Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1156
Peer-review started: October 16, 2020
First decision: November 24, 2020
Revised: December 6, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 16, 2021
Processing time: 106 Days and 8.1 Hours
Pulmonary alveolar proteinosis (PAP) is a rare condition that can cause progressive symptoms including dyspnea, cough and respiratory insufficiency. Secondary PAP is generally associated with hematological malignancies including chronic myelomonocytic leukemia (CMML). To the best of our knowledge, this is the first reported case of PAP occurring secondary to CMML.
We report the case of a 63-year-old male who presented with a recurrent cough and gradually progressive dyspnea in the absence of fever. Based upon clinical symptoms, computed tomography findings, bone marrow aspiration, flow cytometry studies and cytogenetic analyses, the patient was diagnosed with PAP secondary to CMML. He underwent whole lung lavage in March 2016 to alleviate his dyspnea, after which he began combined chemotherapeutic treatment with decitabine and cytarabine. The patient died in January 2020 as a consequence of severe pulmonary infection.
This case offers insight regarding the mechanistic basis for PAP secondary to CMML and highlights potential risk factors.
Core Tip: Pulmonary alveolar proteinosis secondary to chronic myelomonocytic leukemia is a rare disease that has been insufficiently studied to date. Herein, we report a case of pulmonary alveolar proteinosis secondary to chronic myelomonocytic leukemia and perform a comprehensive literature review. We also provide a framework for understanding the mechanistic basis of this disease. It is likely that pulmonary alveolar proteinosis symptoms can be alleviated by achieving primary disease control. Together, these findings may contribute to improvements in clinical practice.
- Citation: Chen C, Huang XL, Gao DQ, Li YW, Qian SX. Chronic myelomonocytic leukemia-associated pulmonary alveolar proteinosis: A case report and review of literature. World J Clin Cases 2021; 9(5): 1156-1167
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1156.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1156
Pulmonary alveolar proteinosis (PAP) is a rare condition wherein patients exhibit excessive surfactant accumulation within the pulmonary alveoli, resulting in progressive symptoms including dyspnea, cough and respiratory insufficiency. The overall incidence of PAP is 8.7 per million[1]. It is classified into three categories: Idiopathic PAP (90% of total cases), secondary PAP (sPAP, less than 10% of total cases), and congenital PAP (2% of total cases). Cases of sPAP are most commonly associated with myelodysplastic syndrome (MDS), acute myelogenous leukemia (AML) and other hematological malignancies[2,3], affecting an estimated 5.3% of individuals with such malignancies[4]. The median age of MDS/sPAP onset is 51 years, and the most common symptoms include fever (45%), dyspnea on exertion (42%) and cough (42%)[5]. The estimated 2-year survival rate for patients with sPAP complicating hematological disorders is 46%[5].
Some evidence suggests that interactions between aspergillosis antigens may interact with lung surfactant to induce PAP. Granulocyte-macrophage colony-stimulating factor (GM-CSF) knockout mice or interleukin-3/GM-CSF/interleukin-5βc chain knockout mice also develop pulmonary involvement similar to that observed in the context of human PAP[6]. This suggests that PAP may arise due to insufficient phospholipid clearance by pulmonary macrophages. In contrast to idiopathic PAP, congenital PAP may be due to a lack of macrophage-mediated surfactant clearance as a consequence of the deletion of the GM-CSF gene[5]. However, sPAP is primarily due to conditions that alter alveolar macrophage numbers or functionality.
Chronic myelomonocytic leukemia (CMML) is an age-related disease characterized by impaired hematopoiesis and myeloid cell dysplasia owing to myeloid cell progenitors being hypersensitive to GM-CSF stimulation[7]. It demonstrates a propensity to transform into AML and has long been considered a form of MDS. A variety of mechanisms may cause PAP in patients with hematologic malignancies including CMML. For example, prior work suggests that changes in cytokines capable of inhibiting GM-CSF synthesis such as IL-10 may cause PAP[8]. Moreover, macrophages develop from monocyte precursors, consistent with the etiology of CMML and sPAP being associated with mononuclear cell abnormalities. There are very few reports to date of patients presenting with both sPAP and CMML. Herein, we describe a case of a patient suffering from both of these conditions, providing further evidence for the relationship between sPAP incidence and mononuclear cell abnormalities.
A 63-year-old male was admitted to our hospital in December 2015 for recurrent cough and gradually progressive dyspnea over the past 3 mo. The condition had become aggravated during the 2 wk prior to admission.
The patient first presented 5 years ago with a cough and dyspnea with exercise that improved with rest. He returned to the clinic for follow-up care at regular intervals. He was admitted to the emergency department due to the gradual aggravation of these symptoms 2 wk ago. Chest computed tomography (CT) scans revealed a pattern of acute bilateral interstitial pneumonia, and as such the patient required hospitalization.
The patient had a 20-year history of hypertension and had been taking amlodipine regularly.
The patient was free of any known congenital disease.
Vital signs were within normal limits at the time of admission, with a heart rate of 106 bpm, blood pressure of 178/71 mmHg, a respiratory rate of 20 breaths/min and a temperature of 37.1 °C. His height was 175 cm, and his weight was 76 kg. Physical examination revealed audible bilateral crackling in the lungs.
Hematological examination revealed a white blood cell count of 11.1 × 109/L, a hemoglobin level of 9.5 g/dL, a platelet count of 769 × 109/L, an absolute monocyte count of 3.8 × 109/L and an absolute eosinophil count of 0.3 × 109/L.
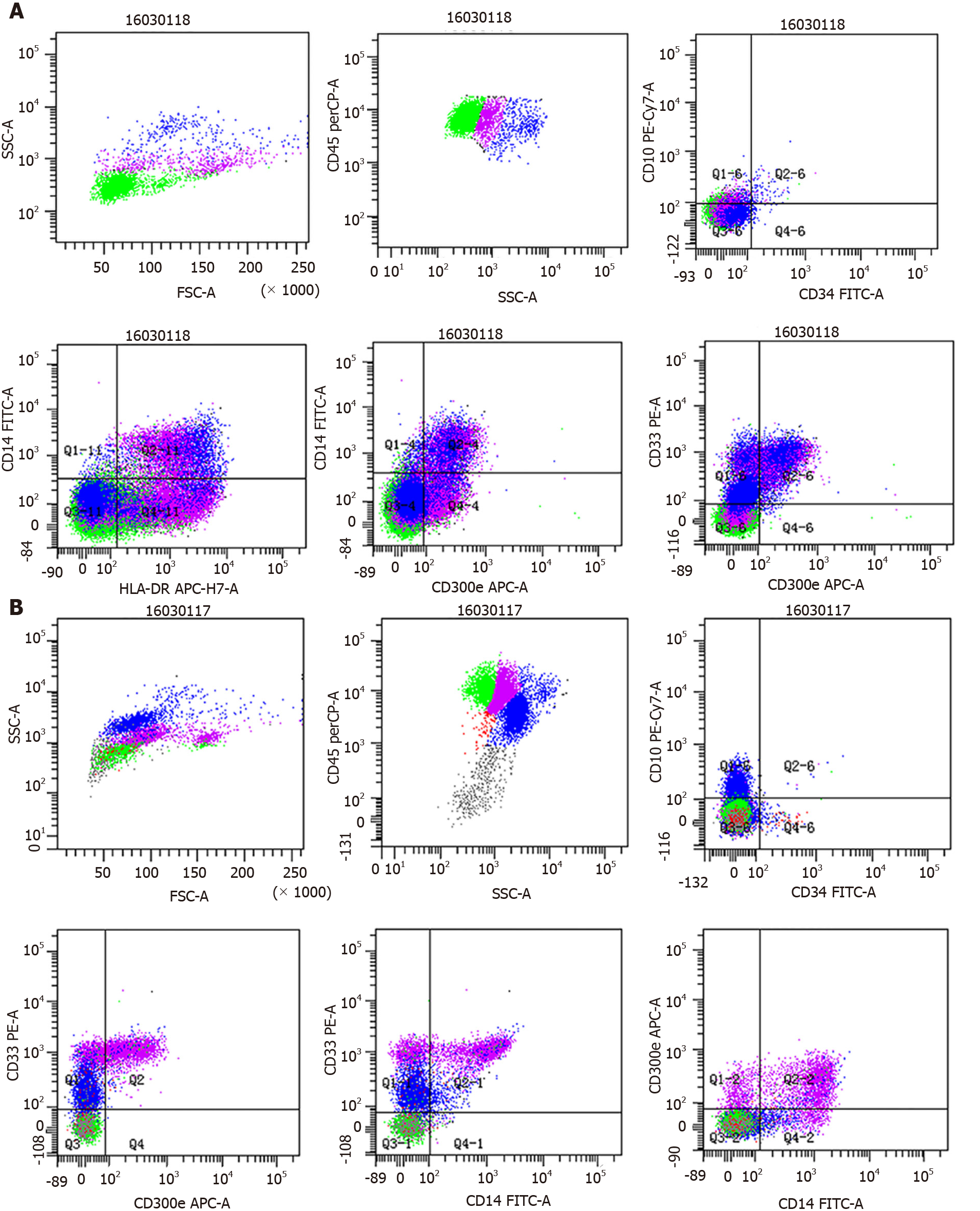
Bronchoalveolar lavage fluid (BALF) appeared light and milky, and differential BALF cell counts revealed the presence of 82% lymphocytes, 11% monocytes and 7% neutrophils. Arterial blood gas analyses under ambient air were performed in an effort to identify the causes of cough and dyspnea, revealing both hypoxemia and hypocapnia. Histological analyses exhibited the thickening of the alveolar septum with fiber hyperplasia, vasodilation, congestion and the infiltration of histiocytes and inflammatory cells. Samples were positive for periodic acid-Schiff staining, and flow cytometry analyses were performed as previously described[9], revealing an elevated frequency of monocytes in the BALF samples (12.4%) with CD33+ CD14+ CD300e+ mature monocytes accounting for 4% of nucleated cells and CD33+ CD14- CD300e+ partially immature monocytes accounting for 8.4% of nucleated cells (Figure 1A).
To better understand the complete blood count abnormalities observed in this patient, we additionally conducted bone marrow aspiration and biopsy. Marked monocytic (30%) hyperplasia was detected with 7.4% monocytic blasts, while myeloid blasts were within the normal range (0.5%). Flow cytometry analyses of bone marrow samples additionally revealed elevated frequencies of monocytes (44% of nucleated cells) and of CD33bright CD14- CD300e+ immature monocytes (7.4% of nucleated cells) (Figure 1B). Samples were negative for BCR/ABL, PDGFRA or PDGFRB cytogenetic aberrations but were positive for JAK2-V617F. CMML molecular studies revealed the presence of synonymous ASXL and SRSF mutations without any corresponding TET2 mutations. These examinations were performed as previously described[9]. Karyotyping revealed the inversion of chromosome 6.
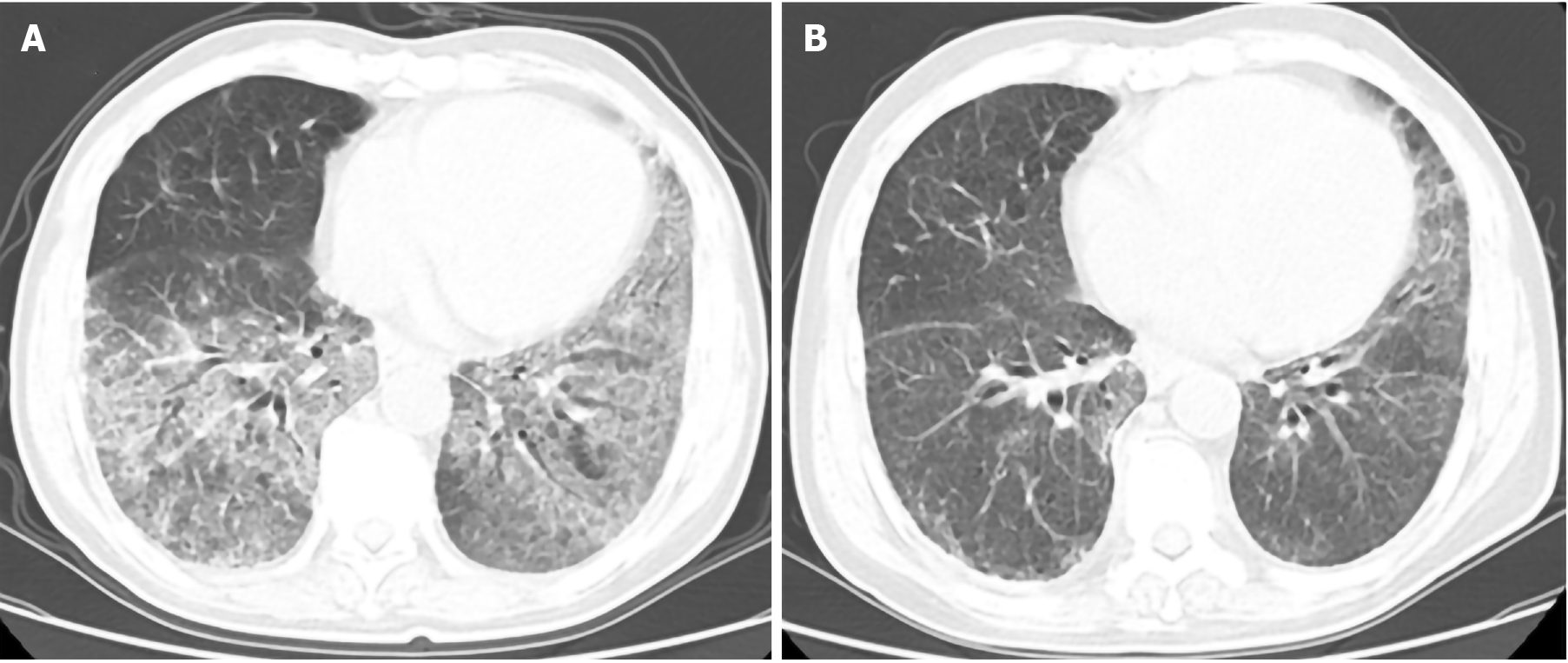
Ultrasonographic analyses revealed mild splenomegaly. High creatinine levels were also detected. CT scans identified bilateral regions of ground-glass opacity (GGO) in the lungs of this patient (Figure 2A). Lung function tests revealed moderate reductions in the diffusing capacity of the lungs for carbon monoxide.
The patient was diagnosed with CMML-1 without eosinophilia, and his revised International Prognostic Scoring System score was 3 points.
The patient underwent whole lung lavage in March 2016 to alleviate his dyspnea, after which he began combined chemotherapeutic treatment with decitabine (30 mg qd for 5 d) and cytarabine (30 mg qd for 14 d). His white blood cell counts rapidly declined, and bronchoalveolar lavage was no longer required after one cycle of chemotherapy. This therapeutic regimen was maintained, and the patient underwent six total cycles of monthly chemotherapy. During cycles 2-4 of treatment, the patient developed anemia that progressed from moderate to severe, while his platelet counts rose to (700-1000) × 109/L. Subsequent bone marrow biopsy was indicative of myelofibrosis. The patient was then treated for 2 mo with ruxolitinib without any response.
After five cycles of chemotherapy, a lung CT scan was performed to assess sPAP status in this patient, revealing marked improvements in analyzed lung abnormalities (Figure 2B). The patient required transfusion therapy 1-2 timer per month followed by maintenance iron removal therapy.
From January 2018 through April 2019, the patient accepted an additional eight cycles of combined azacitidine and cytarabine treatment. At 2 mo following the final round of chemotherapy, the disease had progressed to CMML-2 with 16% blasts in the peripheral blood. Treatment with a Bcl-2 inhibitor was recommended, but the patient was unable to accept this treatment for financial reasons. The patient died in January 2020 as a consequence of severe pulmonary infection.
PAP is a rare pulmonary disorder wherein patients exhibit alveolar surfactant-derived lipoprotein accumulation that can cause symptoms ranging from mild shortness of breath to severe respiratory distress. Patients suffering from PAP may exhibit impaired phagocytosis, chemotaxis or phagolysosomal fusion with defective surfactant clearance being linked to impaired alveolar macrophage functionality[10-12]. In mice, GM-CSF or GM-CSF/IL-3/IL-5-receptor common β chain defects result in the development of a PAP-like syndrome, and βc-chain defects impairing GM-CSF receptor expression on circulating mononuclear cells have been detected in those with congenital PAP[13]. Patients with idiopathic PAP have also been found to exhibit serum and BALF neutralizing antibodies specific for GM-CSF, whereas these are not evident in those with sPAP[14]. This indicates that idiopathic PAP is a form of autoimmune disease and that measuring anti-GM-CSF titers can differentiate between sPAP and idiopathic PAP[15].
PAP is reported to affect approximately 8.7 per million persons, with sPAP secondary to MDS affecting fewer than 1 per million persons[16]. Hematologic malignancies are the most common underlying cause of sPAP[17], with PAP secondary to hematologic malignancy being associated with a range of conditions among which hematological malignancies of myeloid origin are the most common[17-19]. In prior studies, MDS was identified as the underlying disease most often associated with PAP secondary to hematologic malignancy, accounting for 16% of such cases[10]. As sPAP secondary to MDS is very rare, only a limited number of case reports and small case series have been published to date. In total, 65 such cases have been reported to date as noted in Table 1[9,20-30] with ages ranging from 18–75-years-old and with a male/female ratio of 27/38. Of the eleven cases of PAP diagnosed prior to MDS, only four, including the present case, were diagnosed as PAP coinciding with MDS. For all secondary cases, PAP likely occurred as a consequence of disease progression.
| Order | Diagnosis | Case number | Age/Gender | Duration from MDS to PAP | Treatment | Anti-GM-CSF Ab | Concomitant disease | Chromosomal abnormality | Genetic abnormality | Survival time after PAP | Outcome | Survival time after MDS | Ref. |
| 1 | MDS | 1 | 39/M | 1 yr after MDS | NA | NA | Pulmonary fungus | NA | NA | Shortly | Died | 1 yr | [21] |
| 2 | MDS | 1 | 65/F | Coincidence | Methylprednisolone and GM-CSF | NA | None | NA | NA | Shortly | Died | Shortly | [21] |
| 3 | RA deteriorated into AL after 2 yr | 1 | 27/M | 1 yr after AL | Antibiotics and anti-tuberculosis agents | NA | None | NA | NA | 4 mo | Died | 3 yr | [10] |
| 4 | RA deteriorated into AL after 1 yr | 1 | 65/F | 1 mo after AL | Fluconazole and miconazole | NA | Pulmonary fungus | NA | NA | 1 mo | Died | 1 yr | [10] |
| 5 | RAEB-T | 1 | 52/F | 2 yr after RAEB-T | Amphotericin B | NA | Pulmonary fungus | NA | NA | 4 mo | Died | 1 yr | [10] |
| 6 | RA deteriorated into AL within 1 yr | 1 | 70/M | 1 yr after AL | Chemotherapy | NA | Malignant melanoma | NA | NA | 4 mo | Died | 2 yr | [10] |
| 7 | RA deteriorated into RAEB after 12 yr | 1 | 50/M | Coincidence with RAEB | Prednisolone | Negative | Hypereosinophil | 46, XY, -1,-14,+2mar[1] and 46, XY[3] at first diagnosis and 47, XY, add (1)(p11), +add(1), t(1;19)(q11;q11), -14, +mar at disease progression | NA | 2 yr | Alive | 14 yr | [22] |
| 8 | MDS | 1 | 66/M | 12.5 yr | Prednisolone and amobroxol | Negative | NA | 47, XY, add(1)(p11), +add(1),t(1;19)(p10; q10), –14, +mar1[20/20] | NA | NA | NA | NA | [23] |
| 9 | MDS | 1 | 47/M | 2 yr | NA | Negative | NA | NA | NA | Shortly | Died | 2 yr | [24] |
| 10 | MDS-RA deteriorated into RAEB after 9 yr | 1 | 36/M | 1 yr after RAEB | Cord blood transplantation and GM-CSF | Negative | None | NA | NA | 1 yr | Alive | 11 yr | [25] |
| 11 | MDS | 1 | 39/F | NA | BAL | NA | NA | NA | NA | NA | Alive | NA | [8] |
| 12 | MDS-RA deteriorated into RAEB after 1 yr | 1 | 48/M | 1 yr with RAEB | WLL | Negative | None | 47, XY, +8 | NA | 2 r | Alive | 3 yr | [26] |
| 13 | RCMD | 1 | 41/F | 3 yr | Prednisolone and antibiotics | NA | None | 46, XX, –20; der (20)del (20)(q11q13) idic(20) (p11), or idic(20q–) | NA | 7 mo | Died | 4 yr | [4] |
| 14 | MDS-RA | 1 | 34/M | 1 yr | Unrelated bone marrow transplantation | Negative | IBD | 47, XY, +8 | NA | 1 yr | Alive | 3 yr | [27] |
| 15 | MDS-RAEB | 1 | 42/F | 4 mo before RAEB | Unrelated cord blood transplantation. | Negative | IBD | 47, XX, +8 | NA | 4 mo | Died | Shortly | [27] |
| 16 | MDS-RAEB | 1 | 52/F | 8 yr before RAEB | Unrelated bone marrow transplantation | Negative | BD and IBD | 47, XX, +8 | NA | 10 yr | Alive | 2 yr | [27] |
| 17 | MDS | 1 | 34/M | 2 yr before MDS | BAL | NA | NA | NA | NA | NA | NA | NA | [17] |
| 18 | MDS-MLD | 1 | 38/M | NA | NA | Negative | NA | NA | NA | NA | Died | < 2 yr | [15] |
| 19 | MDS-SLD | 1 | 26/M | NA | NA | Negative | NA | NA | NA | NA | Died | < 2 yr | [15] |
| 20 | MDS-U | 1 | 37/M | NA | NA | Negative | NA | NA | NA | NA | Died | < 2 yr | [15] |
| 21 | MDS-EB | 1 | 33/M | NA | NA | Negative | NA | NA | NA | NA | Died | < 2 yr | [15] |
| 22 | Very low + low MDS | 13 | Age: 45 (30-67); Gender: 7 M/6 F | MDS to sPAP: 0 to 168 mo 2 case before | NA | Negative | NA | Good: 2; Intermediate: 11 | NA | 13 mo | 7 Died; 6 Alive | NA | [5] |
| 34 | Inter-high + very high | 18 | Age: 50 (27-57); Gender: 12 M/6 F | MDS to sPAP: 0 to 228 mo 6 cases before | NA | Negative | NA | Intermediate: 13; Poor: 5 | NA | 15 mo | 10 Died; 8 Alive | NA | [5] |
| 51 | MDS-U deteriorated into RCMD after 11 mo and deteriorated into RAEB-1 after 13 mo | 1 | 75/F | Coincidence with RAEB1 | Prednisolone | Negative | Organizing pneumonia | Normal karyotype | NA | 6 mo | Died | 19 mo | [16] |
| 52 | MDS-RA | 1 | 46/F | 3 yr | Steroid pulse, cyclosporine A and infliximab | NA | BD and myelofibrosis | Trisomy 8 | NA | 30 mo | Died | 66 mo | [28] |
| 53 | MDS-RA | 1 | 31/M | 2 yr | Steroid | NA | BD | Trisomy 8 | NA | 2 mo | Died | 2 yr | [28] |
| 54 | MDS-RAEB2 | 1 | 50/F | Coincidence with RAEB | WLL | NA | BD | Trisomy 8 | NA | 2 yr | Alive | 2 yr | [28] |
| 55 | MDS | 1 | 40/M | NA | Stem cell transplantation | NA | NA | NA | NA | NA | Alive | NA | [19] |
| 56 | MDS | 1 | 51/F | NA | NA | Negative | LGL, metastatic melanoma, DVT | Normal karyotype | GATA2 | NA | Died | 12 yr | [29] |
| 57 | MDS/AML | 1 | 33/M | Less than 1 yr | NA | Negative | None | -7 | GATA2 | < 1 yr | Died | < 1 yr | [29] |
| 58 | MDS | 1 | 26/F | Less than 1 yr | NA | Negative | Hypothyroidism | Normal karyotype | GATA2 | < 1 yr | Died | < 1 yr | [29] |
| 59 | MDS | 1 | 26/F | NA | NA | Negative | LGL, breast cancer, miscarriage | Normal karyotype | GATA2 | NA | Died | 27 yr | [29] |
| 60 | MDS/AML | 1 | 19/F | NA | NA | Negative | LGL/DVT | Normal karyotype | GATA2 | NA | Died | 25 yr | [29] |
| 61 | MDS | 1 | 28/M | NA | NA | Negative | None | Trisomy 8 | GATA2 | NA | Alive | 6 yr | [29] |
| 62 | MDS | 1 | 18/F | NA | NA | Negative | LGL, miscarriage | Normal karyotype | GATA2 | NA | Died | 31 yr | [29] |
| 63 | CMML | 1 | 48/F | NA | NA | Negative | LGL, HT, Pancreatic cancer | Normal karyotype | GATA2 | NA | Died | 11 yr | [29] |
| 64 | MDS | 1 | 59/F | Very soon | NA | Negative | NA | NA | GATA2 | Shortly | Died | Shortly | [30] |
| 65 | CMML | 1 | 63/M | Coincidence with CMML-1 | Chemotherapy | NA | None | inv(6) | JAK2-V617F | 5 yr | Died | 5 yr | Current study |
Several factors are potentially associated with the development of PAP secondary to MDS. Monocytes can be separated into three primary subtypes via flow cytometry: classical (CD14+/CD16-, MO1), intermediate (CD14+/CD16+, MO2) and nonclassical (CD14dim/CD16+, MO3). Normally, classical monocytes account for 94% of total circulating monocytes, and an MO1 frequency ≥ 94% can be used to differentiate between CMML and reactive monocytosis with over 90% sensitivity and specificity[31]. Circulating nonclassical monocyte frequencies are elevated in the context of a range of conditions including sepsis, human immunodeficiency virus infection or cancer. Yoshioka et al[23] reported a case of MDS and sPAP in a patient exhibiting elevated CD14dimCD16+ monocyte counts, suggesting that these nonclassical monocytes may influence the development of pulmonary lesions in PAP patients. However, further studies of additional patients will be required to fully resolve the role of these nonclassical monocytes in PAP secondary to MDS.
Several chromosomal abnormalities have been linked to PAP secondary to MDS, with trisomy 8 being the most common karyotypic finding in these patients[27]. Four of the seven reported cases of trisomy 8 in patients with sPAP and MDS were associated with Behçet’s disease (BD), while two of these seven patients were diagnosed with inflammatory bowel disease. One prior meta-analysis detected high frequencies of intestinal lesions (66%) and trisomy 8 (80%) in patients with MDS complicated by confirmed or suspected BD[28]. Immunosuppressive treatment of BD has the potential to cause sPAP and/or MDS, although such drugs were not used in the majority (2/3) of previously reported cases. BD and inflammatory bowel disease are both autoimmune diseases, and as such sPAP and MDS may also arise as a consequence of autoimmune diseases not secondary to immunosuppressive drug use. As trisomy 8 only affects 10%-15% of patients with MDS, this condition may be linked to a high risk of autoimmune diseases or PAP.
A karyotype analysis of the patient in the present case report revealed the inversion of chromosome 6, which is associated with myeloid neoplasms but has not been previously reported in the context of MDS complicated by sPAP. No gene abnormalities were found to be associated with CMML in this patient. GATA2 deficiency has recently been described as a condition resulting from heterozygous mutations and consequent GATA2 haploinsufficiency, resulting in a range of clinical manifestations including virus infections, CMML, MDS or AML[29]. Spinner et al[29] conducted a systematic review of 57 patients with GATA2 deficiency and found that 84%, 14% and 8% suffered from MDS, AML and CMML, respectively. The 10 patients with biopsy-confirmed PAP in their study did not exhibit autoantibodies specific for GM-CSF, and GATA2 mutation status was not assessed for this patient owing to laboratory limitations. GATA2 is a zinc finger transcription factor that serves as a key regulator of hematopoietic cell gene expression[32] while also regulating alveolar macrophage phagocytosis[33]. Patients with a GATA2 deficiency are more susceptible to both MDS and PAP. As there were no reductions in alveolar macrophage counts in the BALF of these patients, this may suggest that there is a functional defect in these alveolar macrophages thus explaining the link between PAP and hematologic malignancies[32].
Unlike other forms of lung disease, PAP has been studied only rarely and is often only diagnosed upon postmortem examination[10]. In the present case, the patient presented with dyspnea. Owing to the detection of leukocytosis, pulmonary infection was first considered as a possible diagnosis. Following a systematic evaluation, the diagnosis of PAP was confirmed after BALF examination. While dry cough and dyspnea were consistent with PAP, they were nonspecific findings. PAP can be difficult to differentiate from infections via chest X-ray, and even following chest CT-mediated detection of GGO regions it was difficult to exclude leukemic infiltration or noncardiogenic edema.
In general, leukemia infiltration is more likely to present with smooth or nodular thickening of the bronchovascular bundles[34]. Nodular disease can also present with prelymphatic, GGO, centrilobular or random radiological patterns[35]. As sensitive or specific biomarkers of this condition are lacking, invasive approaches such as lung biopsy, BAL or bronchoscopy are necessary in order to definitively diagnose PAP. Most patients suffering from hematologic malignancies exhibit severe thrombocytopenia, potentially precluding the implementation of these invasive procedures. Given that our patient presented with GGO findings upon chest CT scan and had been diagnosed with CMML, further classification of his lung disease would have been challenging in the absence of invasive diagnostic evaluation. Fortunately, this patient was able to tolerate BAL treatment. As the patient was found to be negative for leukemic infiltration and periodic acid-Schiff staining was positive, a diagnosis of PAP was confirmed. Flow cytometry analyses of BALF samples further provided strong evidence of a model wherein sPAP is primarily a result of abnormal alveolar macrophage numbers and functionality. In patients with hematologic malignancies, sPAP is thought to be underestimated as a driver of respiratory failure[3]. PAP should therefore be considered in the differential diagnosis when evaluating patients suffering from hematologic malignancies accompanied with pulmonary symptoms or abnormal radiographic findings[11].
CMML treatment is challenging and is largely influenced by patient age, with hypomethylating agents such as 5-azacitidine and decitabine often being used in this therapeutic context. The patient in this report was treated with a low dose combination of cytarabine and decitabine with whole lung lavage and exhibited a good response to this treatment. After one cycle of treatment, the patient’s pulmonary lesions were significantly improved, and he was free of hypoxia. An analysis of prior cases of sPAP and MDS revealed that pulmonary function could only be restored when MDS was controlled, consistent with the present case. These findings suggest that the treatment of underlying disease is essential when attempting to alleviate PAP symptoms. Whole lung lavage can also effectively alleviate patient symptoms.
In summary, the present case offers evidence regarding the mechanistic basis for PAP secondary to CMML. As PAP is associated with a high mortality rate, early detection is essential. No predictive models are currently available to gauge the risk of PAP development, and genetic analyses of GATA2 may offer value as a means of more precisely diagnosing underlying hematological conditions in affected patients. When patients present with unexplained dyspnea and GGO findings upon chest CT while suffering from hematologic disorders, PAP should be considered as a possible diagnosis. Patients with GATA2 deficiencies or primary disease progression are also at a high risk of PAP development. Other factors including trisomy 8 and autoimmune diseases. High levels of CD14dimCD16+ monocytes may also be indicative of an increased PAP risk. Future prospective or high-quality objective studies are needed to better evaluate risk factors associated with sPAP and to confirm related prior findings.
The authors gratefully appreciate the staff of the Medical Records Room of Hangzhou First People’s Hospital for the support of this study. The authors are also thankful to all the patients for their understanding and cooperation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kontos CK S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 517] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 581] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Cordonnier C, Fleury-Feith J, Escudier E, Atassi K, Bernaudin JF. Secondary alveolar proteinosis is a reversible cause of respiratory failure in leukemic patients. Am J Respir Crit Care Med. 1994;149:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Xue Y, Han Y, Li T, Chen S, Zhang J, Pan J, Wu Y, Wang Y, Shen J. Pulmonary alveolar proteinosis as a terminal complication in a case of myelodysplastic syndrome with idic(20q-). Acta Haematol. 2010;123:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ishii H, Seymour JF, Tazawa R, Inoue Y, Uchida N, Nishida A, Kogure Y, Saraya T, Tomii K, Takada T, Itoh Y, Hojo M, Ichiwata T, Goto H, Nakata K. Secondary pulmonary alveolar proteinosis complicating myelodysplastic syndrome results in worsening of prognosis: a retrospective cohort study in Japan. BMC Pulm Med. 2014;14:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Robb L, Drinkwater CC, Metcalf D, Li R, Köntgen F, Nicola NA, Begley CG. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci USA. 1995;92:9565-9569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 218] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Solary E, Itzykson R. How I treat chronic myelomonocytic leukemia. Blood. 2017;130:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Pollack SM, Gutierrez G, Ascensao J. Pulmonary alveolar proteinosis with myeloproliferative syndrome with myelodysplasia: bronchoalveolar lavage reduces white blood cell count. Am J Hematol. 2006;81:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Can Chen, Kuang Chen, Xilian Huang, Kaile Wang, Shenxian Qian. Concurrent eosinophilia and IgG4-related disease in a child: A case report and review of the literature. Exp Ther Med. 2018;2739-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Shoji N, Ito Y, Kimura Y, Nishimaki J, Kuriyama Y, Tauchi T, Yaguchi M, Payzulla D, Ebihara Y, Ohyashiki K. Pulmonary alveolar proteinosis as a terminal complication in myelodysplastic syndromes: a report of four cases detected on autopsy. Leuk Res. 2002;26:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Chaulagain CP, Pilichowska M, Brinckerhoff L, Tabba M, Erban JK. Secondary pulmonary alveolar proteinosis in hematologic malignancies. Hematol Oncol Stem Cell Ther. 2014;7:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax. 2000;55:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, Murray R, Burdach S. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J Clin Invest. 1997;100:2211-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 381] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Zhang D, Tian X, Feng R, Guo X, Wang P, Situ Y, Xiao Y, Xu KF. Secondary pulmonary alveolar proteinosis: a single-center retrospective study (a case series and literature review). BMC Pulm Med. 2018;18:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Inoue D, Marumo S, Ishii H, Fukui M. Secondary pulmonary alveolar proteinosis during corticosteroid therapy for organising pneumonia associated with myelodysplastic syndrome. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Zhao Y, Xiong W, Wu X. A case of secondary pulmonary alveolar proteinosis, but prior to myelodysplastic syndrome. Respirol Case Rep. 2013;1:58-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Gacouin A, Le Tulzo Y, Suprin E, Briens E, Bernard M, Camus C, Thomas R. Acute respiratory failure caused by secondary alveolar proteinosis in a patient with acute myeloid leukemia: a case report. Intensive Care Med. 1998;24:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Chung JH, Pipavath SJ, Myerson DH, Godwin D. Secondary pulmonary alveolar proteinosis: a confusing and potentially serious complication of hematologic malignancy. J Thorac Imaging. 2009;24:115-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ohmachi K, Ogiya D, Morita F, Kojima M, Tsuboi K, Tazume K, Komatsu M, Hayama N, Kumaki N, Ogawa Y, Ando K. Secondary pulmonary alveolar proteinosis in a patient with chronic myeloid leukemia in the accelerated phase. Tokai J Exp Clin Med. 2008;33:146-149. [PubMed] |
| 21. | Liu Y, Chen LL, Qiu YY, Xiao YL, Cai HR. Clinical features of secondary pulmonary alveolar proteinosis associated with myelodysplastic syndrome: Two case reports. Medicine (Baltimore). 2017;96:e8481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Ando J, Tamayose K, Sugimoto K, Oshimi K. Late appearance of t(1;19)(q11;q11) in myelodysplastic syndrome associated with dysplastic eosinophilia and pulmonary alveolar proteinosis. Cancer Genet Cytogenet. 2002;139:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Yoshioka Y, Ohwada A, Harada N, Satoh N, Sakuraba S, Dambara T, Fukuchi Y. Increased circulating CD16+ CD14dim monocytes in a patient with pulmonary alveolar proteinosis. Respirology. 2002;7:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Sawada K, Yamada G, Shijubo N, Takagi-Takahashi Y, Ohnishi T, Saitoh E, Takahashi H, Watanabe A, Satoh M, Abe S. Biphasic pulmonary blastoma presenting as endobronchial polyp with a long stalk. Intern Med. 2005;44:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Fukuno K, Tomonari A, Tsukada N, Takahashi S, Ooi J, Konuma T, Uchiyama M, Fujii T, Endo T, Iwamoto A, Oyaizu N, Nakata K, Moriwaki H, Tojo A, Asano S. Successful cord blood transplantation for myelodysplastic syndrome resulting in resolution of pulmonary alveolar proteinosis. Bone Marrow Transplant. 2006;38:581-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Tabata S, Shimoji S, Murase K, Takiuchi Y, Inoue D, Kimura T, Nagai Y, Mori M, Togami K, Kurata M, Ito K, Hashimoto H, Matushita A, Nagai K, Takahashi T. Successful allogeneic bone marrow transplantation for myelodysplastic syndrome complicated by severe pulmonary alveolar proteinosis. Int J Hematol. 2009;90:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Nishida A, Miyamoto A, Yamamaoto H, Uchida N, Izutsu K, Wake A, Ohta Y, Fujii T, Araoka H, Taniguchi S, Kishi K. Possible association of trisomy 8 with secondary pulmonary alveolar proteinosis in myelodysplastic syndrome. Am J Respir Crit Care Med. 2011;184:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Handa T, Nakatsue T, Baba M, Takada T, Nakata K, Ishii H. Clinical features of three cases with pulmonary alveolar proteinosis secondary to myelodysplastic syndrome developed during the course of Behçet's disease. Respir Investig. 2014;52:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 518] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 30. | Griese M, Zarbock R, Costabel U, Hildebrandt J, Theegarten D, Albert M, Thiel A, Schams A, Lange J, Krenke K, Wesselak T, Schön C, Kappler M, Blum H, Krebs S, Jung A, Kröner C, Klein C, Campo I, Luisetti M, Bonella F. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm Med. 2015;15:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, Germing U, Onida F, Padron E, Platzbecker U, Santini V, Sanz GF, Solary E, Van de Loosdrecht A, Malcovati L. Diagnosis and Treatment of Chronic Myelomonocytic Leukemias in Adults: Recommendations From the European Hematology Association and the European LeukemiaNet. Hemasphere. 2018;2:e150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819-5831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Lasbury ME, Tang X, Durant PJ, Lee CH. Effect of the transcription factor GATA-2 on phagocytic activity of alveolar macrophages from Pneumocystis carinii-infected hosts. J Eukaryot Microbiol. 2001;Suppl:158S-159S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Tanaka N, Matsumoto T, Miura G, Emoto T, Matsunaga N, Satoh Y, Oka Y. CT findings of leukemic pulmonary infiltration with pathologic correlation. Eur Radiol. 2002;12:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Heyneman LE, Johkoh T, Ward S, Honda O, Yoshida S, Müller NL. Pulmonary leukemic infiltrates: high-resolution CT findings in 10 patients. AJR Am J Roentgenol. 2000;174:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |