Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10472
Peer-review started: March 27, 2021
First decision: August 18, 2021
Revised: August 31, 2021
Accepted: October 24, 2021
Article in press: October 24, 2021
Published online: December 6, 2021
Processing time: 247 Days and 23.6 Hours
Chronic atrophic gastritis (AG) with intestinal metaplasia (IM) significantly increases the risk of gastric cancer. Some medicines have showed definite therapeutic effects in AG and IM regression.
To validate the efficacy of Lamb’s tripe extract and vitamin B12 capsule (LTEVB12) initial therapy and celecoxib rescue therapy for IM and AG.
A total of 255 patients were included to receive LTEVB12 initial therapy (2 capsules each time, three times daily for 6 mo) in hospital in this study. The patients with failure of IM regression continued to receive celecoxib rescue therapy (200 mg, once daily for 6 mo). After each therapy finished, the patients underwent endoscopy and biopsy examination. The regression efficiency was assessed by the operative link on gastritis assessment (OLGA) and the operative link on the gastric intestinal metaplasia assessment (OLGIM) staging system. Logistic regression analysis was applied to identify factors associated with the curative effect.
For LTEVB12 initial therapy, the reversal rates of IM and AG were 52.95% and 48.24%, respectively. Analogously, for celecoxib rescue therapy, the effective rates for IM and AG were 56.25% and 51.56%, respectively. The IM regression rate of complete therapy was up to 85.03%. In different OLGA and OLGIM stages of IM patients, therapeutic efficiency showed a significant difference in each group (P < 0.05). For both therapies, patients with high stages (III or IV) of both the OLGA and OLGIM evaluation systems showed a higher IM or AG regression rate than those with low stages (I or II). Among patients with high stages (OLGIM III and IV), the IM regression rate was above 70% for each therapy. Eating habits, fresh vegetable intake, and high-salt diet were identified as independent factors for the IM reversal effect of LTEVB12 therapy, especially high-salt diet (odds ratio = 1.852, P < 0.05).
Monotherapy could reverse IM and AG. LTEVB12 initial therapy and celecoxib rescue therapy significantly increase the regression effect. IM may not be the point of no return among gastric precancerous lesions.
Core Tip: First, we used the operative link on the gastric intestinal metaplasia (IM) assessment and the operative link on the gastritis assessment staging systems to assess IM and atrophic gastritis regression of individual lesions. Monotherapy with either Lamb’s tripe extract and vitamin B12 capsule or celecoxib could reverse IM and AG. Additionally, the results proved that the integrative therapy combining Chinese and Western medicine had better regression effects. Last but not least, the results counter the argument that IM may not be the point of no return about gastric mucosal lesions.
- Citation: Wu SR, Liu J, Zhang LF, Wang N, Zhang LY, Wu Q, Liu JY, Shi YQ. Lamb’s tripe extract and vitamin B12 capsule plus celecoxib reverses intestinal metaplasia and atrophy: A retrospective cohort study. World J Clin Cases 2021; 9(34): 10472-10483
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10472.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10472
Gastric cancer (GC), one of the most common malignant tumors, has a high incidence around the world[1]. In China, GC ranks second in both the morbidity and mortality of malignant tumors. According to previous reports in 2015, approximately 498000 Chinese people died from GC per year[2]. The Correa model revealed a successive stepwise development of premalignant gastric lesions, which resulted in GC, especially for the intestinal type[3]. From decades of research, intestinal metaplasia (IM) and severe atrophic gastritis (AG) have proved to form the backdrop of dysplasia and intestinal-type gastric adenocarcinoma, so that they were considered high risk factors for GC occurrence[4,5]. Even in the low GC risk population cohort, IM and AG obtained 6.2 and 4.5 hazard ratios, respectively, compared with the normal group[6]. The view of the point of no return among gastric precancerous lesions was revealed at the end of the last century[7]. There were some meta-analyses supporting this view. They suggested that Helicobacter pylori (H. pylori) eradication did not reverse IM but did have an effect on chronic AG[8-10]. However, there was still some evidence that did not support this conclusion. Some studies supported that H. pylori eradication actually could reverse the IM in the long-term follow-up, which has made the debate about the point of no return among gastric precancerous lesions persisting[11-13].
There are still some studies that found the IM reversal effect of medicine. In recent years, there have also been many reports related to drugs, including Western medicine and traditional Chinese medicine, that could reverse IM. Lamb’s tripe extract and vitamin B12 capsule (LTEVB12) is the combination of the lamb sheep's fourth tripe extracted at low temperature, vitamin B1, and excipients. The extract of lamb's fourth tripe contains many active substances, such as renin, pepsin, mucin, and bifidus factor. It has been proven to promote the growth and propagation of bifidobacteria in vitro[14]. Recently, many studies have shown that both the application of LTEVB12 alone or in combination with other medication could reverse IM and AG[15,16]. Moreover, our recent study found that the use of LTEVB12 alone for 6 mo and 12 mo reversed IM. Among all related studies, the IM regression rate of LTEVB12 alone was reported to be up to 55.71%[16].
Cyclooxygenase-2 (COX-2), an enzyme that acts as a catalyst in the transformation of arachidonic acid into prostaglandins, has been found to participate in H. pylori-associated gastric carcinogenesis[17]. COX-2 is overexpressed in gastric carcinoma and premalignant lesions[18,19]. A selective COX-2 inhibitor, celecoxib, can observably decrease the risk of colon, lung, breast, and prostate cancers[20]. Many studies have shown that both long-term and short-term applications of celecoxib can reverse IM and AG and even other gastric premalignant lesions[21-25]. However, these studies reported that the reversal rate of IM was approximately 40%.
On the basis of such evidence, monotherapy with either LTEVB12 or celecoxib did not show an ideal efficiency with regard to IM regression. We conducted a retrospective cohort study to assess whether LTEVB12 initial therapy and celecoxib rescue therapy can prevent progression or enhance the regression of IM and AG.
From October 2016 to July 2019, 345 patients diagnosed with IM with or without low-grade intraepithelial neoplasia (LGIN) by upper gastrointestinal endoscopy and histopathological biopsy were enrolled and followed at the Department of Gastroenterology, Xijing Hospital, Air Force Military Medical University. The inclusion criteria were: (1) Patients aged from 18 to 75 years old; (2) IM patients with or without LGIN diagnosed by upper gastrointestinal endoscopy and histopathological biopsy; and (3) Patients without H. pylori infection confirmed by 13C-urea breath test (UBT) or patients with H. pylori infection who completed the bismuth-containing quadruple program and had confirmed successful eradication by 13C-UBT. The exclusion criteria were: (1) Previously diagnosed malignant tumor; (2) History of stomach surgery; (3) Breastfeeding or pregnancy; (4) Hypothyroidism, adrenal insufficiency, systemic lupus erythematosus, ankylosing spondylitis, and other endocrine diseases or autoimmune diseases; (5) Severe mental illness; (6) Refusal of drug treatment; (7) Diagnosis of GC or high grade intraepithelial neoplasia (HGIN) by upper gastrointestinal endoscopy and pathological examination; and (8) Severe liver and kidney dysfunction. The general situation, eating habits, behavioral characteristics (smoking and drinking), disease history, medication history, and other data of the patients were collected at the inception of the study. When the therapy was accomplished, they underwent upper gastrointestinal endoscopy and histopathological biopsy. This study was performed in accordance with the ethical principles for medical research as outlined in the Declaration of Helsinki. The study was approved by the institutional research ethics committee of the First Affiliated Hospital, the Air Force Medical University (KY20212048-C-1).
The participants received LTEVB12 initial treatment (2 capsules each time, three times daily; GMP, the Xinjiang Uygur Autonomous Region, China) for 6 mo at first. Some participants with IM regression failure in initial therapy could choose to continue the next rescue therapy or not. The patients deciding to accept it received celecoxib rescue therapy (200 mg, once daily; Pfizer, New York, NY, United States) for 6 mo. This study is a retrospective cohort study. We determined whether the patients needed to accept celecoxib rescue therapy depending on the change of the operative link on the gastric intestinal metaplasia assessment (OLGIM) stage score before and after treatment. The study size was decided by comparing with similar studies.
Participants with a prior diagnosis of IM and dysplasia accepted upper gastrointestinal endoscopy surveillance with a standard video endoscope (Olympus GIF-Q160, Tokyo, Japan). Comprehensive biopsy samples for histological examination were obtained from five standardized sites: Two from the antrum, two from the corpus (one from the lesser curvature and one from the greater curvature), and one from the angulus. In the case of endoscopically visible lesions, additional targeted biopsy samples were obtained.
Three pathologists blinded to the patient clinical information independently reviewed the histology of the collected samples. The grades of IM and AG were classified according to the updated Sydney system, which scored as 0 (absent), 1 (mild), 2 (moderate), or 3 (marked). Dysplasia was assessed based on the revised Vienna classification[26,27]. Of antrum and angulus biopsy samples, the severer one was on behalf of the distal antrum mucosa score. The same method applied to corpus greater and lesser curvature biopsy samples for the corpus mucosa score. According to the standardized sites, the AG, IM, and inflammation stages in all five biopsy specimens were evaluated on the basis of the operative link on gastritis assessment (OLGA) staging system[28] and the OLGIM staging system[29]. Combining the antrum and corpus scores for AG, IM, and inflammation resulted in OLGA and OLGIM staging scores (range: 0-4, respectively). For an inconsistent diagnosis, the final decision was depended on the majority diagnosis: At least two of three pathologists agreed.
The hypothesis tested in this study was that LTEVB12 initial treatment and celecoxib rescue therapy could promote the reversal of IM and AG. The main observation indexes were OLGIM and OLGA stage changes. To evaluate the effects of the therapies, each subject was assigned a stage score before the therapy (A) and at the end point (B) according to OLGIM and OLGA stages. We choose to use the result of B-A to verdict the development status of gastric mucosal lesions. If B-A was > 1, = 0, and < 0, the subject was considered as progression, no-change, and regression, respectively. Regression was deemed to be effective; the others were clarified to be ineffective.
Data were analyzed with SPSS 26.0 software. Continuous variables are described as medians and interquartile ranges. Count variables are described as numbers and percentages. Comparison of the effective rate between different therapies and different OLGA and OLGIM stages was evaluated by the chi-square test or Fisher exact method. Comparison of the proportion among different OLGA and OLGIM stages before and after treatment was evaluated by rank sum test. The influence of the various factors on the efficacy was computed by univariate and multivariate logistic regression analyses with P values, odd ratios (ORs), and 95% confidence interval (CIs).
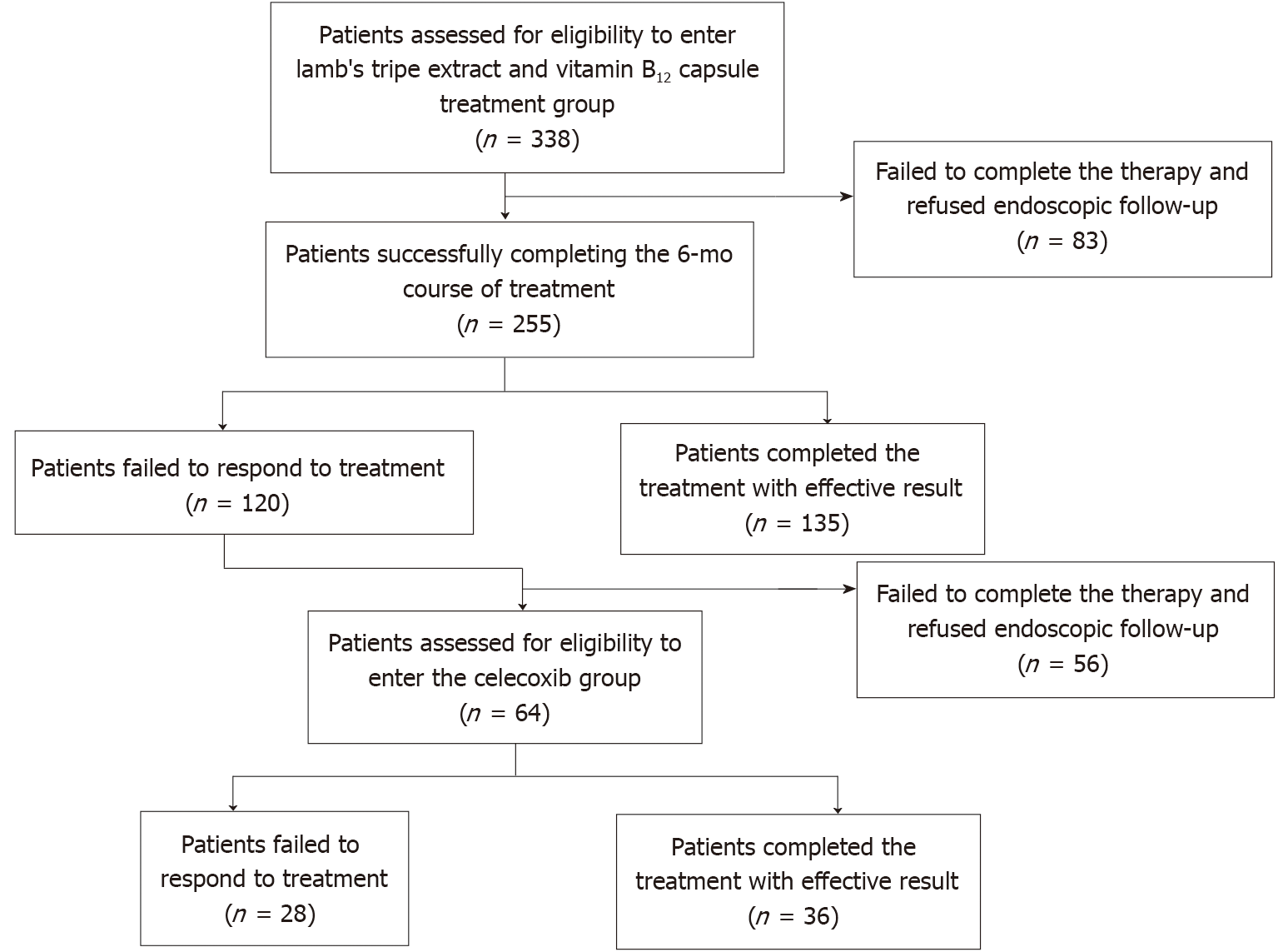
There were 338 IM patients consecutively enrolled in this study during a total follow-up period of 34 mo (median, 15 mo). Eighty-three patients (20 who did not complete the treatment and 63 who refused endoscopic follow-up) in the LTEVB12 initial therapy group and 56 (17 who did not complete the treatment and 39 who refused endoscopic follow-up) in the celecoxib rescue therapy group dropped out of the study (Figure 1). A total of 255 patients finished LTEVB12 initial treatment. For 120 patients, treatment was viewed as ineffective (IM regression failure). Finally, 64 patients completed rescue therapy, 28 of whom were invalid (Figure 1). For the patients receiving LTEVB12 and celecoxib therapy, the demographic parameters, pretreatment histological features, and baseline data are shown in the supplementary materials (Supplementary Table 1). Before LTEVB12 initial treatment, the OLGIM stages in 255 patients from I to IV were 64, 110, 62, and 19, respectively, and the OLGA stages from 0 to IV were 23, 14, 102, 81, and 35, respectively. Twenty-one had LGIN at baseline. Before celecoxib rescue therapy, the OLGIM stages from I to IV in 64 patients were 6, 32, 16, and 10, and the OLGA stages from 0 to IV were 1, 0, 20, 23, and 20, respectively. One had LGIN.
There were 255 cases in the LTEVB12 initial therapy group and 64 cases in the celecoxib rescue therapy group that were enrolled for per-protocol (PP) analysis. The rates of IM and AG regression at 6 mo according to intention-to-treat (ITT) or PP analysis are shown in Table 1. The results of comparing the same patients before and after the therapies by OLGA and OLGIM stages were significantly different (for the two therapies and both stages, P < 0.01). For the LTEVB12 initial therapy group, about half of the patients (52.95%) in the PP analysis showed IM regression after 6 mo of initial LTEVB12 therapy. Forty-eight percent of patients showed an effect with regard to AG regression. In addition, 19 out of 21 patients with LGIN had lesions that disappeared. For 6-mo celecoxib rescue therapy, in 64 patients, the regression rate of IM was up to 56.25%. Approximately 51.56% of patients had AG regression. The lesion of the only LGIN patient disappeared. For complete therapy, 85.93% of participants in the PP analysis had IM regression. Efficiency did not differ between the two monotherapies (P > 0.05) but showed a significant difference when comparing either monotherpay to complete therapy (P < 0.05).
| Group | IM regression (%) | AG regression (%) | IM or AG regression (%) | |||
| ITT analysis | PP analysis | ITT analysis | PP analysis | ITT analysis | PP analysis | |
| LTEVB12 initial therapy | 39.94 | 52.95 | 36.39 | 48.24 | 53.55 | 70.98 |
| Celecoxib rescue therapy | 30.00 | 56.25 | 27.50 | 51.56 | 39.17 | 78.33 |
| Complete therapy | 50.59 | 85.93 | - | - | - | - |
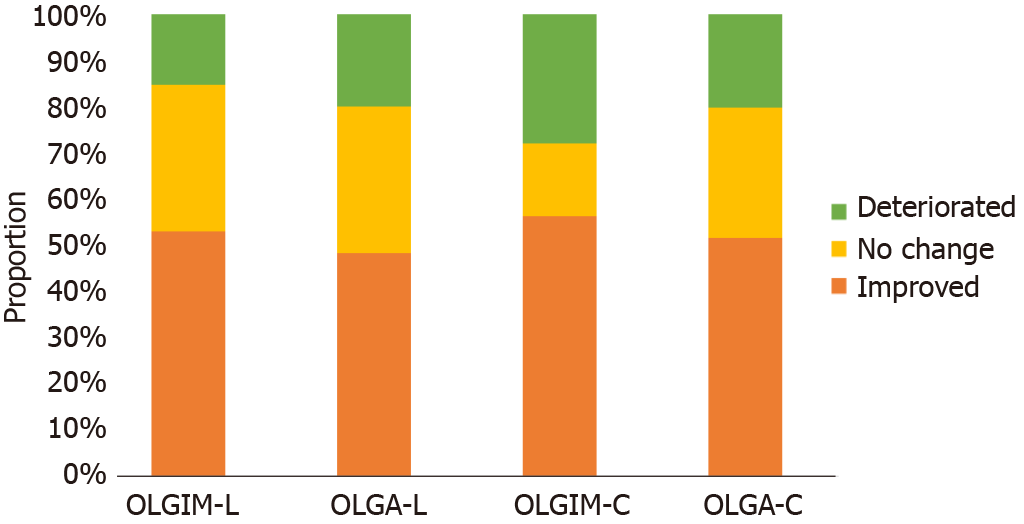
Figure 2 shows the proportions of patients who had IM and achieved improvement of IM after 6 mo of LTEVB12 and celecoxib therapy. For LTEVB12, 11.76% (30/255) and 16.08% (41/255) of patients had complete disappearance of IM and AG, respectively. A total of 41.18% (105/255) and 31.16% (82/255) of patients were found to have decreased OLGIM and OLGA stages, respectively. Similarly, the rates of complete IM and AG disappearance were 6.25% (4/64) and 14.06% (9/64), respectively, following celecoxib rescue therapy. Fifty percent (32/64) and 37.5% (24/64) of patients were found to have decreased OLGIM and OLGA stages, respectively.
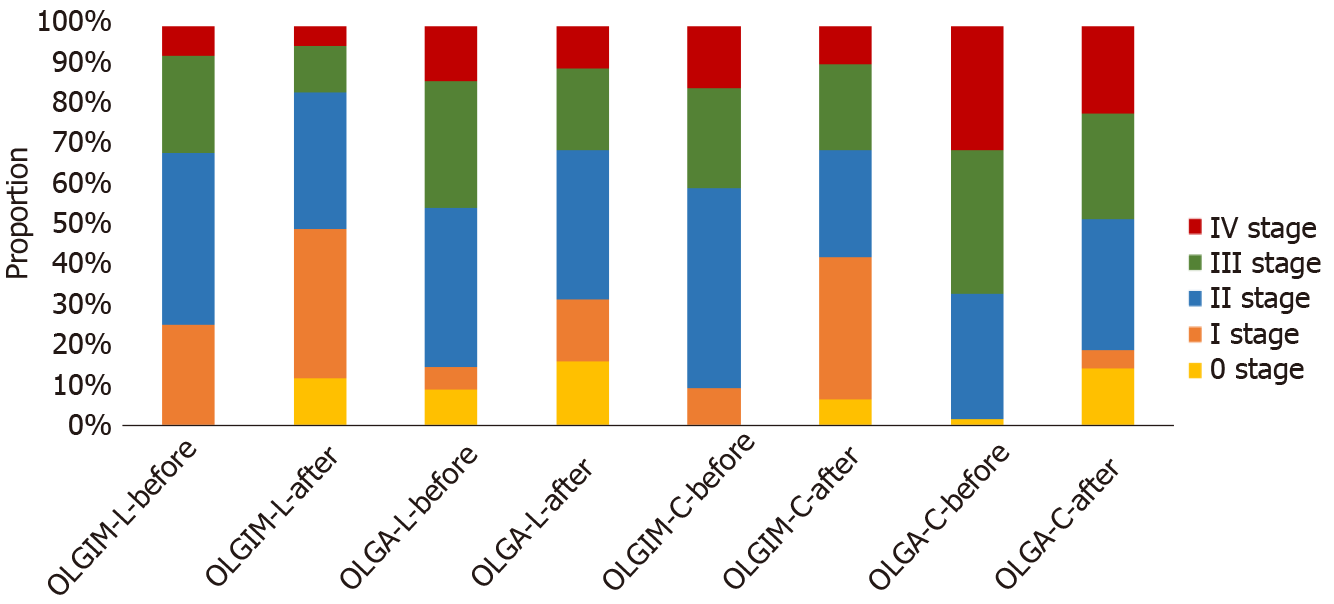
Depending on the OLGA and OLGIM stages, the proportions of different stages showed significant differences before and after therapies (for the two therapies and both stages, P < 0.01). Figure 3 shows the different OLGA and OLGIM stages changing after each therapy. The proportion of high stages was decreased by therapies. The proportion of low stages, by contrast, obviously increased. In OLGIM stages III and IV, which were viewed as a high risk for GC, the IM regression rates were all above 70% for each therapy. LTEVB12 initial therapy reversed IM in 89.47% of OLGIM stage IV patients. For high-risk OLGA stages III and IV patients, the AG regression rate ranged from 50% to 100%.
In different OLGA and OLGIM stages of IM patients, therapeutic efficiency showed a significant difference in each group (Table 2). For LTEVB12 therapy, patients with OLGIM or OLGA stage IV disease had higher IM and AG regression rates than those with stages I and II disease (IM: 89.5% vs 17.2% and 57.3%; AG: 100% vs 33.3% and 40.6%; P < 0.05). For celecoxib therapy, both IM and AG regression rates showed significant differences between high stages (III and IV) and stage II (IM: 71.6% and 71.4% vs 31.4%; AG: 76.2%, 50% vs 30%; P < 0.05). In summary, each therapy had more efficiency for patients with high OLGA or OLGIM stages.
The influencing factors of two therapies were assessed by univariate and multivariate logistic regression analyses. Many factors were included in the analysis, such as sex, age, body mass index, family history of GC, smoking and alcohol status, disease history, medication history, and eating habits. The results of univariate logistic regression analysis are shown in the supplementary materials (Supplementary Tables 2 and 3). After univariate logistic regression analysis, as shown in Table 3, some factors were included in the multivariate analysis according to the inclusion criteria (P < 0.10). Eating habits, fresh vegetable intake, and high-salt diet were viewed as independent factors for the IM reversal effect of LTEVB12 therapy, especially high-salt diet. Nearly twice as many patients with a high-salt diet as those without a high-salt diet benefited from LTEVB12 therapy in IM regression (OR = 1.852, 95%CI: 1.044-3.285). For AG regression, patients with low education levels (OR = 0.480, 95%CI: 0.255-0.903) may benefit more from LTEVB12 therapy than patients with high education levels (P < 0.05). For celecoxib therapy, income level (≥ 5000 yuan/mo) was the independent influencing factor for the IM regression, which suggested that celecoxib therapy for IM regression may be more effective in patients with high income levels (P < 0.10). In addition, for male patients with LTEVB12 therapy, the inflammation score (score > 2) before therapy (OR = 0.448, 95%CI: 0.223-0.898) at baseline and fresh fruit intake (OR = 2.784, 95%CI: 1.131-6.852) were associated with the effect (P < 0.05).
| Regression effect | Factor | LTEVB12 | Celecoxib | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| IM | Fresh vegetable intake (> 100 g/d) | 0.497 | 0.224-1.105 | 0.087 | - | - | - |
| High salt diet | 1.852 | 1.044-3.285 | 0.035 | - | - | - | |
| Tea intake (> 100g/d) | - | - | - | 2.295 | 0.736-7.158 | 0.152 | |
| Education level (≥ senior high school) | - | - | - | 1.672 | 0.495-5.643 | 0.408 | |
| Income level (≥ 5000 yuan/mo) | - | - | - | 3.097 | 0.902-10.638 | 0.073 | |
| AG | Education level (≥ senior high school) | 0.480 | 0.255-0.903 | 0.023 | - | - | - |
| Tea intake (> 100g/d) | 0.678 | 0.388-1.185 | 0.173 | - | - | - | |
| Profession (ref: Farmer) | 0.169 | - | |||||
| Officer | 0.784 | 0.313-1.965 | 0.603 | - | - | - | |
| Medic | - | - | - | - | - | - | |
| Teacher | 1.897 | 0.620-5.804 | 0.262 | - | - | - | |
| Merchant | 1.028 | 0.361-2.933 | 0.958 | - | - | - | |
| Technical staff | 3.305 | 1.275-8.565 | 0.014 | - | - | - | |
| Retired | 1.878 | 0.791-4.458 | 0.153 | - | - | - | |
| Unemployed | 1.570 | 0.612-4.027 | 0.348 | - | - | - | |
| Blood type (ref: A) | - | 0.123 | |||||
| B | - | - | - | 2.021 | 0.240-17.033 | 0.518 | |
| O | - | - | - | 0.322 | 0.064-1.617 | 0.169 | |
| AB | - | - | - | 0.118 | 0.010-1.460 | 0.096 | |
| Unknown | - | - | - | 0.389 | 0.128-1.182 | 0.096 | |
Through this study, we found that both LTEVB12 and celecoxib monotherapies could reverse IM and AG, and the addition of celecoxib rescue therapy to LTEVB12 initial therapy further increased the regression rate of IM. After LTEVB12 initial therapy and celecoxib rescue therapy, the regression rate of IM depending on the OLGIM stage was up to 85.93%. These results suggested that this complete therapy could be applied in the clinical setting to reverse precancerous lesions, especially IM.
LTEVB12 is a kind of traditional Chinese medicine extract. Several studies have shown that it has an effect on IM and AG[15,16]. In addition, it was effective for clinical symptoms such as abdominal distension and lack of appetite, with an effective rate up to 91%. In contrast with studies that evaluated IM regression after 6 mo or 12 mo of LTEVB12 treatment, the effective rates were 56.25% vs 55.71% and 32.9% and 41.8%, respectively. In other studies, few adverse effect or toxic side effects was found during the treatments[14-16]. The participants in our research did not show severe adverse effect and toxic side effects. Some studies have indicated that the application of aspirin or other non-steroidal anti-inflammatory drugs could restrain the development of GC[30,31]. Several studies have reported the effect of celecoxib on IM regression. COX-2 was suggested to cause H. pylori-related gastric lesions though various mechanisms. Overexpression of COX-2 and the prostaglandin cascade induced by H. pylori-induced inflammation during carcinogenesis could lead to cell proliferation, mutagenesis, mitogenesis, and inhibition of apoptosis. As a COX-2 inhibitor, celecoxib could restrain the processes as mentioned above so that it may inhibit the development of GC[17,20]. Moreover, nuclear factor kappa B activation, which acts as a major mediator of H. pylori-related inflammation, was reported to be inhibited by celecoxib[32,33]. Wong et al[25] reported that the combination of anti-H. pylori therapy and celecoxib did not show better effects than anti-H. pylori therapy alone. However, two studies found that after eradication therapy (1 year and 3 years apart), celecoxib still had an effect on IM and AG regression[21,22]. This might contribute to the persistent existence of the tumor microenvironment even after H. pylori eradication[34,35]. In contrast with other studies that evaluated IM regression after 12 mo or 2 mo of celecoxib treatment, the rate was 56.25% vs 42% and 51.3% and 28.6%, respectively. It was also significantly different even when compared with the nondrug group (16.1% and 20.0%)[21-23].
The complete therapy used traditional Chinese medicine extract and Western medicine in turn, which could combine the advantage of both sides. In Taipei consensus on integrative traditional Chinese and Western medicine, Western medicine was deemed to play a part in the disease diagnosis and therapy, yet was still not perfect with deficiency. Traditional Chinese medicine had complementary and alternative effect which is indispensable[36]. A meta-analysis indicated that traditional Chinese medicine is more effective than current routine pharmacotherapy in clinical symptom relief, H. pylori eradication, and efficacy under endoscopy[37]. Thus, LTEVB12 initial therapy and celecoxib rescue therapy actually enhance the regression rate of gastric mucosal lesions with few adverse effects and toxic side effects.
Generally, the effective rate of each monotherapy for IM in our study was better or at least not inferior to that in other studies. Following the complete therapy, the IM regression rate was up to 85.93%, obviously improving the efficacy of IM regression. In addition, in contrast to previous studies, we had a larger sample in this study. Moreover, in our study, we chose the OLGIM and OGLA stages to assess the effect of IM and AG regression. Some studies chose other methods, such as the mean IM score (MIM), to assess the IM and AG regression effects[24]. MIM was the sum of all IM scores from all samples divided by the number of tissues, which led to the assessment of the effect of regression by lesions, not patients. Compared with other evaluation methodologies of histological examination, OLGA and OLGIM stages exhibited superior capability to assess the individuals. OLGA and OLGIM stages combines the location and degree of gastric mucosal atrophy and IM, which could better reflect the severity of gastric mucosal lesions. What’s more, the OLGA and OLGIM stages have been applied to evaluate the risk of GC in the clinic, which could be much more persuasive[38,39]. In each monotherapy in this study, high GC risk patients with OLGIM or OLGA stages III and IV disease had good effects compared with low-risk patients with low stage disease. Kang et al[40] conducted a 3-year follow-up study in Korea and found that the severe grade of IM was associated with the improvement of IM in the body (OR = 5.14; P < 0.05). This result may be attributed to some reasons. Patients with high OLGIM or OLGA stage usually had more serious inflammation, which may show better efficacy when receiving the therapy particularly the celecoxib therapy. Besides, the patients with advanced OLGIM stage would preferably follow the doctor's advice than other patients and complete the whole course of treatment. Many factors associated with efficacy were included in our study for analysis. The results of logistic regression analysis showed that eating habits are an independent factor for the IM reversal effect of LTEVB12 therapy, which suggested that patients should change unhealthy eating habits while receiving treatment. At the end of the follow-up, four patients were diagnosed with HGIN and recommended to undergo digestive endoscopy surgery. Thus, the efficacy of complete therapy on such advanced lesions may be limited. A drawback of the study was that we did not obtain complete data for clinical symptoms, so we could not evaluate the effect. However, many studies have reported that celecoxib treatment did not increase adverse reactions or affect renal function[22]. This study was conducted in a single center, the sample size was limited, and the follow-up was only applied when 6 mo of therapy was finished, so the conclusion needs to be verified by a long-term follow-up prospective study.
Based on our findings, it is inappropriate to regard IM as the point of no return of gastric mucosal lesions. Correa et al[41] reported in 1990 that in the long-term follow-up, IM could reverse spontaneously in a few patients (0.044/person-year). Hwang et al[42] suggested that in the 10-year follow-up, eradication therapy of H. pylori could reverse 60% of IM lesions. Although some meta-analyses have shown that IM cannot be reversed after H. pylori eradication, there were still some clinical studies with long-term follow-up showed that IM could be reversed after H. pylori eradication. Correa et al[43] reported that eradication therapy could reverse not only the degree of AG, but also the IM in a randomized controlled trial. Leung et al[44] demonstrated that H. pylori eradication could inhibit the development of IM based on a randomized controlled trial with a 5-year follow-up. Kong et al[11] conducted a meta-analysis with the inclusion criteria using the Sydney system or the updated Sydney system. The result showed that H. pylori eradication was significantly related to improvement in IM in the antrum. Kodama et al[12] conducted a prospective 10-year follow-up of patients with IM after H. pylori eradication. Biopsy specimens were taken from five points of the stomach, as recommended by the updated Sydney system. IM scores of the lesser curvature of the corpus decreased gradually in the whole observation period and showed a significant decline after 6-year follow-up. These studies showed the importance of standardized methods of biopsy depending on OLGIM stage and long-term follow-up. Besides, Western and Chinese traditional medicines have a great effect on IM regression. In addition to LTEVB12 and celecoxib mentioned in this study, our group recently also found that resveratrol could reduce IM through the PI3K/AKT/p-FoxO4 signaling pathway and had a potential reversing effect on those IM lesions especially caused by bile acid reflux[45]. Vitamins and other traditional Chinese medicines, such as Moluodan, have also been reported to have reversal effects on gastric precancerous lesions[46,47]. In general, IM should not be viewed as a point of no return of gastric mucosal lesions. The reversal effect of medicine is important for the prevention of GC and is beneficial for reducing the heavy burden of endoscopic follow-up of IM in China.
LTEVB12 initial therapy and celecoxib rescue therapy can effectively decrease the OLGA and OLGIM stages of IM patients to reduce the risk of GC. This therapy with integrative Chinese and western medicine may have good clinical application value in the prevention of GC. Moreover, this finding supports the insight that IM is not the point of no return among gastric precancerous lesions.
A large number of intestinal metaplasia (IM) patients need to be effectively treated, which can successfully reduce the risk of gastric cancer (GC). Some medicines have showed the potential to reverse the IM lesion. It would help doctors in clinical practice and refute the concept that IM could not be reversed.
Lamb’s tripe extract and vitamin B12 capsule (LTEVB12) and celecoxib have been proved to reverse IM in past studies. But the IM regression effect of LTEVB12 and celecoxib still have to be evaluated thoroughly by operative link on gastritis assessment (OLGA) and operative link on the gastric intestinal metaplasia assessment (OLGIM) stages. What’s more, the combination of these two kinds of drugs may enhance the effect of IM regression.
This study aimed to validate the efficacy of LTEVB12 initial therapy and celecoxib rescue therapy on IM.
This study was a retrospective cohort study. A total of 255 patients were included to receive LTEVB12 initial therapy in this study. The patients with failure of IM regression continued to celecoxib receive rescue therapy. After each therapy finished, patients underwent endoscopy and biopsy examination. OLGA and OLGIM stages were applied to evaluate the reversal of atrophic gastritis (AG) and IM.
For LTEVB12 initial therapy, the reversal rates of IM and AG were 52.95% and 48.24%, respectively. For celecoxib rescue therapy, the effective rates for IM and AG were 56.25% and 51.56%, respectively. The IM regression rate of complete therapy was up to 85.03% (P < 0.05). For both therapies, patients with high stages (III or IV) of both OLGA and OLGIM evaluation systems showed a higher IM or AG regression rate than those patients with low stages (I or II). Among high stage (OLGIM III and IV) patients, the IM regression rate was above 70% for each therapy.
Each monotherapy could effectively reverse IM and AG. The LTEVB12 initial therapy and celecoxib rescue therapy, significantly increased the regression effect, which showed strong potential to reduce the risk of GC. IM may be not the point of no return among gastric precancerous lesions.
LTEVB12 initial therapy and celecoxib rescue therapy can achieve better effect on IM regression compared with either monotherapy. IM could be reversed by clinical intervention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wakatsuki T S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4902] [Article Influence: 700.3] [Reference Citation Analysis (1)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 3. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 4. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 5. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 6. | Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Wright NA. Gastric carcinogenesis: when is the point of no return? CA: Springer, 1998. |
| 8. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Kong YJ, Yi HG, Dai JC, Wei MX. Histological changes of gastric mucosa after Helicobacter pylori eradication: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:5903-5911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Kodama M, Murakami K, Okimoto T, Abe T, Nakagawa Y, Mizukami K, Uchida M, Inoue K, Fujioka T. Helicobacter pylori eradication improves gastric atrophy and intestinal metaplasia in long-term observation. Digestion. 2012;85:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wang SR, Zhang HW, Zhang KY. Biological activity of bifudus factor in the extracts from animal gastric mucosa. Zhongguo Yaojixue Zazhi. 1994;15:41-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Gou X, Liu L, Wang Q, Chen L, Dou DC. Treatment of chronic atrophic gastritis in middle aged and senile patients by gastropylor complex capsules combined with folic acid. Xinan Guofangjun Yixue Zazhi. 2013;23:40-42. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 16. | He H, Liu F, Li FF, Re YL, Chen WG. Clinical effect of gastropylor complex capsules in treatment of chronic atrophic gastritis with intestinal metaplasia. Zhonghua Weichangganbingxue Zazhi. 2015;24:1116-1118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Saukkonen K, Rintahaka J, Sivula A, Buskens CJ, Van Rees BP, Rio MC, Haglund C, Van Lanschot JJ, Offerhaus GJ, Ristimaki A. Cyclooxygenase-2 and gastric carcinogenesis. APMIS. 2003;111:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Liu F, Pan K, Zhang X, Zhang Y, Zhang L, Ma J, Dong C, Shen L, Li J, Deng D, Lin D, You W. Genetic variants in cyclooxygenase-2: Expression and risk of gastric cancer and its precursors in a Chinese population. Gastroenterology. 2006;130:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Hung KH, Yang HB, Cheng HC, Wu JJ, Sheu BS. Short-term celecoxib to regress long-term persistent gastric intestinal metaplasia after Helicobacter pylori eradication. J Gastroenterol Hepatol. 2010;25:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sheu BS, Tsai YC, Wu CT, Chang WL, Cheng HC, Yang HB. Long-term celecoxib can prevent the progression of persistent gastric intestinal metaplasia After H. pylori eradication. Helicobacter. 2013;18:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Yang HB, Cheng HC, Sheu BS, Hung KH, Liou MF, Wu JJ. Chronic celecoxib users more often show regression of gastric intestinal metaplasia after Helicobacter pylori eradication. Aliment Pharmacol Ther. 2007;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Zhang LJ, Wang SY, Huo XH, Zhu ZL, Chu JK, Ma JC, Cui DS, Gu P, Zhao ZR, Wang MW, Yu J. Anti-Helicobacter pylori therapy followed by celecoxib on progression of gastric precancerous lesions. World J Gastroenterol. 2009;15:2731-2738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, Liu WD, Feng GS, Zhang XD, Li J, Lu AP, Xia HH, Lam S, You WC. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3558] [Article Influence: 122.7] [Reference Citation Analysis (3)] |
| 27. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 499] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 28. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 30. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Aspirin use and the risk of gastric cancer: a meta-analysis. Dig Dis Sci. 2010;55:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Futagami S, Suzuki K, Hiratsuka T, Shindo T, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis K, Tsukui T, Sakamoto C. Chemopreventive effect of celecoxib in gastric cancer. Inflammopharmacology. 2007;15:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Fu SL, Wu YL, Zhang YP, Qiao MM, Chen Y. Anti-cancer effects of COX-2 inhibitors and their correlation with angiogenesis and invasion in gastric cancer. World J Gastroenterol. 2004;10:1971-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Nam KT, Hahm KB, Oh SY, Yeo M, Han SU, Ahn B, Kim YB, Kang JS, Jang DD, Yang KH, Kim DY. The selective cyclooxygenase-2 inhibitor nimesulide prevents Helicobacter pylori-associated gastric cancer development in a mouse model. Clin Cancer Res. 2004;10:8105-8113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719-31.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 36. | Lue HC, Su YC, Lin SJ, Huang YC, Chang YH, Lin IH, Yang SP. Taipei consensus on integrative traditional Chinese and Western Medicine. J Formos Med Assoc. 2021;120:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Chen X, Dai YK, Zhang YZ, Liu FB, Lan SY, Wang SS, Hu L, Li PW. Efficacy of traditional Chinese Medicine for gastric precancerous lesion: A meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2020;38:101075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1984] [Article Influence: 248.0] [Reference Citation Analysis (1)] |
| 39. | Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 40. | Kang JM, Kim N, Shin CM, Lee HS, Lee DH, Jung HC, Song IS. Predictive factors for improvement of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: a three-year follow-up study in Korea. Helicobacter. 2012;17:86-95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737-4740. [PubMed] |
| 42. | Hwang YJ, Kim N, Lee HS, Lee JB, Choi YJ, Yoon H, Shin CM, Park YS, Lee DH. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018;47:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 43. | Satoh K, Kimura K, Takimoto T, Kihira K. A follow-up study of atrophic gastritis and intestinal metaplasia after eradication of Helicobacter pylori. Helicobacter. 1998;3:236-240. [PubMed] |
| 44. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 45. | Lu W, Ni Z, Jiang S, Tong M, Zhang J, Zhao J, Feng C, Jia Q, Wang J, Yao T, Ning H, Shi Y. Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway. Phytother Res. 2021;35:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 483] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 47. | Tang XD, Zhou LY, Zhang ST, Xu YQ, Cui QC, Li L, Lu JJ, Li P, Lu F, Wang FY, Wang P, Bian LQ, Bian ZX. Randomized double-blind clinical trial of Moluodan () for the treatment of chronic atrophic gastritis with dysplasia. Chin J Integr Med. 2016;22:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |