Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11419
Peer-review started: July 4, 2021
First decision: September 28, 2021
Revised: September 29, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 26, 2021
Processing time: 172 Days and 5.2 Hours
The advent of immune checkpoint inhibitors (ICIs) has revolutionized the management of several types of solid cancers, including lung cancer, by boosting the body's natural tumor killing response. However, it is undeniable that only a small proportion of non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations can achieve long-term responses and benefit from immunotherapy.
Herein, we report the case of a 48-year-old man diagnosed with stage IV lung adenocarcinoma with an EGFR L858R mutation who was administered pembrolizumab monotherapy followed by pemetrexed and achieved a 10-month progre
We suggest that patients with EGFR-mutated NSCLC with high PD-L1 expression (defined as ≥ 25%), the L858R mutation, smoking history, or pemetrexed pre
Core Tip: In this paper, we report a patient with metastatic epidermal growth factor receptor-mutant non-small cell lung cancer showed dramatic response to immunotherapy after pemetrexed plus carboplatin and achieved a durable disease control over 10 mo. We aimed to analyze the potential reasons why the patient can benefit from immunotherapy and explore the strategy that should be adopted in the future.
- Citation: Li D, Cheng C, Song WP, Ni PZ, Zhang WZ, Wu X. Dramatic response to immunotherapy in an epidermal growth factor receptor-mutant non-small cell lung cancer: A case report. World J Clin Cases 2021; 9(36): 11419-11424
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11419.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11419
Epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitors (TKIs) can significantly prolong the median progression-free survival (PFS) benefit with very manageable toxicity profiles in advanced non-small cell lung cancer (NSCLC) patients harboring sensitive EGFR mutations. However, although this strategy is effective, the treatment response lacks durability, and disease progression frequently occurs after a median of 10 mo to 14 mo of EGFR-TKI therapy[1]. When resistance develops, systemic chemotherapy is administered as a second-line treatment following standard medical instructions for patients without T790M-positive NSCLC[2]. After the standard first- and second-line treatments, there are no effective strategies for the third-line therapy or beyond that improve patient overall survival outcomes. Immune checkpoint inhibitors, particularly inhibitors of the programmed death-1 (PD-1)/PD-ligand 1 pathways, have led to substantial modifications of NSCLC treatment strategies[3]. However, patients with metastatic EGFR-mutated NSCLC show a poor response to anti-PD-1/PD-L1 treatment[4]. In this paper, we report the case of a patient with metastatic EGFR-mutant NSCLC who showed a good response to immunotherapy after a dramatic response to gefitinib and pemetrexed plus car
A 48-year-old man without a history of active or passive smoking presented to our hospital complaining of intermittent cough, bloody sputum, and chest pain in November 2017.
The patient had intermittent cough, bloody sputum, and chest pain for 1 wk.
The patient had no history of smoking and no underlying disease.
He had no personal or family history of other diseases.
For physical examination, the patient presented with intermittent cough, bloody sputum and percussion pain in the chest area (+).
His blood count showed a WBC of 8.23 × 109/L, neutrophil count of 2.12 × 109/L, Hb of 125 g/L, and platelet count of 210 × 109/L.
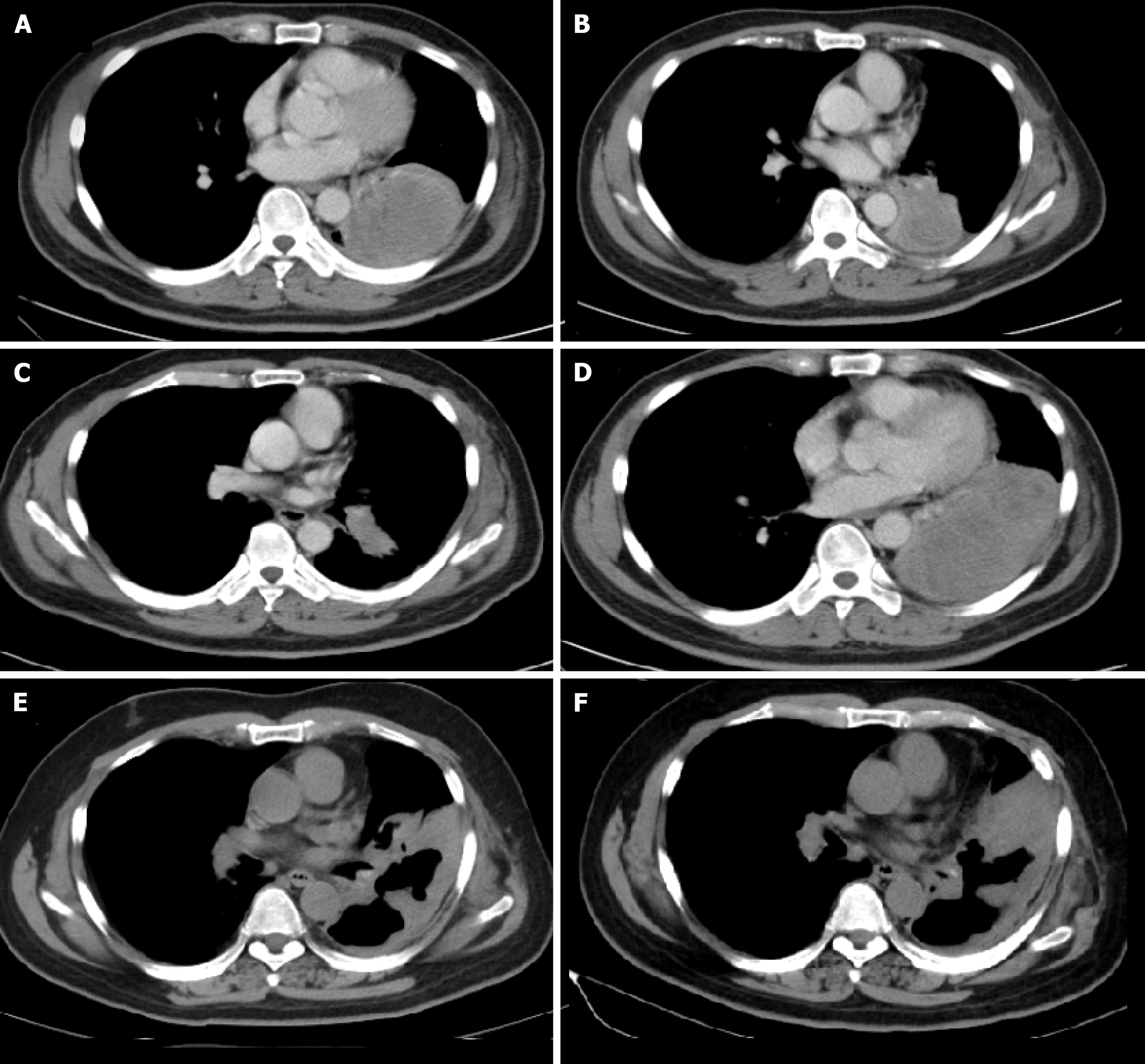
A chest computed tomography scan showed a nodule sized 57 mm × 52 mm, pleural infiltration, and mediastinal lymphadenopathy; therefore, surgery was not indicated (Figure 1A).
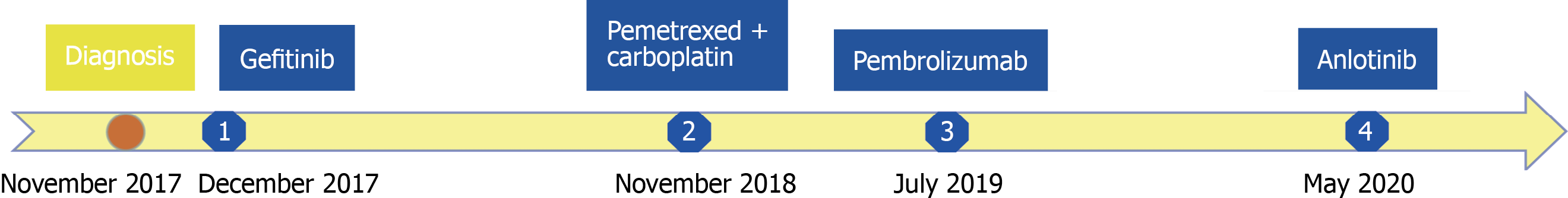
Subsequently, bronchoscopic biopsy suggested the diagnosis of adenocarcinoma. The EGFR exon 21 L858R mutation (with an abundance of 31.5%) was detected by droplet digital polymerase chain reaction of the biopsy sample. Finally, the patient was diagnosed with stage IV lung adenocarcinoma with pleural involvement harboring the EGFR exon 21 L858R mutation.
After 11 mo of gefitinib (250 mg once daily) as the first-line treatment, his disease progressed without evidence of an EGFR T790M mutation (Figure 1B). Then, the patient received four cycles of pemetrexed (500 mg/m2) plus carboplatin (at the target AUC = 5) and achieved a partial response (Figure 1C).
However, after 5 cycles of maintenance treatment with pemetrexed alone, the primary lung lesion enlarged, and the patient was found to have progressive disease (Figure 1D). Hence, pembrolizumab alone was applied at a dose of 200 mg every three weeks and was well tolerated without grade 3 or 4 adverse events during the treatment. After 4 cycles of treatment, a partial response was achieved and was maintained for 10 mo. However, the nodule in the lung enlarged and increased slightly after 14 cycles of pembrolizumab treatment.
The patient inevitably experienced disease progression and received anlotinib (12 mg once daily on days 1-14 of a 21-d cycle) as the fourth-line treatment in May 2020. The treatment timeline of this NSCLC patient is summarized in Figure 2.
In recent decades, PD-1/PD-L1 inhibitors, such as pembrolizumab and nivolumab, have been approved worldwide as treatments for advanced NSCLC and have been hailed as an important addition to the management of this patient population. The results of several phase III trials revealed that immune checkpoint inhibitors provide long-term survival benefits over chemotherapy for patients with advanced NSCLC[5-8]. However, a pooled analysis designed to compare several checkpoint inhibitors with traditional chemotherapy indicated that patients with EGFR-mutated NSCLC obtained no survival benefit from PD-1/PD-L1 inhibitors compared with that achieved with single-agent chemotherapy[9]. Mechanistic and additional confirmatory studies are ongoing. However, potential reasons for this lack of survival benefit have been proposed based on the role of EGFR in tumor cells and the effects of EGFR on immunologic effector cells. Regulatory T cells, which account for the main characteristics of tumors, play an important role in maintaining peripheral tolerance. EGFR signaling pathway activation can promote the generation of regulatory T cells via amphiregulin acting as a ligand of EGFR[10-12]. Nevertheless, EGFR signaling pathway activation can also promote the generation of tolerogenic dendritic cells to maintain immune tolerance via the negative selection of autoreactive T cells[13]. The activation of STAT3, an important downstream signaling molecule of the EGFR signaling pathway, plays an important role in the immune suppression of myeloid-derived suppressor cells to promote myeloid-derived suppressor cell-mediated immune suppression in lung cancer[14].
Most patients with NSCLC and EGFR mutations do not benefit from immunotherapy. However, based on the result of the ATLANTIC phase 2 clinical trial, patients with EGFR-mutated NSCLC and PD-L1 expression ≥ 25% have encouraging outcomes with an objective response rate (ORR) of 14.1% with durvalumab monotherapy, while EGFR-mutated NSCLC patients with PD-L1 expression < 25% showed a substantially lower ORR of 3.6%[15]. Additionally, the results of a multicenter, retrospective study showed that patients with the L858R mutation achieved a comparable ORR to those with wild-type EGFR (7 of 44, 16%, vs 47 of 212, 22%, respectively, P = 0.42), while patients with the 19 exon deletion showed a lower response rate than those with wild-type EGFR (5 of 76, 7% vs 47 of 212, 22%, respectively, P = 0.002). However, whether the different tumor mutation burdens could be the cause of the various efficacies of immunotherapy in patients in terms of the subtypes of EGFR mutations remains uncertain[16]. There is no definitive conclusion on the correlation between clinical factors, such as smoking history or duration of response to prior target therapy, and the survival outcomes of patients receiving immunotherapy[17]. However, it is undeniable that a small proportion of patients with EGFR mutations could benefit from immunotherapy[18]. Further studies into the heterogeneity of EGFR-mutated tumors are needed to enhance the benefits and uses of PD-L1 therapies for patients with these mutations.
Meanwhile, Cavazzoni et al[19] indicated that only pemetrexed could increase PD-L1 Levels by activating both mTOR/P70S6K and STAT3 pathways and induce the secretion of cytokines by activated peripheral blood mononuclear cells, which further stimulated the expression of PD-L1[19]. Therefore, according to the results of previous studies, EGFR-mutated NSCLC patients with high PD-L1 expression (defined as ≥ 25%), the L858R mutation, smoking history, or pemetrexed pretreatment may benefit from immunotherapy. Thus, deeper study of these patients may help discover new therapeutic strategies for EGFR-mutated lung cancer patients.
Herein, we report a metastatic NSCLC patient with TKI-resistant EGFR-mutated tumors who progressed after systemic chemotherapy, benefited from pembrolizumab treatment, and achieved a ten-month PFS interval with a very manageable toxicity profile.
Consistent with the data in published reports, our case report also suggests that EGFR-mutated NSCLC patients with high PD-L1 expression (defined as ≥ 25%), the L858R mutation, smoking history, or pemetrexed pretreatment may benefit from immunotherapy, and they should not be excluded from trials or clinical applications of immune checkpoint inhibitors when resistance to TKIs or chemotherapy occurs. Furthermore, more research is needed to determine the subgroup of EGFR-mutated lung cancer patients who may benefit the most from immunotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Abed A, Batyrbekov K, Brat K, Konala VM, Sugimura H S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Piotrowska Z, Sequist LV. Treatment of EGFR-Mutant Lung Cancers After Progression in Patients Receiving First-Line EGFR Tyrosine Kinase Inhibitors : A Review. JAMA Oncol. 2016;2:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Mok TSK, Kim SW, Wu YL, Nakagawa K, Yang JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, Ponce S, Shi X, Rukazenkov Y, Haddad V, Thress KS, Soria JC. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. J Clin Oncol. 2017;35:4027-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S; ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192-iv237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1607] [Article Influence: 229.6] [Reference Citation Analysis (0)] |
| 4. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6335] [Article Influence: 633.5] [Reference Citation Analysis (0)] |
| 5. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4803] [Article Influence: 686.1] [Reference Citation Analysis (0)] |
| 6. | Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR; OAK Study Group. Atezolizumab vs docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 3703] [Article Influence: 462.9] [Reference Citation Analysis (0)] |
| 7. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab vs Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6739] [Article Influence: 673.9] [Reference Citation Analysis (0)] |
| 8. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab vs Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7536] [Article Influence: 753.6] [Reference Citation Analysis (0)] |
| 9. | Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, Herbst RS, Gralla RJ, Mok T, Yang JC. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 441] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 10. | Mascia F, Schloemann DT, Cataisson C, McKinnon KM, Krymskaya L, Wolcott KM, Yuspa SH. Cell autonomous or systemic EGFR blockade alters the immune-environment in squamous cell carcinomas. Int J Cancer. 2016;139:2593-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, Ding Q, Xia J. Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK-3β/Foxp3 Axis. J Biol Chem. 2016;291:21085-21095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Gröne A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 13. | Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 14. | Wu L, Du H, Li Y, Qu P, Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol. 2011;179:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, Bidoli P, Wheatley-Price P, Park K, Soo RA, Huang Y, Wadsworth C, Dennis PA, Rizvi NA; ATLANTIC Investigators. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 468] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 16. | Ichihara E, Harada D, Inoue K, Shibayama T, Hosokawa S, Kishino D, Harita S, Ochi N, Oda N, Hara N, Hotta K, Maeda Y, Kiura K. Characteristics of patients with EGFR-mutant non-small-cell lung cancer who benefited from immune checkpoint inhibitors. Cancer Immunol Immunother. 2021;70:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Chiu M, Lipka MB, Bhateja P, Fu P, Dowlati A. A detailed smoking history and determination of MYC status predict response to checkpoint inhibitors in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2020;9:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, Rizvi H, Lisberg A, Truini A, Lydon CA, Liu Z, Henick BS, Wurtz A, Cai G, Plodkowski AJ, Long NM, Halpenny DF, Killam J, Oliva I, Schultz N, Riely GJ, Arcila ME, Ladanyi M, Zelterman D, Herbst RS, Goldberg SB, Awad MM, Garon EB, Gettinger S, Hellmann MD, Politi K. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol. 2019;30:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 19. | Cavazzoni A, Digiacomo G, Alfieri R, La Monica S, Fumarola C, Galetti M, Bonelli M, Cretella D, Barili V, Zecca A, Giovannetti E, Fiorentino M, Tiseo M, Petronini PG, Ardizzoni A. Pemetrexed Enhances Membrane PD-L1 Expression and Potentiates T Cell-Mediated Cytotoxicity by Anti-PD-L1 Antibody Therapy in Non-Small-Cell Lung Cancer. Cancers (Basel). 2020;12:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |