Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10626
Peer-review started: May 8, 2021
First decision: June 5, 2021
Revised: June 6, 2021
Accepted: August 17, 2021
Article in press: August 17, 2021
Published online: December 6, 2021
Processing time: 206 Days and 6.8 Hours
Post-extubation cough is a common phenomenon in surgical patients undergoing general anesthesia, which can lead to potentially dangerous complications. In this meta-analysis, we evaluated the efficacy and safety of intracuff alkalinized lidocaine in patients with tracheal intubation to prevent cough and other airway complications during the perioperative period.
To perform a systematic review and meta-analysis of intracuff alkalinized lidocaine for the prevention of postoperative airway complications.
PubMed, Embase, Cochrane, and Web of Science were searched for randomized controlled trials (RCTs) that compared intracuff alkalinized lidocaine to placebo. We used risk-of-bias assessment to assess the RCTs, and the quality of evidence was assessed using the grading of recommendations, assessment, development, and evaluations.
Twelve randomized trials (1175 patients) were analyzed. Meta-analysis showed that intracuff alkalinized lidocaine was associated with less cough compared to that produced by placebo [risk ratio (RR): 0.38; 95% confidence interval (CI): 0.23-0.63]. Similarly, intracuff alkalinized lidocaine was more effective than the control in reducing postoperative sore throat at 24 h (RR: 0.19; 95%CI: 0.09-0.41) and postoperative hoarseness (RR: 0.38; 95%CI: 0.21-0.69).
Intracuff alkalinized lidocaine is an effective adjuvant that can decrease airway complications, such as coughing, hoarseness, and sore throat.
Core Tip: Our study is different to previous systematic reviews and meta-analysis. We focused on adult patients and included relevant literature on alkalinized lidocaine in the analysis. In addition, this is the first systematic review and meta-analysis to analyze lubrication of the cuff before intubation in order to eliminate the influence of con
- Citation: Chen ZX, Shi Z, Wang B, Zhang Y. Intracuff alkalinized lidocaine to prevent postoperative airway complications: A meta-analysis. World J Clin Cases 2021; 9(34): 10626-10637
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10626.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10626
Tracheal intubation is the most commonly used airway management method in general anesthesia. Due to its high safety, simple operation, and convenient ma
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for the reporting of meta-analyses of randomized controlled trials (RCTs)[23]. The protocol was registered in the International Prospective Register of Systematic Reviews (trial registration number: CRD42020178143).
A comprehensive literature search of PubMed (until May 2020), Embase (until May 2020), Cochrane (until May 2020), and Web of Science (until May 2020) was performed. We used a combination of free text and database-specific subjects (i.e., MESH or EMTREE headings) to describe ‘lidocaine’. The search strategy is shown in Supplementary material: Appendix A. No language restrictions were placed on inclusion. Non-English studies were translated using online translation. Finally, the references of all articles retrieved from the search were manually scrutinized for any relevant trials not identified using the strategy described above.
Studies were selected if they were: (1) Conducted as an RCT; (2) Compared intracuff alkalinized lidocaine fills with a control (i.e., with air or saline fills) in patients ≥ 18 years old who received tracheal intubation under general anesthesia; and (3) Reported the incidence of cough, hoarseness, sore throat and/or visual analogue scale (VAS) of sore throat, among other outcomes. Selected studies were then excluded if they met one or more of the following criteria: (1) The trial included emergency surgery; (2) It was a small-scale preliminary pilot study; (3) The necessary data could not be ex
The primary outcome was the incidence of post-extubation cough. Secondary outcomes included the following: Incidence of postoperative hoarseness, the incidence of a postoperative sore throat within 24 h, VAS of a postoperative sore throat at 1 h and 24 h.
Two independent reviewers (Chen ZX and Shi Z) screened the retrieved titles and abstracts for potential inclusion, reviewed the full text of potential studies, and extracted the data from studies that met the inclusion criteria. Any discrepancies between the reviewers were resolved through a consensus process. When the two reviewers failed to reach an agreement, the final decision was made by the third reviewer (Wang B). Data extraction was completed by two coauthors (Chen ZX and Shi Z) using a predesigned piloted data extraction form. The data extraction form collected information regarding the year of publication; primary author; country of origin; types of surgery; participant characteristics (gender, age, number, inclusion and exclusion criteria); intervention; lidocaine and placebo group events; severity of postoperative sore throat. Dichotomous data were converted to incidences for data synthesis, and continuous data were recorded using mean ± SD. Any disagreement was resolved through the consensus process discussed previously.
The risk of bias was assessed in duplicate using the method outlined in the Cochrane Risk of Bias Tool for Non-Randomized Studies. The risk of bias was assessed as low, moderate, high, for each selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Any disagreement was resolved by consensus.
The meta-analysis was performed using RevMan 5.3 software (Cochrane Collaboration, Oxford, England). The level of evidence quality of each study was estimated according to the guidelines of the grading of recommendations, assessment, development, and evaluation (GRADE). We examined the following five categories: Risk of bias, consistency, directness, imprecision, and reporting bias. RCTs began as high-quality evidence and were rated down based on the described criteria. The evidence grades were classified as high quality (further research is unlikely to change the confidence in the estimate of effect), moderate quality (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low quality (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate), or very low quality (we are very uncertain about the estimate).
If necessary, standard deviations from the confidence interval (CI) limits, the standard error, or the range values provided in the past studies were estimated. The effect sizes of dichotomous outcomes were reported as risk ratios (RR), and the mean difference (MD) was reported for continuous outcomes.
Heterogeneity was assessed using the Cochrane Q test and I2 statistic. I2 < 50% was considered low heterogeneity, I2 = 50% to 75% as moderate, and I2 = 75% to 100% as high. P values < 0.05 were considered statistically significant. A fixed-effect model was used if heterogeneity was considered low. If I2 statistic ≥ 50% and P < 0.05, a random-effects model was applied to the data. The statistical methods of this study were reviewed by Peng Z from the Department of Maternal, Child, and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, China.
A sensitivity analysis was performed to assess the potential influence in our analysis. We attempted to exclude RCTs with: (1) A high risk of bias; (2) Only female subjects; and (3) Cuff prefilling.
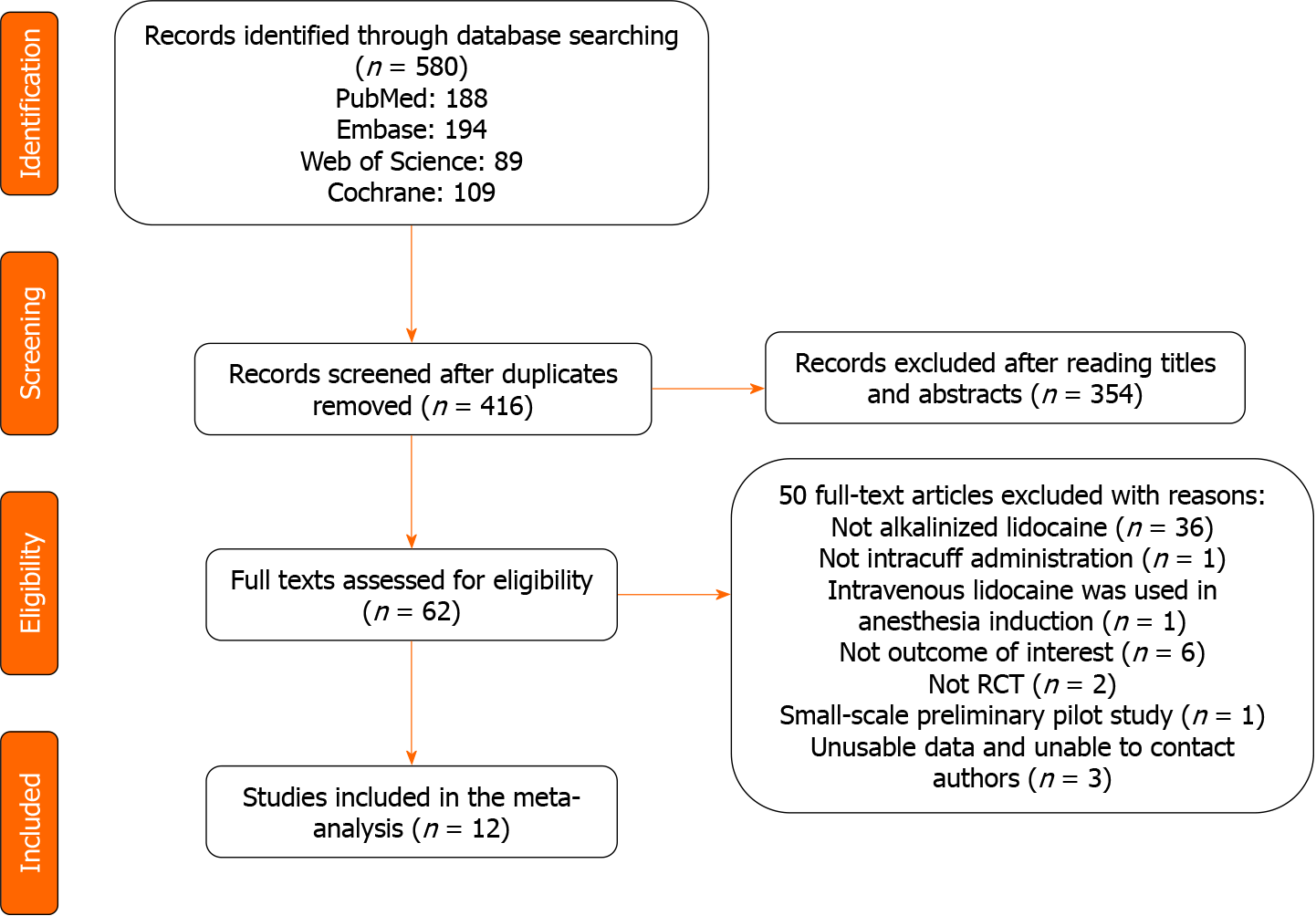
A total of 580 articles were identified from the primary electronic databases (PubMed: 188, Embase: 194, Cochrane: 109, Web of Science: 89. After removing duplicates, we screened 416 studies based on the abstracts, among which 62 full-text articles were assessed for eligibility. Finally, 12 studies were included in the analysis (Figure 1).
The 12 included studies involved a total of 1175 participants with an average age ranging from 36.71 years to 52.00 years, and gender ratios ranging from 0-1.96. All studies were RCTs with placebo or no treatment control arms. Seven trials included patients with an ASA status of I and II[24-30]. Five trials included patients with an ASA status of I, II, and III[31-35]. The included surgeries were: One gynecological surgery[30]; two lumbar surgeries[33,34]; one orthopedic, spine and general surgery[25]; one gynecological, orthopedic, or plastic surgery[26]; one gynecological or plastic surgery[28]; one thyroidectomy surgery[29]. Four studies did not report the type of surgery[24,27,32,35]. Opioids were used in all studies except one which did not report on the use of anesthetics[24]. Saline and air were used in the control group in three and seven experiments[24,25,28,29,33-35], respectively. Two experiments used both saline and air[27,31]. One of the experiments using air in the control group also used 1.4%NaHCO3 as an intervention[29]. In addition, 5 studies lubricated the cuff of the tracheal tube with sterile water or water-soluble gel or normal saline spray before intubation[26,29,30,33,34], and 2 studies performed prefilled at least 90 min before tracheal intubation[32,35] (Table 1).
| Trial | Country | Age (yr) | Male/ Female | Sample size | ASA status | Surgery | Intervention/comparator | Outcomes |
| Rizvanović et al[31], 2019 | Bosnia and Herzegovina | 49.4 (18-65) | 44/46 | 90 | I, II, III | Elective surgery | 1 Alkalinized 2% lidocaine; 2 0.9% saline; 3 Air | IPOST |
| Nath et al[32], 2018 | USA | 52 (18-80) | 73/127 | 200 | I, II, III | NR | 1 Alkalinized 2% lidocaine; 2 0.9% saline | IPC |
| Gaur et al[24], 2017 | Arabia | 44.62 (18-65) | 51/49 | 100 | I, II | NR | 1 Alkalinized 2% lidocaine; 2 Air | IPOST |
| Suma et al[25], 2015 | India | 37.66 (18-65) | NR | 200 | I, II | Elective orthopedic, spine, and general surgery | 1 Alkalinized 2% lidocaine; 2 Air | VAS of POST |
| Navarro et al[26], 2012 | Brazil | NR (≥ 18) | 13/37 | 50 | I, II | Elective gynecological, orthopedic, or plastic surgery | 1 Alkalinized 4% lidocaine; 2 0.9% saline | IPC, IPH, IPOST |
| Shroff and Patil[27], 2009 | UK | 36.71 (18-60) | 51/99 | 150 | I, II | NR | 1 Alkalinized 2% lidocaine; 2 0.9% saline 3 Air | IPC, IPH |
| Navarro et al[28], 2007 | Brazil | 45.15 (18-65) | NR | 50 | I, II | Gynecological surgery or plastic surgery | 1 Alkalinized 2% lidocaine; 2 Air | IPOST |
| Estebe et al[29], 2005 | USA | 47.67 ( ≥ 18) | 13/47 | 60 | I, II | Thyroidectomy surgery | 1 Alkalinized 2% lidocaine (8.4%NaHCO3); 2 Alkalinized 2% lidocaine (1.4%NaHCO3); 3 Air | IPC, IPH, VAS of POST |
| Estebe et al[33], 2004 | UK | 49.67 ( ≥ 18) | 39/21 | 60 | I, II, III | Lumbar spinal surgery | 1 Alkalinized 2% lidocaine (lubricated with sterile water); 2 Alkalinized 2% lidocaine (lubricated with water-soluble gel); 3 Air | IPC, IPH, VAS of POST |
| Estebe et al[34], 2002 | USA | 46.5 ( ≥ 18) | 27/23 | 50 | I, II, III | Lumbar spinal surgery | 1 Alkalinized 2% lidocaine; 2 2% Lidocaine; 3 Air | IPC, IPH, VAS of POST |
| Navarro et al[35], 1997 | USA | 40.15 (NR) | 18/88 | 106 | I, II, III | NR | 1 Alkalinized 2% Lidocaine; 2 Air | IPOST, VAS of POST |
| D’Aragon et al[30], 2013 | Canada | 41.8 ( ≥ 18) | 0/59 | 59 | I, II | Elective gynecological surgery | 1 Alkalinized 2% lidocaine; 2 0.9% saline | IPC |
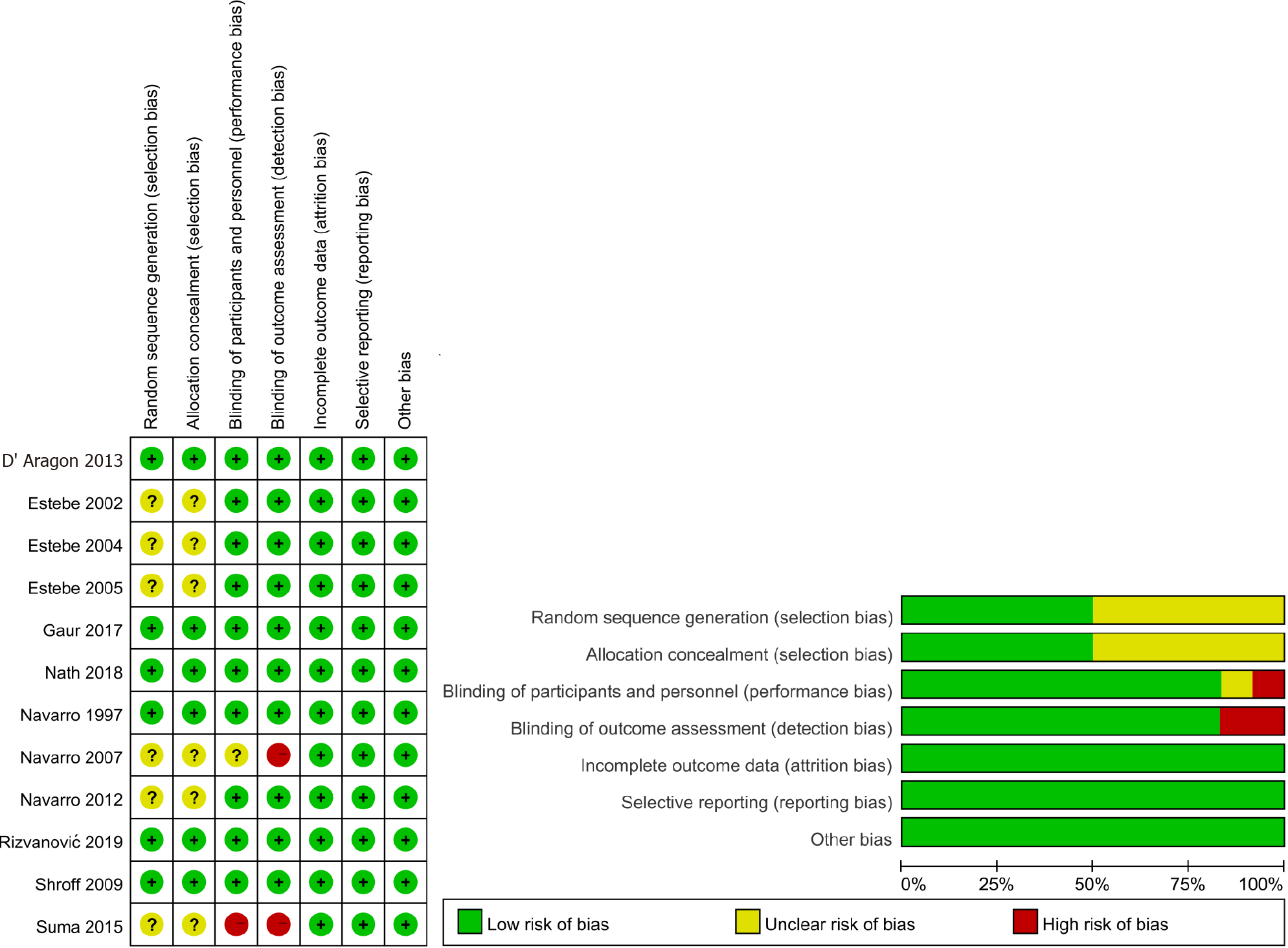
Six trials were judged to be a low risk of bias in all domains. Six trials had an unclear risk of bias, mostly related to random sequence generation and allocation concealment. Two trials had a high risk of bias. The domain that was judged to have a high risk of bias was performance bias and detection bias (Figure 2). The GRADE assessment demonstrated an overall high quality of evidence for the incidence of post-extubation cough and hoarseness. The quality of evidence for the following outcomes was considered moderate: incidence of a postoperative sore throat at 24 h, VAS of a postoperative sore throat at 1 h and 24 h (Supplementary material: Appendix B).
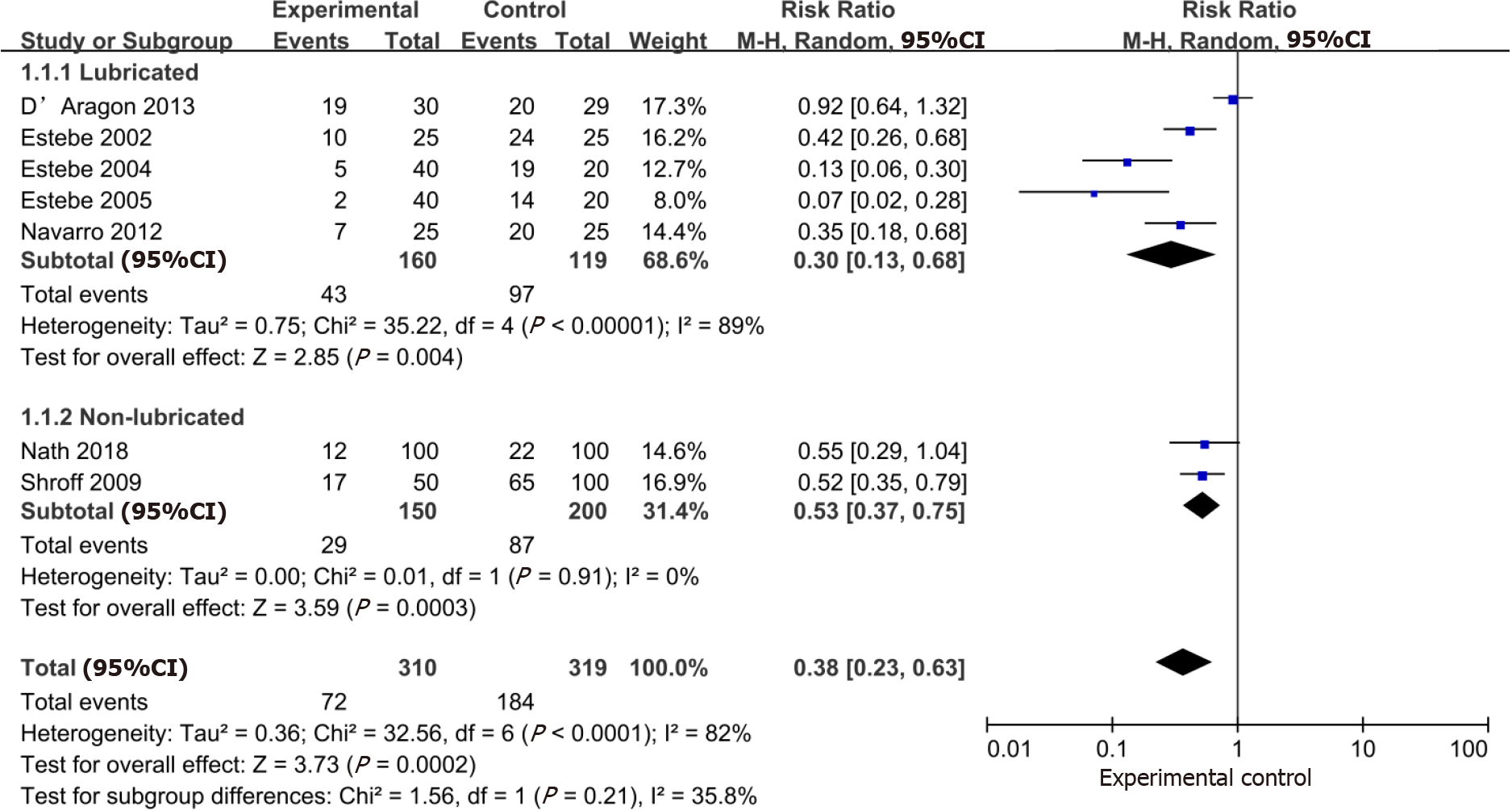
The aggregate effect of the 7 studies (n = 629) evaluating the effect of intracuff alkalinized lidocaine on the incidence of post-extubation cough was in favor of lidocaine over the control (RR: 0.38; 95%CI: 0.23-0.63). Subgroup analysis revealed that the use of intracuff alkalinized lidocaine could also result in a large reduction in post-extubation cough regardless of the lubricated cuff. Concerning subgroup analysis, a significant effect in the reduction of the post-extubation cough was found in the lubricated group (RR: 0.30; 95%CI: 0.13, 0.68) compared with the non-lubricated group (RR: 0.53; 95%CI: 0.37-0.75). However, there was no subgroup difference in relation to lubrication of the cuff (P = 0.21) (Figure 3).
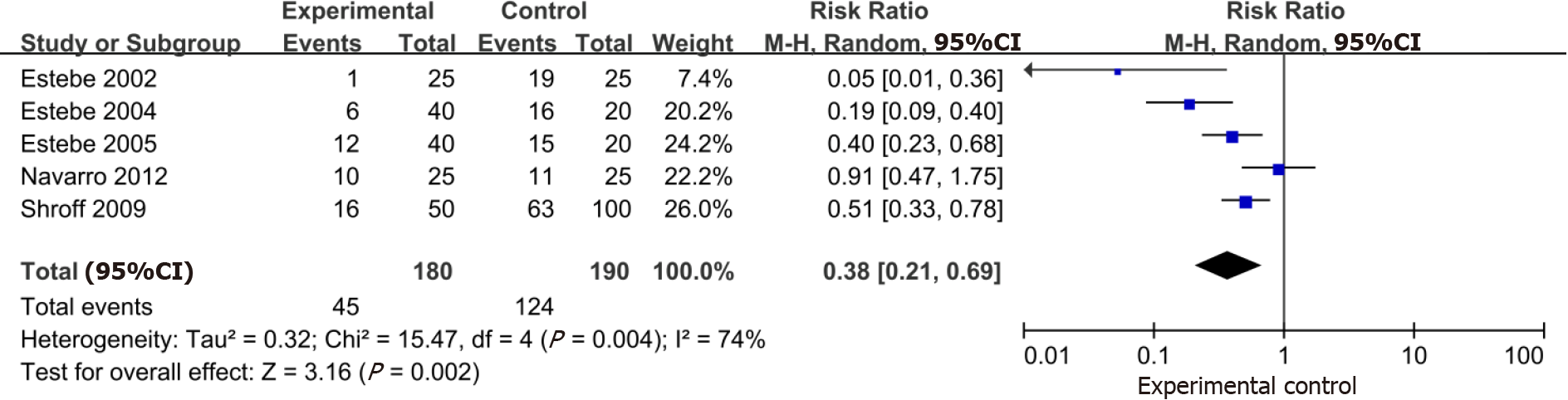
Based on the data pooled from 5 trials (n = 370), the use of intracuff alkalinized lidocaine demonstrated a large reduction in postoperative hoarseness (RR: 0.38; 95%CI: 0.21-0.69) (Figure 4).
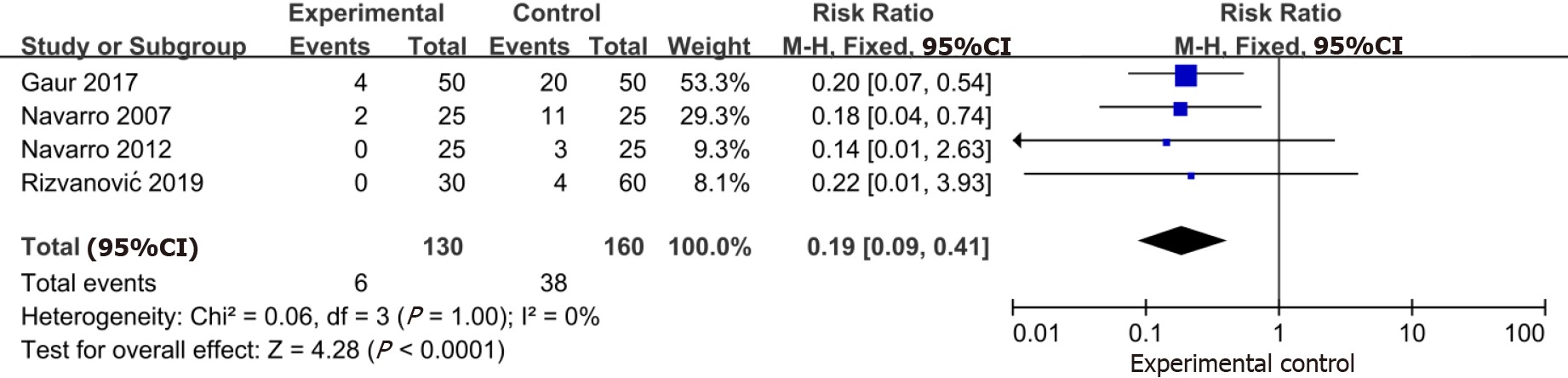
The data from four trials (n = 290) indicated that intracuff alkalinized lidocaine reduced the incidence of a postoperative sore throat within 24 h (RR: 0.19; 95%CI: 0.09-0.41) (Figure 5).
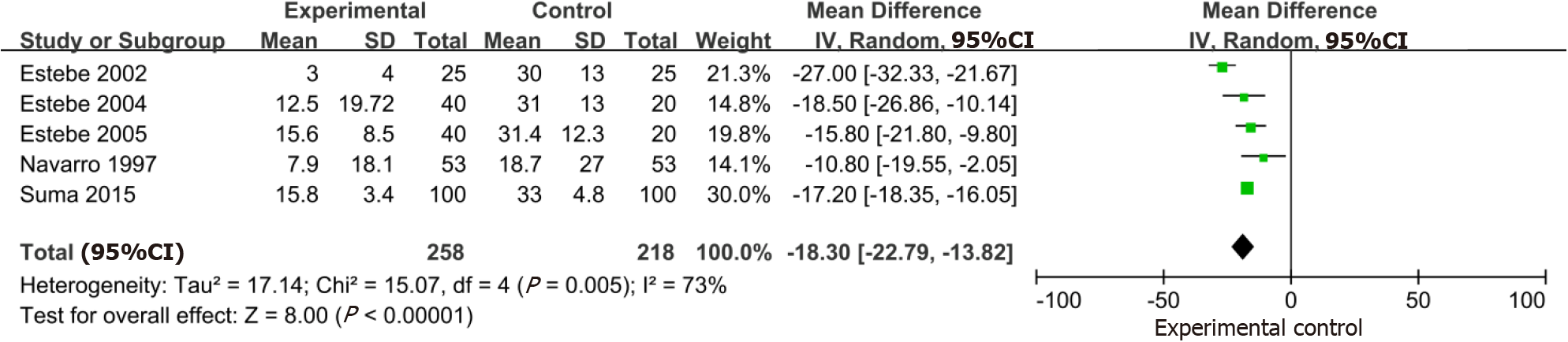
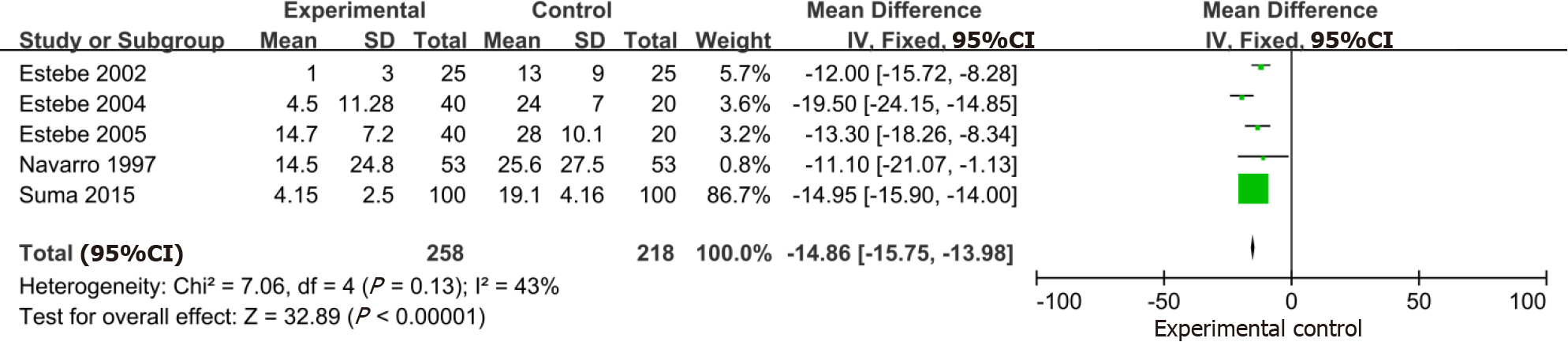
Five trials that included 476 participants demonstrated a large reduction in the VAS of a postoperative sore throat at 1 h with the use of intracuff alkalinized lidocaine (MD: -18.30; 95%CI: -22.79, -13.82) (Figure 6). Similarly, in the 5 studies (n = 476) that evaluated intracuff alkalinized lidocaine on the VAS of a postoperative sore throat at 24 h, a significant benefit of alkalinized lidocaine compared with the control was identified (MD: -14.86; 95%CI: -15.75, -13.98) (Figure 7).
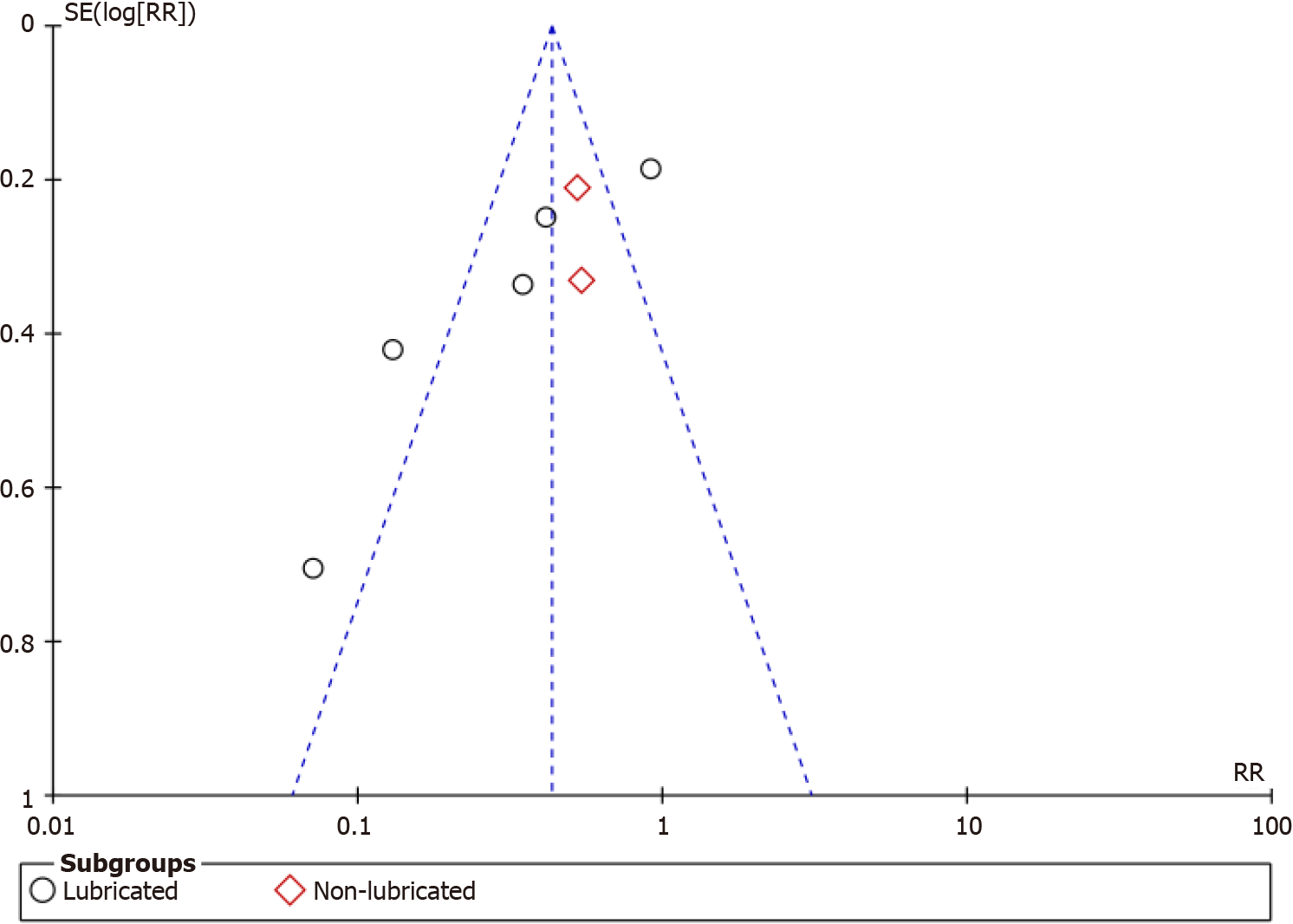
The funnel plot for the included studies showed an asymmetrical characteristic, which suggested a possible publication bias (Figure 8).
In this study, 12 RCTs on the prevention of postoperative airway complications with intracuff alkalinized lidocaine were included. This meta-analysis showed that intracuff alkalinized lidocaine could significantly reduce the incidence of post-extubation cough with high quality of evidence, compared with the control group, and many surgical patients may benefit from the application of intracuff alkalinized lidocaine in clinical practice. At the same time, subgroup analysis showed that saline lubrication before intubation reduced the incidence of post-extubation cough; nonetheless, the observed differences were not statistically significant. Therefore, the evidence in this study strongly suggests that intracuff alkalinized lidocaine can prevent post-extubation cough, and further study is unlikely to change this conclusion. Regarding the VAS of a postoperative sore throat at 1 h and 24 h, intracuff alkalinized lidocaine reduced the severity of postoperative sore throat at 1 h or 24 h. Similarly, the use of intracuff alkalinized lidocaine demonstrated a large reduction in postoperative hoarseness.
In terms of safety, there is no doubt that the usage of intracuff alkalinized lidocaine is much safer than the usage of intravenous lidocaine. In terms of the extubation time, no study has shown that intracuff alkalinized lidocaine may prolong the extubation time. None of the trials reported any adverse events directly related to the use of intracuff alkalinized lidocaine.
In the present study, we performed a detailed literature search, which included the main RCTs on the subject of intracuff alkalinized lidocaine. Our methodology followed strict guidelines developed by the Cochrane Collaboration. A bias risk assessment was conducted for each trial. Also, the quality of available evidence was assessed using grading criteria. The robustness of the obtained results was also tested by sensitivity analysis.
Two previous systematic reviews and meta-analyses have also demonstrated the effectiveness of intracuff alkalinized lidocaine for the prevention of airway complications in patients with tracheal intubation; nonetheless, our study is different to these studies. First of all, previous studies did not distinguish between adult and pediatric patients, while the present study focused on adult patients. Secondly, based on the huge difference in diffusion velocity between alkalinized lidocaine and non-alkalinized lidocaine in tracheal intubation, in the present study, we only included the relevant literature on alkalinized lidocaine in the analysis. At the same time, the previous systematic review and meta-analysis were not limited, although they also carried out the corresponding subgroup analysis. Thirdly, regardless of whether lubricating the cuff before intubation may have an important impact on postoperative airway complications in patients undergoing endotracheal intubation, it is necessary to analyze lubrication of the cuff before intubation in order to eliminate the influence of confounding factors on the results. Finally, 12 RCTs were included in this study, while the previous study only included 9 RCTs[36] and 8 RCTs[37] in the alkalized lidocaine subgroup. Additional trials will provide more data and information to support the conclusions of this study.
Our results revealed that intracuff alkalinized lidocaine decreased postoperative airway complications. To achieve a significant therapeutic effect, large doses of lidocaine may be necessary without alkalinization[37]. According to Estebe et al[34], plasma lidocaine levels confirmed the increased diffusion of lidocaine through the cuff when lidocaine was alkalinized. Moreover, this increased diffusion did not lead to vocal cord palsy. Therefore, the use of a small dose of alkalinized lidocaine (40 mg) is a relatively easy and safe practice that avoids the use of large doses of lidocaine.
The main limitation and disadvantage of this study is the obvious heterogeneity, although a pre-defined subgroup analysis was performed. Clinically, opioids, inhalational anesthetics, and the depth of anesthesia during extubation may have an impact on cough, hoarseness, and sore throat after extubation. In order to minimize these possible confounding factors, we conducted a special sensitivity analysis. Meta-analysis still showed the effectiveness of intracuff alkalinized lidocaine when we excluded RCTs with: (1) A high risk of bias; (2) Only female subjects; (3) Cuff prefilling (Table 2). However, there are still many variables, such as operation time, inflation volume of the tracheal catheter cuff, anesthesiologist’s expertise and ability, which were not systematically reported in the selected studies, thus cannot be analyzed. In addition, only 7 studies with primary outcome were eligible for inclusion, and the funnel plot results are not accurate. To sum up, the use of intracuff alkalinized lidocaine after tracheal intubation is a simple, economical and safe choice to prevent postoperative cough, sore throat, and hoarseness in adult patients. Anesthesiologists can use this technique in clinical patients.
| Potential bias or limitations excluded | IPC RR (95%CI), I2 | IPH RR (95%CI), I2 | IPOST within 24 h RR (95%CI), I2 | VAS of POST at 1 h RR (95%CI), I2 | VAS of POST at 24 h RR (95% CI), I2 |
| Overall | 0.38 (0.23, 0.63), 82% | 0.38 (0.21, 0.69), 74% | 0.19 (0.09, 0.41), 0% | -18.30 (-22.79, -13.82), 73% | -14.86 (-15.75, -13.98), 43% |
| Only females | 0.33 (0.20, 0.52), 69% | NE | NE | ||
| Cuff prefilling | 0.35 (0.19, 0.63), 85% | NE | NE | -17.59 (-18.69, -16.49), 77% | -14.81 (-17.22, -12.41), 54% |
| A high risk of bias | NE | NE | 0.19 (0.08, 0.48), 0% | -18.45 (-25.61, -11.29), 77% | -14.37 (-18.31, -10.43), 56% |
This meta-analysis revealed that intracuff alkalinized lidocaine decreased postope
Tracheal intubation is the most commonly used airway management method in general anesthesia. However, this approach has been associated with some problems, such as postoperative airway complications, which are common phenomena and adverse reactions in patients who underwent elective general anesthesia. To reduce the occurrence of postoperative airway-related complications, many interventions have been proposed. Among these, intracuff alkalinized lidocaine can be used as local anesthesia, to reduce complications during extubation, and to avoid the side effects of lidocaine on the circulation and central nervous system during general application.
Intracuff lidocaine can be used as local anesthesia, to reduce complications during extubation, and to avoid the side effects of lidocaine on the circulation and central nervous system during general application. Nevertheless, lidocaine is not easy to diffuse in the cuff, and adding sodium bicarbonate can greatly enhance the diffusion ability of lidocaine, to achieve better action on the tracheal mucosa.
Perform a systematic review and meta-analysis to summarize the efficacy of intracuff alkalinized lidocaine in the prevention of postoperative airway-related complications.
A comprehensive literature search of Pubmed (until May 2020), Embase (until May 2020), Cochrane (until May 2020), and Web of Science (until May 2020) was performed. Heterogeneity was assessed using the Cochrane Q test and I2 statistic. A fixed-effect model was used if heterogeneity was considered low. If I2 statistic ≥ 50% and P < 0.05, a random-effects model was applied to the data.
Twelve randomized trials (1175 patients) met the inclusion criteria. The meta-analysis showed that intracuff alkalinized lidocaine was associated with less cough compared to that produced by placebo. Similarly, intracuff alkalinized lidocaine was more effective than the control in reducing postoperative sore throat at 24 h and post
Intracuff alkalinized lidocaine decreased postoperative airway complications, in
The use of intracuff alkalinized lidocaine after tracheal intubation is a simple, eco
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sikiric P S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1672] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 2. | Asai T, Koga K, Vaughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Pitts T. Airway protective mechanisms. Lung. 2014;192:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Thomas S, Beevi S. Dexamethasone reduces the severity of postoperative sore throat. Can J Anaesth. 2007;54:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Boes CJ, Matharu MS, Goadsby PJ. Benign cough headache. Cephalalgia. 2002;22:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Mattle HP, Nirkko AC, Baumgartner RW, Sturzenegger M. Transient cerebral circulatory arrest coincides with fainting in cough syncope. Neurology. 1995;45:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Leech P, Barker J, Fitch W. Proceedings: Changes in intracranial pressure and systemic arterial pressure during the termination of anaesthesia. Br J Anaesth. 1974;46:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | El-Boghdadly K, Bailey CR, Wiles MD. Postoperative sore throat: a systematic review. Anaesthesia. 2016;71:706-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 9. | Yamanaka H, Hayashi Y, Watanabe Y, Uematu H, Mashimo T. Prolonged hoarseness and arytenoid cartilage dislocation after tracheal intubation. Br J Anaesth. 2009;103:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Tung A, Fergusson NA, Ng N, Hu V, Dormuth C, Griesdale DEG. Medications to reduce emergence coughing after general anaesthesia with tracheal intubation: a systematic review and network meta-analysis. Br J Anaesth. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 11. | Kuriyama A, Maeda H, Sun R. Topical application of magnesium to prevent intubation-related sore throat in adult surgical patients: a systematic review and meta-analysis. Can J Anaesth. 2019;66:1082-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Neelakanta G, Miller J. Minimum alveolar concentration of isoflurane for tracheal extubation in deeply anesthetized children. Anesthesiology. 1994;80:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Antoniades N, Worsnop C. Topical lidocaine through the bronchoscope reduces cough rate during bronchoscopy. Respirology. 2009;14:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Diachun CA, Tunink BP, Brock-Utne JG. Suppression of cough during emergence from general anesthesia: laryngotracheal lidocaine through a modified endotracheal tube. J Clin Anesth. 2001;13:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Altintaş F, Bozkurt P, Kaya G, Akkan G. Lidocaine 10% in the endotracheal tube cuff: blood concentrations, haemodynamic and clinical effects. Eur J Anaesthesiol. 2000;17:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Fagan C, Frizelle HP, Laffey J, Hannon V, Carey M. The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth Analg. 2000;91:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Soltani HA, Aghadavoudi O. The effect of different lidocaine application methods on postoperative cough and sore throat. J Clin Anesth. 2002;14:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Khezri M, Jalili S, Asefzadeh S, Kayalha H. Comparison of intratracheal and intravenous lidocaine effects on bucking, cough, and emergence time at the end of anesthesia. Pak J Med Sci. 2011;27:793-796. [DOI] [Full Text] |
| 19. | Yang SS, Wang NN, Postonogova T, Yang GJ, McGillion M, Beique F, Schricker T. Intravenous lidocaine to prevent postoperative airway complications in adults: a systematic review and meta-analysis. Br J Anaesth. 2020;124:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004;99:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Tanaka Y, Nakayama T, Nishimori M, Tsujimura Y, Kawaguchi M, Sato Y. Lidocaine for preventing postoperative sore throat. Cochrane Database Syst Rev. 2015;CD004081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Dollo G, Estebe JP, Le Corre P, Chevanne F, Ecoffey C, Le Verge R. Endotracheal tube cuffs filled with lidocaine as a drug delivery system: in vitro and in vivo investigations. Eur J Pharm Sci. 2001;13:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47158] [Article Influence: 2947.4] [Reference Citation Analysis (0)] |
| 24. | Gaur P, Ubale P, Khadanga P. Efficacy and Safety of Using Air Versus Alkalinized 2% Lignocaine for Inflating Endotracheal Tube Cuff and Its Pressure Effects on Incidence of Postoperative Coughing and Sore Throat. Anesth Essays Res. 2017;11:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Suma KV, Bhaskar KU. Prevention of post intubation sore throat by inflating endotracheal tube cuff with alkalinized lignocaine. Indian Journal of Public Health Research and Development. 2015;6:200-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Navarro LH, Lima RM, Aguiar AS, Braz JR, Carness JM, Módolo NS. The effect of intracuff alkalinized 2% lidocaine on emergence coughing, sore throat, and hoarseness in smokers. Rev Assoc Med Bras (1992). 2012;58:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 27. | Shroff PP, Patil V. Efficacy of cuff inflation media to prevent postintubation-related emergence phenomenon: air, saline and alkalinized lignocaine. Eur J Anaesthesiol. 2009;26:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Navarro LH, Braz JR, Nakamura G, Lima RM, Silva Fde P, Módolo NS. Effectiveness and safety of endotracheal tube cuffs filled with air vs filled with alkalinized lidocaine: a randomized clinical trial. Sao Paulo Med J. 2007;125:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Estebe JP, Gentili M, Le Corre P, Dollo G, Chevanne F, Ecoffey C. Alkalinization of intracuff lidocaine: efficacy and safety. Anesth Analg. 2005;101:1536-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 30. | D'Aragon F, Beaudet N, Gagnon V, Martin R, Sansoucy Y. The effects of lidocaine spray and intracuff alkalinized lidocaine on the occurrence of cough at extubation: a double-blind randomized controlled trial. Can J Anaesth. 2013;60:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Rizvanović N, Čaušević S, Hrnčić N, Hatibović H. Effect of intracuff alkalinized 2% lidocaine on endotracheal tube cuff pressure and postoperative throat symptoms in anaesthesia maintained by nitrous oxide. Med Glas (Zenica). 2019;16:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Nath P, Williams S, Herrera Méndez LF, Massicotte N, Girard F, Ruel M. Alkalinized Lidocaine Preloaded Endotracheal Tube Cuffs Reduce Emergence Cough After Brief Surgery: A Prospective Randomized Trial. Anesth Analg. 2018;126:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Estebe JP, Delahaye S, Le Corre P, Dollo G, Le Naoures A, Chevanne F, Ecoffey C. Alkalinization of intra-cuff lidocaine and use of gel lubrication protect against tracheal tube-induced emergence phenomena. Br J Anaesth. 2004;92:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Estebe JP, Dollo G, Le Corre P, Le Naoures A, Chevanne F, Le Verge R, Ecoffey C. Alkalinization of intracuff lidocaine improves endotracheal tube-induced emergence phenomena. Anesth Analg. 2002;94:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Navarro RM, Baughman VL. Lidocaine in the endotracheal tube cuff reduces postoperative sore throat. J Clin Anesth. 1997;9:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Lam F, Lin YC, Tsai HC, Chen TL, Tam KW, Chen CY. Effect of Intracuff Lidocaine on Postoperative Sore Throat and the Emergence Phenomenon: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2015;10:e0136184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Peng F, Wang M, Yang H, Yang X, Long M. Efficacy of intracuff lidocaine in reducing coughing on tube: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060520901872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |