Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1329
Peer-review started: September 8, 2020
First decision: November 20, 2020
Revised: December 3, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: February 26, 2021
Processing time: 150 Days and 18.3 Hours
The most common EGFR mutations are in-frame deletions in exon 19 and point mutations in exon 21. Cases with classical EGFR mutations show a good response to EGFR tyrosine kinase inhibitors (TKIs), the standard first-line treatment. With the development of next generation sequencing, some uncommon genomic mutations have been detected. However, the effect of TKIs on such uncommon EGFR mutations remains unclear.
Here, we report a case of rare EGFR co-mutation in non-small cell lung cancer and the efficacy of afatinib on this EGFR co-mutation. A 64-year-old woman was diagnosed with thoracolumbar and bilateral local rib bone metastases, bilateral pulmonary nodules, and pericardial and left pleural effusion. The pathological diagnosis was lung adenocarcinoma. To seek potential therapeutic regimens, rare co-mutation comprising rare EGFR G724S/R776H mutations and amplification were identified. The patient experienced a significant clinical response with a progression-free survival of 17 mo.
A case of non-small cell lung cancer with rare EGFR G724S/R776H mutations and EGFR amplification responds well to TKI treatment.
Core Tip: EGFR represents the first identified targetable oncogenic driver discovered in non-small cell lung cancer (NSCLC). The most common EGFR mutations are in-frame deletions in exon 19 and point mutations in exon 21. However, rare mutations were found in nearly 10%-15% of EGFR-positive NSCLC and NSCLC with rare co-mutations had significantly different responses to EGFR tyrosine kinase inhibitor. Herein, we describe a rare case of rare EGFR G724S/R776H mutations and amplification in a NSCLC responding to afatinib.
- Citation: He SY, Lin QF, Chen J, Yu GP, Zhang JL, Shen D. Efficacy of afatinib in a patient with rare EGFR (G724S/R776H) mutations and amplification in lung adenocarcinoma: A case report. World J Clin Cases 2021; 9(6): 1329-1335
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1329.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1329
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death, especially in developing countries such as China[1]. A recent study shows that in 2015 there were about 733000 newly diagnosed cases of NSCLC in China, with approximately 610000 Chinese patients dying from the disease[2]. NSCLC accounts for the majority (75%) of clinical lung cancer cases. Adenocarcinoma is the most common histological type of NSCLC and can be subdivided into different clinically relevant molecular subtypes according to the type of driver gene mutation.
EGFR was the first identified targetable oncogenic driver discovered in NSCLC[3]. Approximately 40% of Asian patients with newly diagnosed metastatic NSCLC harbor a somatic mutation in the EGFR gene[4]. The most common EGFR mutations are in-frame deletions in exon 19 and point mutations in exon 21. However, rare mutations were found in nearly 10%-15% of EGFR-positive NSCLC cases[5,6]. Although it has been reported that afatinib is effective against rare EGFR mutations, there are significant differences in progression-free survival (PFS) and overall survival (OS) among patients with different rare EGFR mutations[7]. Here, we describe a rare case of EGFR G724S/R776H mutations and EGFR amplification in an NSCLC patient responding to afatinib.
A 64-year-old nonsmoking woman visited our hospital on April 26, 2019 for further treatment because she could not tolerate the side effects of previous chemotherapy for lung adenocarcinoma, including myelosuppression and cardiac and renal insufficiency.
Chest computed tomography (CT) showed bone metastases in the thoracolumbar spine and bilateral local ribs, nodules in both lungs, and pericardial and left pleural effusion.
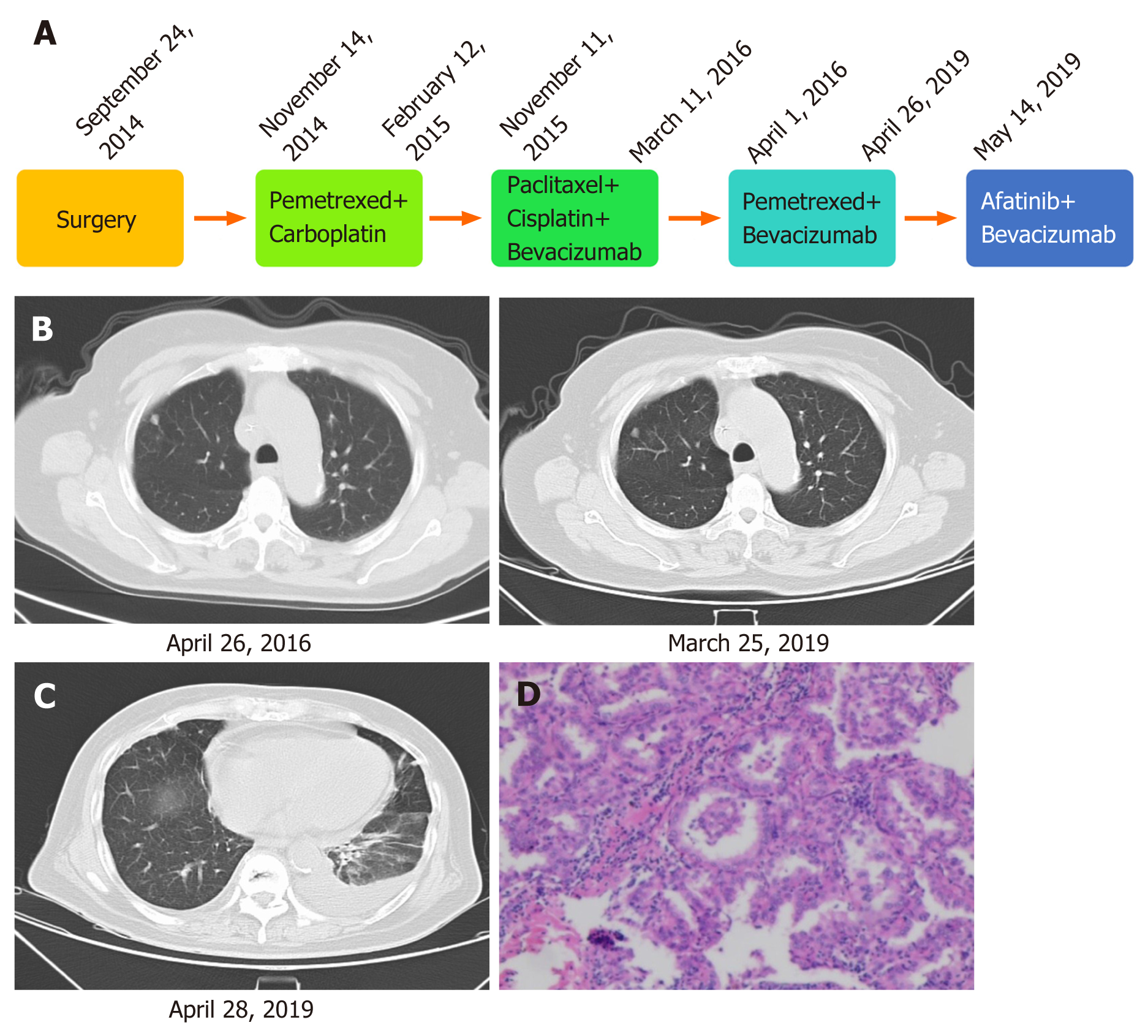
On September 10, 2014, the patient went to a local hospital because of sudden glossolalia with right lower limb numbness, and was diagnosed with stage IIIA lung adenocarcinoma, and then she underwent resection of the upper lobe on September 24, 2014 (Figure 1A). The patient received pemetrexed combined with carboplatin for four cycles of chemotherapy. In November 2015, the disease progressed. The patient was given paclitaxel plus cisplatin combined with bevacizumab for six cycles from November 11, 2015 to March 11, 2016. From April 1, 2016 to April 26, 2019, the patient received pemetrexed combined with bevacizumab, and his condition remained stable (Figure 1B). After the last cycle of treatment with pemetrexed plus bevacizumab, chest CT showed bone metastases in the thoracolumbar spine and bilateral local ribs, nodules in both lungs, and pericardial and left pleural effusion (Figure 1C).
The previous pathological diagnosis was lung adenocarcinoma (Figure 1D).
After the last cycle of treatment with pemetrexed plus bevacizumab, CT showed bone metastases in the thoracolumbar spine and bilateral local ribs, nodules in both lungs, and pericardial and left pleural effusion (Figure 1C).
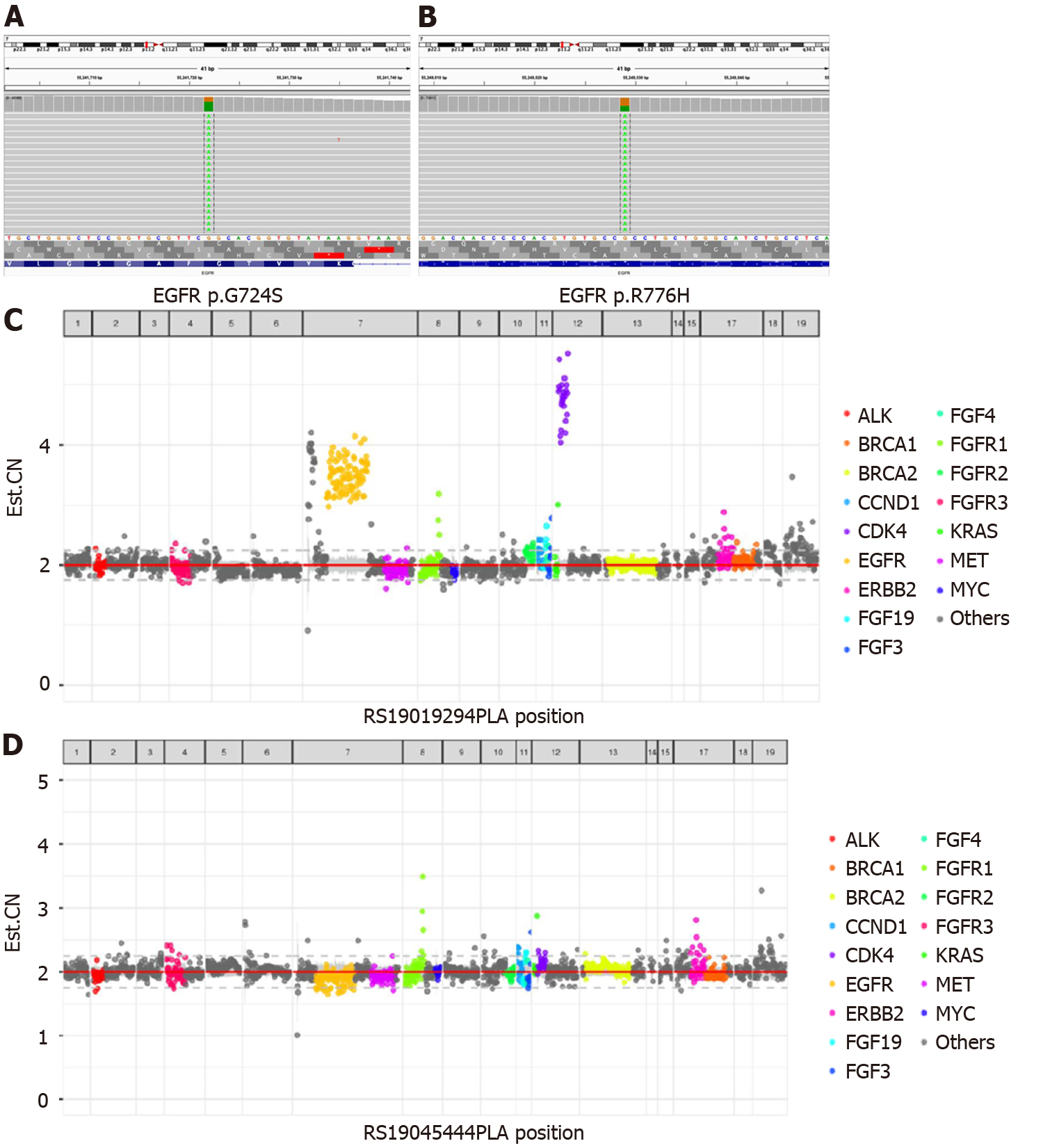
Because the patient could not tolerate the side effects of chemotherapy, potential therapeutic regimens were sought. Her blood was subjected to NGS analysis, and a rare EGFR G724S [mutant allele frequency (MAF): 67.59%] mutation in exon 18 and R776H (MAF: 40.54%) mutation in exon 20 as well as amplification was identified (Figure 2). Therefore, the patient was finally diagnosed with lung adenocarcinoma with rare EGFR G724S and R776H mutations and amplification.
Based on the above findings, the patient was administered with afatinib (30 mg qd) combined with bevacizumab and followed regularly.
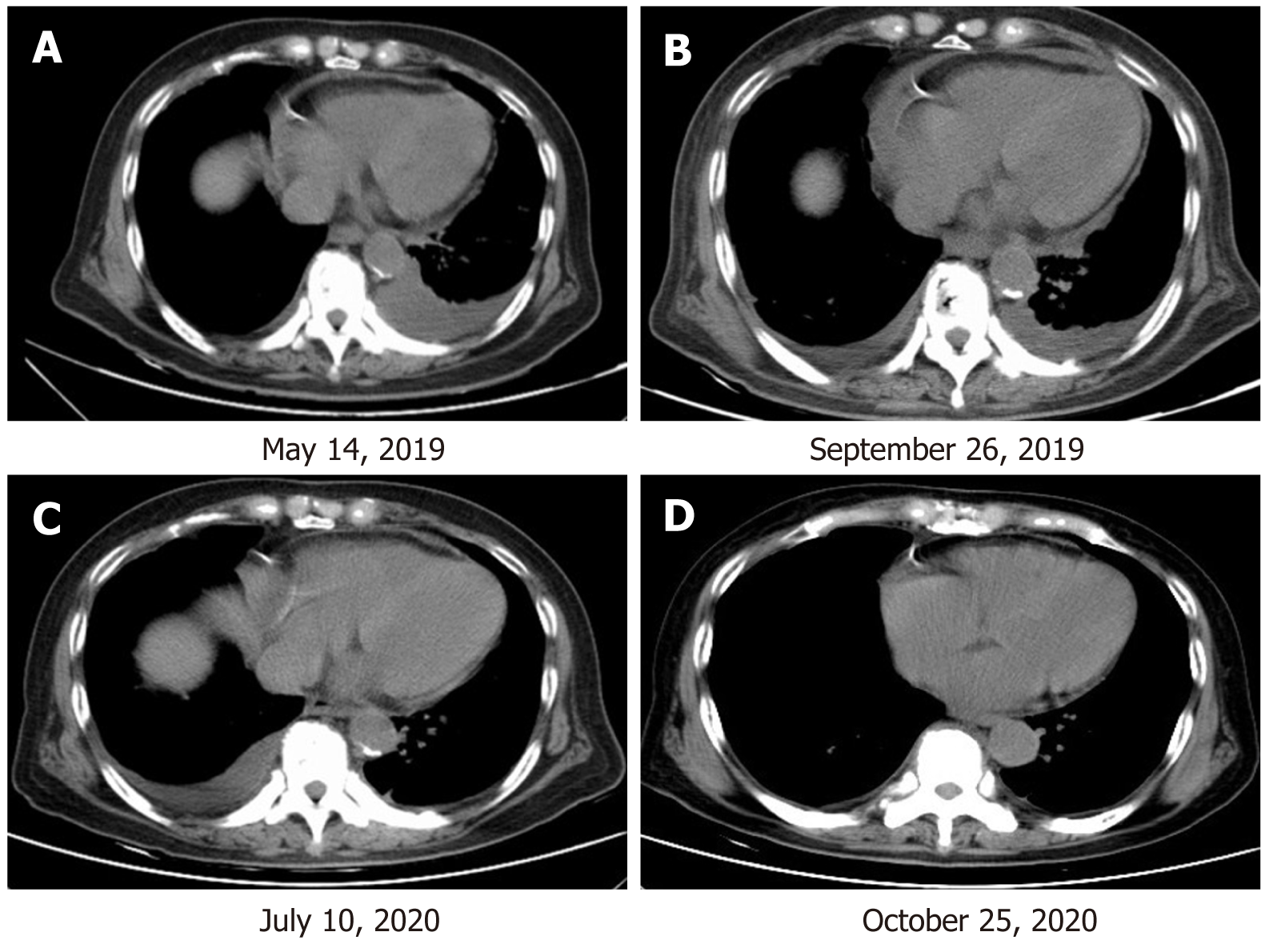
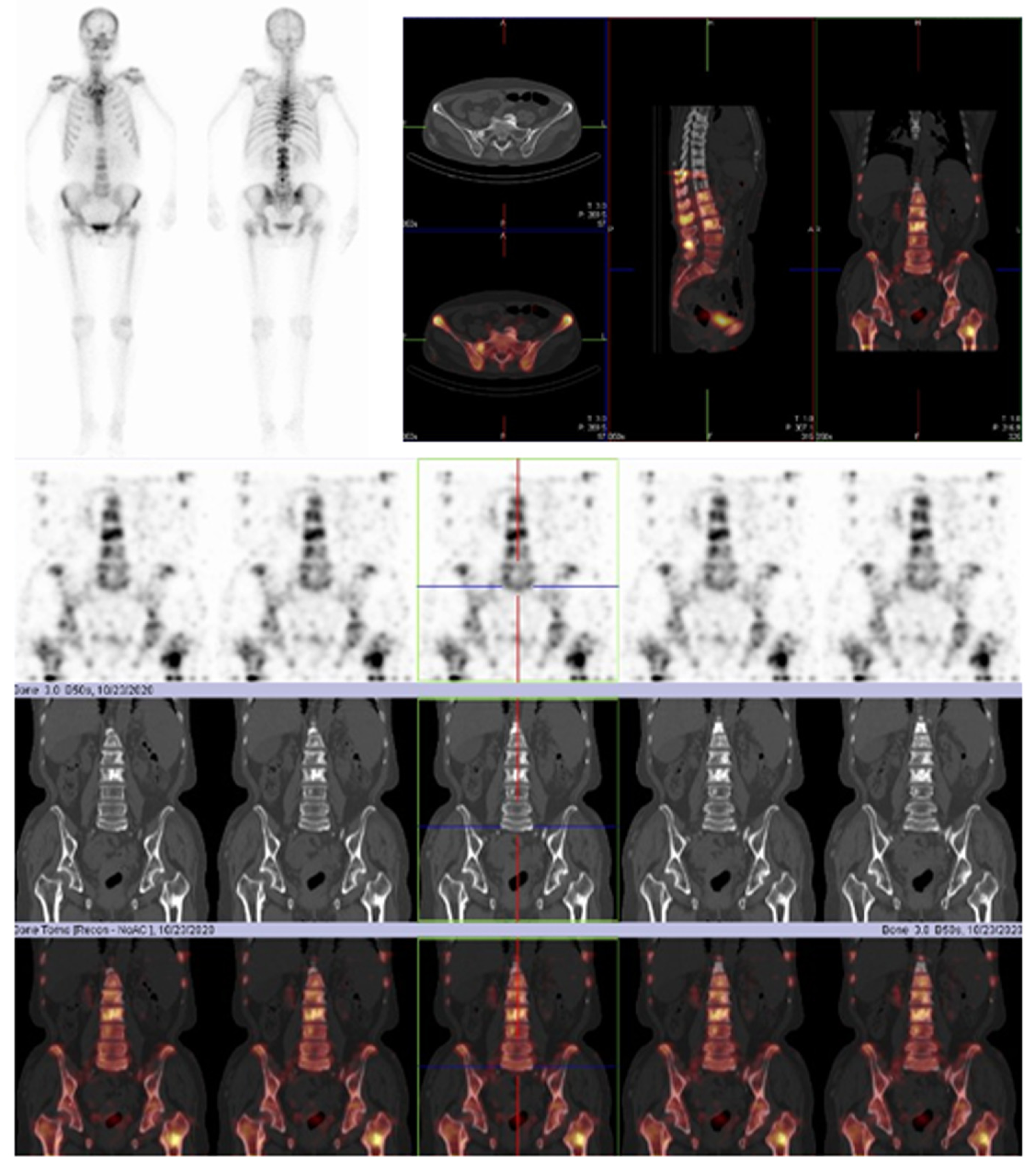
After 4 mo of treatment, the left pleural effusion and pericardial effusion were significantly reduced and the patient showed SD according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (Figure 3). The MAFs for both R776H and G724S were also decreased (R776H from 40.54% to 0.16% and G724S from 67.59% to undetected). During this period, zoledronic acid was irregularly given for anti-bone metastasis therapy. The patient was followed several times, and CT performed on July 10, 2020 showed that the tumor lesion of the right lung remained stable (Figure 3). However, the reexamination on October 25, 2020 revealed disease progression with multiple bone metastases (Figure 4). Imaging studies indicated progressive disease (PD), and the patient's final PFS was 17 mo. There were no obvious adverse reactions during the treatment.
In the era of precision medicine, EGFR genotyping has become the standard practice for NSCLC, but the identification of rare mutations does not necessarily imply clear targeted therapeutic action. Due to the small number and high heterogeneity of patients with rare mutations, the efficacy of EGFR TKIs in patients with rare EGFR mutations remains unclear. However, a large number of clinical studies have shown significant differences in the efficacy of EGFR TKIs in patients with rare mutations in EGFR. Therefore, these patients should be analyzed separately in clinical studies to provide them with more effective individualized treatment.
As a second-generation EGFR TKI, afatinib is more effective than chemotherapies and first-generation EGFR-TKIs[8,9]. In LUX-Lung 7 and LUX-Lung 8 studies, it was found that patients treated with afatinib as both first-line treatment (compared with gefitinib) and second-line treatment (compared with erlotinib) resulted in a longer PFS or OS[10,11]. However, most patients with rare or complex EGFR mutations had a shorter PFS than patients with exon 19 deletion (16.0 mo vs 9.0 mo; HR, 0.34; 95%CI, 0.13-0.94, P = 0.037)[12]. In addition, it has been reported that patients aged ≥ 65 years with rare mutations have significantly longer PFS than patients aged < 65 years after receiving EGFR TKIs (median PFS: 10.5 mo vs 5.5 mo, P = 0.0320)[13]. Patients with rare EGFR mutations are often excluded from clinical trials. However, these adverse characteristics are frequently encountered in clinical practice, and in particular, rare mutations of EGFR (two or more EGFR mutations at the same time) are generally considered a relatively rare event representing a unique and highly heterogeneous subset of NSCLC.
In summary, we report a rare case of NSCLC with EGFR G724S/R776H and amplification, which has never been reported before. The successful use of afatinib in this case may provide a new treatment option for this type of EGFR co-mutation, especially for patients who decline or are not suitable for chemotherapy. By deepening our understanding of functional and structural differences between rare subtypes of EGFR variation, the different responses to EGFR TKIs and overall survival rates of patients with these mutations need to be further studied. This case provides valuable insights for future clinical cancer treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Farshadpour F, Higuera-de la Tijera F, Ishizawa K S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15461] [Article Influence: 2576.8] [Reference Citation Analysis (2)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13200] [Article Influence: 1466.7] [Reference Citation Analysis (3)] |
| 3. | Baek JH, Sun JM, Min YJ, Cho EK, Cho BC, Kim JH, Ahn MJ, Park K. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: a retrospective analysis in Korea. Lung Cancer. 2015;87:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Fogli S, Polini B, Del Re M, Petrini I, Passaro A, Crucitta S, Rofi E, Danesi R. EGFR-TKIs in non-small-cell lung cancer: focus on clinical pharmacology and mechanisms of resistance. Pharmacogenomics. 2018;19:727-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH, Zheng MY, Bai XY, Wang Z, Su J, Chen ZH, Zhang XC, Dong ZY, Wu SP, Jiang BY, Chen HJ, Wang BC, Xu CR, Zhou Q, Mei P, Luo DL, Zhong WZ, Yang XN, Wu YL. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812-3821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Saxon JA, Sholl LM, Jänne PA. EGFR L858M/L861Q cis Mutations Confer Selective Sensitivity to Afatinib. J Thorac Oncol. 2017;12:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2568] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 9. | Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1577] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 10. | Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 892] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 11. | Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, Göker E, Georgoulias V, Li W, Isla D, Guclu SZ, Morabito A, Min YJ, Ardizzoni A, Gadgeel SM, Wang B, Chand VK, Goss GD; LUX-Lung 8 Investigators. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 12. | Ho GF, Chai CS, Alip A, Wahid MIA, Abdullah MM, Foo YC, How SH, Zaatar A, Lam KS, Leong KW, Low JS, Yusof MM, Lee EC, Toh YY, Liam CK. Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer. 2019;19:896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Tsai MJ, Hung JY, Lee MH, Kuo CY, Tsai YC, Tsai YM, Liu TC, Yang CJ, Huang MS, Chong IW. Better Progression-Free Survival in Elderly Patients with Stage IV Lung Adenocarcinoma Harboring Uncommon Epidermal Growth Factor Receptor Mutations Treated with the First-line Tyrosine Kinase Inhibitors. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |