSUD, and most of them focus on imaging manifestations. Only a few reports have examined the application of MRI in follow-up of MSUD patients. Vogel et al[28] confirmed that biochemical indicators in the blood cannot accurately reflect the contents of these metabolites in the brain. Therefore, in addition to blood tandem mass spectrometry, MRI should be performed when evaluating the treatment effect on patients. Cheng et al[18] showed that along with increases in the normal myelination area with age, the hyperintense range on DWI also expands. Moreover, this study found that DWI hyperintensity could disappear with appropriate treatment, which supports the hypothesis that MSUD edema on DWI is intramedullary edema rather than cytotoxic edema. Sato et al[29] reported the specificity of magnetic resonance spectroscopy (MRS) of MSUD, that is, a BCAA methyl peak can be observed at 0.9-1.0 PPM. Additionally, this peak can be reduced during the stabile period of disease, which can be used as an effective means to evaluate the stable period of MSUD.
We present three cases of MSUD. In our patients, MRI showed typical features, in concordance with the available literature. Early detection and timely treatment are very helpful for the prognosis of MSUD patients, but the clinical manifestations of MSUD are non-specific and similar to some neonatal brain diseases. Therefore, MRI examination should be performed in neonates who manifest poor feeding, vomiting, poor weight gain, and increasing lethargy after birth.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Strauss KA, Puffenberger EG, Morton DH. "Maple syrup urine disease." GeneReviews®. [Internet]. University of Washington, Seattle, 2013. |
| 2. | Puffenberger EG. Genetic heritage of the Old Order Mennonites of southeastern Pennsylvania. Am J Med Genet C Semin Med Genet. 2003;121C:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, Cheng KC, Lanoue KF, Flanagan JM. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Chuang DT. Maple syrup urine disease: it has come a long way. J Pediatr. 1998;132:S17-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, Strauss KA. Biochemical correlates of neuropsychiatric illness in maple syrup urine disease. J Clin Invest. 2013;123:1809-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Rodríguez-Pombo P, Navarrete R, Merinero B, Gómez-Puertas P, Ugarte M. Mutational spectrum of maple syrup urine disease in Spain. Hum Mutat. 2006;27:715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, Shellmer D, Moser AB, Morton DH. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Jain A, Jagdeesh K, Mane R, Singla S. Imaging in classic form of maple syrup urine disease: a rare metabolic central nervous system. J Clin Neonatol. 2013;2:98-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Righini A, Ramenghi LA, Parini R, Triulzi F, Mosca F. Water apparent diffusion coefficient and T2 changes in the acute stage of maple syrup urine disease: evidence of intramyelinic and vasogenic-interstitial edema. J Neuroimaging. 2003;13:162-165. [PubMed] |
| 10. | Jan W, Zimmerman RA, Wang ZJ, Berry GT, Kaplan PB, Kaye EM. MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology. 2003;45:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Ha JS, Kim TK, Eun BL, Lee HS, Lee KY, Seol HY, Cha SH. Maple syrup urine disease encephalopathy: a follow-up study in the acute stage using diffusion-weighted MRI. Pediatr Radiol. 2004;34:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Harper PA, Healy PJ, Dennis JA. Ultrastructural findings in maple syrup urine disease in Poll Hereford calves. Acta Neuropathol. 1986;71:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Brismar J, Aqeel A, Brismar G, Coates R, Gascon G, Ozand P. Maple syrup urine disease: findings on CT and MR scans of the brain in 10 infants. AJNR Am J Neuroradiol. 1990;11:1219-1228. [PubMed] |
| 14. | Sakai M, Inoue Y, Oba H. Age dependence of diffusion-weighted magnetic resonance imaging findings in maple syrup urine disease encephalopathy. J Comput Assist Tomogr. 2005;29:524-527. |
| 15. | Wang HW, Wang XM, Guo QY, Fu JH. Maple syrup urine disease encephalopathy in neonate: A case report. Zhonghua Fangshe Xue Zazhi. 2008;42:1221-1222. |
| 16. | Lungarotti MS, Calabro A, Signorini E, Garibaldi LR. Cerebral edema in maple syrup urine disease. Am J Dis Child. 1982;136:648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Xia W, Yang W. Diffusion-weighted magnetic resonance imaging in a case of severe classic maple syrup urine disease. J Pediatr Endocrinol Metab. 2015;28:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Cheng A, Han L, Feng Y, Li H, Yao R, Wang D, Jin B. MRI and clinical features of maple syrup urine disease: preliminary results in 10 cases. Diagn Interv Radiol. 2017;23:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Hou L, Han LS, Chi RM, Wang DB. Brain MRI and clinical characteristics of maple syrup urine disease. Shiyong Fangshe Xue Zazhi. 2016;10:1582-1585. [DOI] [Full Text] |
| 20. | Sener RN. Maple syrup urine disease: diffusion MRI, and proton MR spectroscopy findings. Comput Med Imaging Graph. 2007;31:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Cavalleri F, Berardi A, Burlina AB, Ferrari F, Mavilla L. Diffusion-weighted MRI of maple syrup urine disease encephalopathy. Neuroradiology. 2002;44:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Shah T, Purohit S, Raval M. Imaging in Maple Syrup Urine Disease. Indian J Pediatr. 2018;85:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Jain P, Sharma S, Sankhyan N, Gupta N, Kabra M, Gulati S. Imaging in neonatal maple syrup urine disease. Indian J Pediatr. 2013;80:87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Cornelius LP, Kannan B, Saravanan V, Venkatesan EP. Intramyelinic edema in maple syrup urine disease. Ann Indian Acad Neurol. 2014;17:211-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kilicarslan R, Alkan A, Demirkol D, Toprak H, Sharifov R. Maple syrup urine disease: diffusion-weighted MRI findings during acute metabolic encephalopathic crisis. Jpn J Radiol. 2012;30:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Indiran V, Gunaseelan RE. Neuroradiological findings in maple syrup urine disease. J Pediatr Neurosci. 2013;8:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kathait AS, Puac P, Castillo M. Imaging Findings in Maple Syrup Urine Disease: A Case Report. J Pediatr Neurosci. 2018;13:103-105. [PubMed] |
| 28. | Vogel KR, Arning E, Wasek BL, McPherson S, Bottiglieri T, Gibson KM. Brain-blood amino acid correlates following protein restriction in murine maple syrup urine disease. Orphanet J Rare Dis. 2014;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Sato T, Muroya K, Hanakawa J, Asakura Y, Aida N, Tomiyasu M, Tajima G, Hasegawa T, Adachi M. Neonatal case of classic maple syrup urine disease: usefulness of (1) H-MRS in early diagnosis. Pediatr Int. 2014;56:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |