Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10418
Peer-review started: March 18, 2021
First decision: July 3, 2021
Revised: July 16, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 6, 2021
Processing time: 257 Days and 5.6 Hours
Acute pancreatitis (AP) is a very common acute disease, and the mortality rate of severe AP (SAP) is between 15% and 35%. The main causes of death are multiple organ dysfunction syndrome and infections. The mortality rate of patients with SAP related to liver failure is as high as 83%, and approximately 5% of the SAP patients have fulminant liver failure. Liver function is closely related to the progr
Core Tip: Several studies have contributed to the pathophysiology and clinical trials of liver dysfunction associated with acute pancreatitis (AP). However, great progress has been made on liver injury-associated AP (LIAAP) based on the published literature. This review aims to summarize the research progress of LIAAP's clinical manifestations and underlying mechanisms, which can provide new insights for further understanding and better treatment of AP and LIAAP.
- Citation: Liu W, Du JJ, Li ZH, Zhang XY, Zuo HD. Liver injury associated with acute pancreatitis: The current status of clinical evaluation and involved mechanisms. World J Clin Cases 2021; 9(34): 10418-10429
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10418.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10418
Acute pancreatitis (AP) is a common disease that often requires hospitalization. Over the past decades, as knowledge of pancreatitis has increased, the death rate of pancreatitis has decreased dramatically, but the mortality rate of patients with organ failure during severe AP (SAP) is still high. Global estimates of the incidence and mortality rate for AP were 33.74 cases (95%CI: 23.33-48.81) per 100000 people and 1.60 deaths (95%CI: 0.85-1.58) per 100000 people each year[1]. SAP, characterized by severe pro
AP can cause damage to the liver[4]; however, liver injury can also aggravate the severity of AP[5]. LIAAP often presents with abnormal serum biochemical indicators, abnormal liver perfusion, and fatty liver.
In AP, the serum biochemical indexes of the liver often change, and changes in liver function will affect the severity and prognosis of AP.
The level of serum bilirubin reflects the ability of hepatocytes to uptake, bind, and excrete bilirubin through the liver reticuloendothelial system. When the hepatocytes are damaged, the ability of the liver to clear bilirubin is decreased, and the level of blood bilirubin is increased. When the level of bilirubin in serum is too high, the patient will develop jaundice. Serum total bilirubin, albumin (ALB), and ALB-bilirubin scores are independent risk factors for SAP and can predict hospital mortality in SAP[6,7].
The increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) usually indicates the severity of the liver disease, which usually precedes the appearance of abnormal clinical symptoms, but with the aggravation of liver injury, a large number of hepatocytes become necrotic and die, eventually causing aminotransferase to become exhausted. Consequently, the decrease in aminotransferase occurs in the severe stage of severe liver disease, so the elevated level of the aminotransferase cannot accurately reflect the severity of liver disease or be used to evaluate the prognosis[8]. The serum levels of ALT and AST are positively correlated with the severity of pancreatitis, and the serum levels of ALT and AST return to normal after pancreatitis is resolved[9].
Serum ALB is only synthesized by the liver, with a half-life of 20 d, which can reflect the synthetic function of the liver within a certain period of time. When a large number of hepatocytes are necrotic and the residual function cannot be fully compensated, the level of ALB may decrease. ALB is an independent prognostic factor of persistent organ failure (POF) and can predict POF in AP[10].
Prothrombin time (PT) can be an indicator of the function of the exogenous coagulation system. When severe liver parenchyma cell damage occurs, it can lead to the disturbance in the synthesis and biological activities of coagulation factors Ⅰ, Ⅱ, Ⅴ, Ⅶ, and X, which results in the prolongation of PT. Dynamic changes in coagulation and fibrinolytic markers (such as PT) are good predictors of AP-related mortality and organ failure in patients with AP[11].
Alkaline phosphatase (ALP) mainly exists in the bile capillaries of the liver, bone, kidney, and placenta, while γ-glutamyl transpeptidase (GGT) mainly exists in the cell membrane of the liver, pancreas, spleen, kidney, heart, and brain. The presence of liver parenchymal damage, cholestasis, or biliary obstruction from the capillary bile duct to any level of common bile duct opening can lead to an increase in ALP and GGT. In gallstone AP, patients with higher levels of ALT, bilirubin, and ALP had longer hospital stays than patients without these elevations[12].
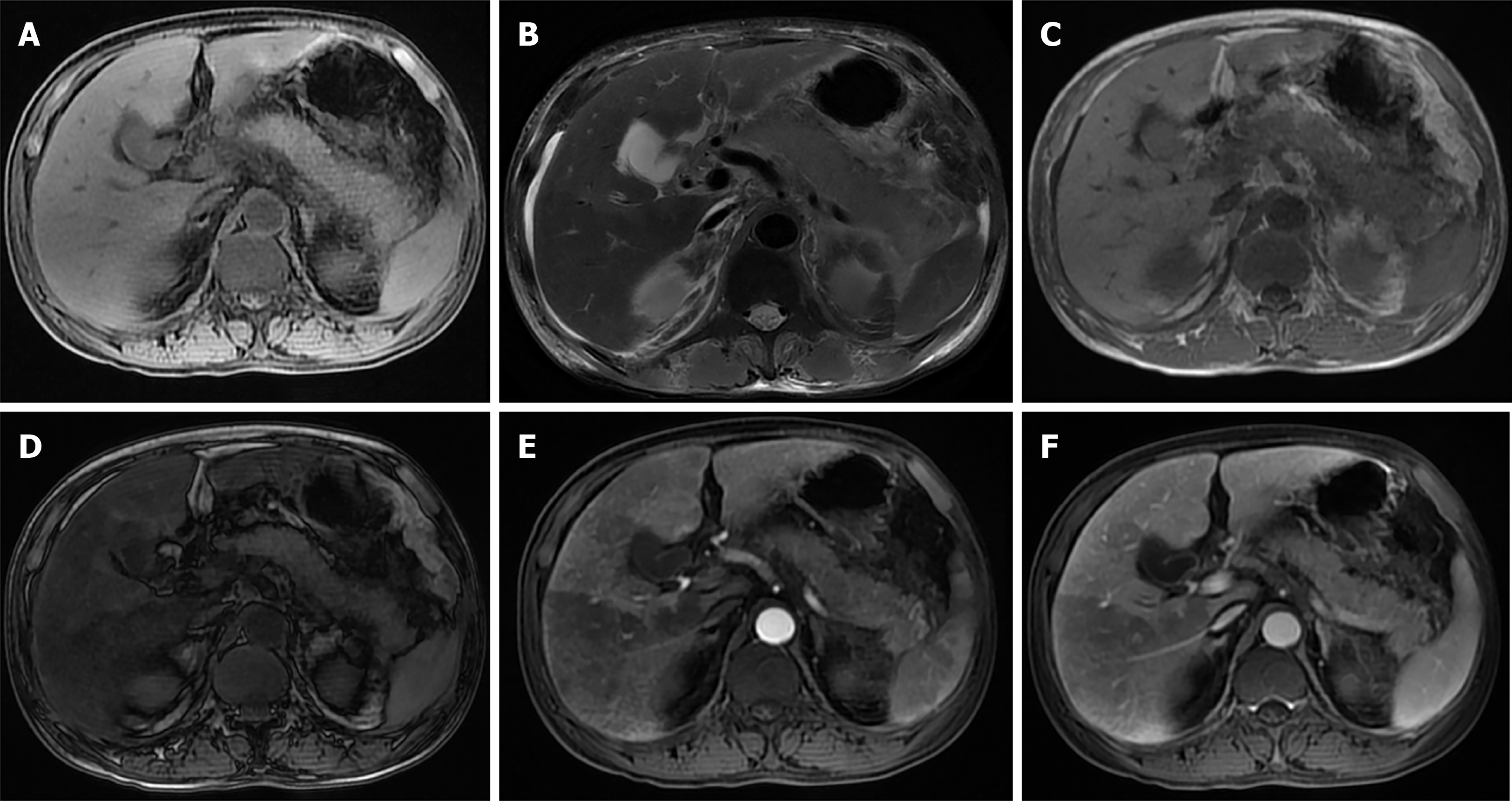
Fatty liver and AP also interact with each other. Researchers have retrospectively collected data from patients with AP after a magnetic resonance (MR) examination and found that there was a significant correlation between the difference in liver signal intensity on in-phase (IP)/out-phase (OP) images and the MR severity index (MRSI) score in AP patients with fatty liver. The difference in liver signal intensity increased with an increasing MRSI score. During the follow-up, it was found that fatty liver could disappear or be relieved after the patient recovered. On the one hand, fatty liver occurs or is aggravated in AP; on the other hand, fatty liver also improves after AP improves[4]. In AP, the incidence of complications, organ failure, metabolic disorder, SIRS, infection, death, and hospital stay were increased in patients with fatty liver[5,13-16] (Figure 1A-D).
There is a correlation between the blood perfusion noted on computed tomography (CT) and the severity of liver injury[17], and there are obvious perfusion changes in liver tissue caused by AP. In perfusion changes, systemic mediators seem to be as effective as local inflammatory changes[18]. It has been reported that a perfusion CT performed within the first 24 h after the onset of AP can predict the severity of AP by revealing liver perfusion abnormalities[19].
The inflammatory process of AP may spread through the hepatoduodenal ligament or the gastrohepatic ligament to the hilum to the liver and eventually along the Glisson sheath[20,21]. Liver perfusion abnormalities in AP may be caused by an increase in the arterial blood flow due to inflammation of the liver lobes or gallbladder[22]. AP can spread to the vesicle and can easily enter the left lobe of the liver around the gastrohepatic ligamentum because the pancreatic body is usually close to the left lobe of the liver[23]. However, an interesting finding is that in an experiment in rats with AP induced by the intraperitoneal administration of caerulein, perfusion changes were more obvious in the right lobe[18]. This has also been demonstrated in human patients[24]. Perfusion changes may be dependent not only on local inflammatory processes but also on the effect of systemic mediators. At the end of the experiment, the rats were euthanized, the liver was resected, and the liver tissue was sent for histopathological analysis. The results showed hepatocyte destruction, sinusoid dilatation, focal necrosis, KCs proliferation, and central venous congestion. It can be seen from this that systemic mediators seem to have effects similar to the local inflammatory changes in regards to perfusion[18]. Finally, liver perfusion abnormalities in AP may be related to a low blood volume and the high metabolism caused by the inflammation. Portal venous blood flow decreases by 50% in AP and increases by 50% after fluid resuscitation. Visceral insufficiency is present in the early stages of acute hemorrhagic pancreatitis, and signs of insufficient blood perfusion can be prevented by fluid resuscitation[25]. Liver blood volume is estimated to be 1/4 of the cardiac output and is important for maintaining a normal liver function. A decreased perfusion within the liver impairs and inhibits the function of the blood/hepatocyte replacement process (Figure 1E and F).
In addition, the ratio of liver volume measured by CT to the standard liver volume reflects the changes in the liver volume during the occurrence and development of the acute liver failure, and this ratio can be used to judge the prognosis of the acute liver failure. The results show that in acute liver failure, CT-derived liver volume/ standardized liver volume measured by CT < 83.9% indicates a poor prognosis[26]. Whether LIAAP will cause changes in liver volume needs further study.
In AP, MODS is closely related to the prognosis of the disease, and the liver is one of the key extrapancreatic organs. For patients with LIAAP, the treatment of the liver injury can also improve the prognosis of AP. Therefore, it is urgent to determine the potential mechanisms of LIAAP.
Cytokines are divided into proinflammatory and anti-inflammatory cytokines, which are balanced with each other[27]. According to the theory of the inflammatory-anti-inflammatory factor balance, when the body is subjected to a harmful stimulation, the inflammatory response is activated as the protective mechanism of the body by releasing pro-inflammatory cytokines, and the anti-inflammatory system is also activated. The body releases anti-inflammatory cytokines to inhibit and regulate the inflammatory response. This not only makes the body effectively resist the invasion of pathogenic factors but also prevents the overactivation of the inflammatory reaction to prevent alterations of the normal function of the body.
During the progression of AP, activated digestive enzymes attack pancreatic acinar cells and hepatocytes. At the same time, activated digestive enzymes can induce neutrophils to release a large number of inflammatory factors, which can lead to a systemic inflammatory response, thus causing damage to multiple organs. Pancreatic elastase induces KCs to produce cytokines by activating the nuclear transcription factor-κB (NF-κB) pathway during SAP[28]. Because part of the pancreatic blood flows back through the portal vein, the liver is the first extrapancreatic organ attacked by high concentrations of activating enzymes and inflammatory mediators at the beginning of AP.
Tumor necrosis factor-α: Excessive tumor necrosis factor-α (TNF-α) can activate neutrophils and release interleukin-1 (IL-1) β, IL-6, IL-8 and other cytokines, which leads to a wide range of pathological reactions. TNF-α is an important initiator of SAP complications, including liver damage. The mechanisms of liver injury induced by TNF-α include: (1) Direct hepatotoxicity; (2) An excessive production of nitric oxide (NO) and an excessive production of oxygen free radicals (OFR) by hepatic KCs and neutrophils, leading to cytotoxic effects; and (3) Apoptosis of hepatocytes and KCs induced by endotoxin before cell injury. TNF-α alone can cause toxic shock symptoms and lead to MODS[29,30].
IL-6: In AP, IL-6 can promote the activation of B lymphocytes and eventually increase the synthesis of immunoglobulins to promote humoral immunity, promote the proliferation and differentiation of T lymphocytes, and promote acute phase reactions, which lead to liver tissue injury, an increase neutrophil function, and induce inter
IL-8: IL-8 is a neutrophil chemokine produced mainly by neutrophils that mediates inflammation from the pancreas to extrapancreatic organs, such as the liver. IL-8 increases the tissue damage of neutrophils and enhances the phagocytosis and killing effect of NK cells on inflammatory tissues[31].
IL-18: IL-18 belongs to the IL-1 superfamily and is produced mainly by macrophages but also by other cell types. It stimulates various cell types and has pleiotropic functions. IL-18 is a proinflammatory cytokine that triggers a type 1 response. Together with IL-12, it induces cell-mediated immunity after exposure to microbial products, such as lipopolysaccharide (LPS)[32]. IL-18 mediates liver damage through two mechanisms: (1) Inducing Fas ligand(FasL) expression to directly mediate hepatocyte apoptosis; and (2) Through the Janus kinase/signal transducer and activator of transcription(JAK-STAT) signaling pathway[32].
High-mobility group box-1: High mobility group protein B1 (HMGB1) is a highly conserved nuclear protein that is widely distributed in mammalian cells. Mechanically damaged and necrotic cells can release HMGB1 from the nucleus extracellularly to induce an inflammatory response. The coexistence of damaged cells and macrophages can cause NF-κB-induced nuclear transfer of macrophages and produce an inflammatory response similar to that caused by tissue necrosis[33]. Ethyl pyruvate (EP) may have a therapeutic effect on LIAAP by regulating the response of box 1 and other inflammatory cytokines in the high mobility group, which indicates that LIAAP may be related to HMGB1[34].
During SAP, the liver function is affected by the pancreatitis. The liver's ability to remove toxic and biologically active substances is significantly reduced, and it loses its barrier function to prevent endotoxemia, leading to excessive release of endogenous inflammatory mediators, which forms a vicious cycle. A large number of endogenous inflammatory mediators then enter the systemic circulation, causing a continuous systemic inflammatory response, systemic tissue damage, and organ dysfunction. The resulting chain reactions and amplification reactions are called cascade reactions, which lead to SIRS and MODS[35,36].
Microcirculation disturbances are an important pathological process of AP[37]. In the early stage of AP, microcirculatory disorders can occur in the pancreas and liver[38]. Disorders of microcirculation are an important cause of SAP combined with liver injury. In SAP, liver injury is associated with an insufficient blood volume due to the release of vasoactive substances and the insufficient blood circulation to the liver. A decrease in liver blood flow can cause mitochondrial ATP synthesis disorders, and the phosphorylation rates of cytochrome A and B are also decreased[39]. Endothelin (ET) and NO play opposite roles in the regulation of blood flow, and the regulation of ET and NO on blood vessels is in a dynamic balance. TXA2/PGI2 is a pair of vasodilator regulators, and imbalances of these factors can cause pathological changes, such as vasomotor disorder, microthromboses, vascular occlusion, and so on. In LIAAP, the balance of TXA2/PGI2 and ET/NO is damaged. The relationship between liver injury, the vascular endothelial cell secretion of ET and NO, and the arachidonic acid metabolites, thromboxane (TXA2) and prostacyclin (PGI2), have been described[31].
Tissue factor (TF), also known as platelet TF, factor III, or CD142, is a protein encoded by the F3 gene and is present in the subcutaneous tissue and the white blood cells but is mainly expressed in extravascular tissues[40,41]. TF is the main cellular initiator for coagulation due to its interaction with coagulation factor VII[42]. Both the coagulation system and the fibrinolytic system can be activated during AP, but the function of the fibrinolytic system is relatively insufficient[43]. In SAP, monocytes stimulated by LPS, TNF-α or IL-1 can produce high levels of TF expression. TF can be transferred to platelets and participate in the process of pathological coagulation, and an abnormal expression of TF leads to a dysfunction of the blood coagulation system. In the KCs of the SAP mouse model, the expression of TF was also highly upregulated. After the inhibition of KCs, the function of the coagulation system was improved, the levels of serum TF and TF microparticles (TF-mps) were decreased, and SAP-related liver injury was alleviated[43]. The imbalance of TF expression plays an important role in the process of liver injury caused by liver microcirculation disturbances.
Fibrinogen-like protein 2 (FGL2) is a 70 kDa glycoprotein that belongs to the fibrinogen-related superfamily and is involved in blood coagulation, cell adhesion, and transendothelial migration[44]. In SAP, the FGL2 prothrombin gene and protein expression were significantly upregulated. Fgl2 prothrombin is involved in the development of microthromboses, which leads to the disturbance of liver microcirculation and eventually leads to liver injury[45].
Disturbance of the microcirculation of the pancreas and liver not only affects the blood supply of other organs, but also leads to an increase in the concentration of inflammatory factors and active peptides in the tissue and cells, which further aggravates the ischemic and hypoxic state of the cells and tissues of the pancreas and liver. These mechanisms further worsen the functional damage of the pancreas and liver.
Oxidative stress is a state of imbalance between oxidation and the antioxidant activity in the body, with a bias toward oxidation, which results in the inflammatory infiltration of neutrophils, an increase in protease secretion, and the production of large amounts of oxidative intermediates. Oxidative stress is a negative effect produced by free radicals in the body and is considered to be an important factor in aging and disease[46]. Oxidative stress can also cause liver injury through a variety of mechanisms[47-49]. Reactive oxygen species (ROS), including oxygen ions, peroxides, and oxygen-containing free radicals (OFRs), are a byproduct of biological aerobic metabolism. Under physiological conditions, there is a balance between the production of ROS and their elimination through the mechanism of endogenous antioxidants[50]. In AP, the balance between the oxidant and antioxidant system is damaged. In an animal experimental model, a large amount of OFR is produced in the early stage of AP induced by a duct obstruction in rats[51]. Highly active ROS directly attack lipids and proteins in biofilms and cause their dysfunction[52]. Excessive ROS production can oxidize lipids in the cell membrane, proteins in the cell solute, DNA and other macromolecules in the nucleus. When activated leukocytes increase the production of ROS in AP, the intrinsic defense mechanism leads to cytoskeletal alterations and cell membrane damage in acinar cells[53]. The destruction of the cytoskeleton interferes with the transport of digestive enzymes in cells and activates digestive enzymes prematurely in acinar cells[54]. Cell fragmentation and leakage of ROS and activated pancreatic enzymes damage the capillary endothelium, increase the capillary permeability, and lead to tissue edema. Malondialdehyde (MDA) is an oxidation product produced by the OFR after interacting with fat in the body and can be used as a sign of the degree of oxidative damage[55]. The reaction between MDA and DNA leads to mutations or protein reactions, which leads to changes in DNA and protein structure and function, which causes damage to the cell membrane and then damage to hepatocytes. The accumulation of MDA induces a change in mitochondrial membrane permeability, which promotes the release of many apoptosis-related cytokines, such as cytochrome C, apoptosis-inducing factor, and endonuclease G, and finally leads to apoptosis. In animal experiments, researchers have found that in caerulein-induced AP mice, the activity of superoxide dismutase in the pancreatic tissue was decreased, and the concentration of MDA in the pancreatic tissue was significantly increased. Studies have shown that OFR mediate the increase in lipid peroxidation in pancreatic tissue[56]. Caerulein-induced pancreatitis and liver damage are accompanied by a significant increase in the tissue MDA levels. These findings suggest that lipid peroxidation is significantly enhanced not only in the pancreatic tissue but also in the liver tissue. Therefore, lipid peroxidation should be one of the mechanisms of liver injury caused by oxidative stress[57]. Oxidative stress-induced hepatocyte apoptosis is affected by a variety of regulatory mechanisms, including mitogen-activated protein kinase (MAPK), NF-κB, caspase, Bcl-2, and the death receptor (DR)[58]. NLRP3 also appears to be regulated by ROS, and LIAAP may be related to NLRP3[59]. The NLRP3 inflammatory bodies mainly induce IL-1β and aggravate inflammatory liver injury[60].
Based on the mechanisms of liver injury induced by oxidative stress, the antioxidant effects of melatonin, thalidomide, carvol, ascorbic acid, N-acetylcysteine, and L-cysteine can help ameliorate the liver injury induced by AP[57,61-64].
In AP, endotoxin also plays an important role in liver injury[65]. Pancreatitis causes a disruption in the intestinal function, reduces the function of the intestinal barrier, and disrupts the intestinal microenvironment and normal flora, and endotoxin then enters the bloodstream and invades the liver through the circulation. The endotoxin entering the liver activates phospholipase A2 (PLA2) to mediate membrane phospholipid degradation and induce free radicals to mediate lipid peroxidation in liver cells[66]. In addition, it also causes liver damage by interfering with energy metabolism. The Toll-like receptor 4 (TLR4) signaling pathway, transforming growth factor β1 (TGF-β1), and p38 MAPK signaling pathways are involved in the occurrence of PALI[67-69].
Signaling pathways refer to a series of enzymatic reaction pathways that can transfer extracellular molecular signals into the cell through the cell membrane. These extracellular molecular signals (called ligands) include hormones, growth factors, cytokines, neurotransmitters, and other small molecular compounds. NF-κB, JAKSTAT and P38MAPK are mainly involved in the signaling pathways related to LIAAP.
NF-κB: NF-kB is an important nuclear transcription factor in cells. NF-κB participates in the inflammatory response and the immune response and can regulate apoptosis and the stress response. NF-κB is one of several key signaling systems that mediate the proinflammatory signal in AP[70], so it may become a target for drug therapy in inhibiting the NF-kB signal transduction pathway[71]. AP induces liver injury by upregulating Fas/FasL derived from KCs[72]. On the other hand, AP induces KC apoptosis through an NF-κB-dependent pathway. The balance between the upregulated expression of Fas/FasL and the initial apoptosis induced by Fas/FasL may determine the severity of the pancreatitis-related liver injury[73].
JAK-STAT: The JAK-STAT signaling pathway is involved in many important biological processes, such as cell proliferation, differentiation, apoptosis, and immune regulation. Compared with other signaling pathways, the transmission process of this signaling pathway is relatively simple, and it is mainly composed of three components, namely, the tyrosine kinase-related receptor, the tyrosine kinase JAK, and the transcription factor STAT[74].
The JAK-STAT signaling pathway constitutes one of the major pathways for cytokine signal transduction. Researchers found that in SAP rats induced by a retrograde infusion of 4% sodium taurocholate into the cholangiopancreatic duct, the levels of the liver enzymes, TNF-α, IL-6, and IL-18 were all significantly increased, and the protein expression levels of JAK2 and STAT3 were significantly increased. In the SAP with AG490 (inhibition of JAK2) group, AG490 effectively inhibited the activation of JAK2 and STAT3 phosphorylation, the levels of liver enzymes TNF-α, IL-6, and IL-18 were significantly decreased, and the protein expression levels of JAK2 and STAT3 were significantly decreased. This suggests that the activation of JAK2/STAT3 gene expression and the production of inflammatory factors such as TNF-α, IL-6, and IL-18 can lead to a pancreatitis-induced liver injury[75].
P38 MAPKs: P38 MAPKs are a family of MAPKs that respond to stress stimuli (such as cytokines, ultraviolet radiation, heat shock, and osmotic shock) and are involved in cell differentiation, apoptosis, and autophagy. Due to aging, the continuous activation of the p38 MAPK pathway in muscle satellite cells (muscle stem cells) can inhibit muscle regeneration[76,77]. Studies have shown that p38 MAPK plays an important role in the pathogenesis of SAP[78]. P38 MAP kinase modulates the activation of the NF-κB pathway in AP. In addition, KCs can amplify the release of cytokines by activating p38 MAPK, which leads to the liver injury in AP. Therefore, the activation of p38 MAPK in KCs may be the main regulatory mechanism of SAP[79,80].
Hepatocyte apoptosis may be one of the factors leading to liver failure. The roles of liver Bax, Bcl-2, IL-1 invertase inhibitors, and TGF-β in liver injury induced by SAP hepatocyte apoptosis have been previously reviewed[31].
The Ca2+ storage site in the endoplasmic reticulum (ER) in cells is the largest membrane organelle in the cell and controls the processing, modification, and synthesis of a large number of proteins in the cell. When the environment in the ER, which is characterized by oxidation and a high calcium concentration, is destroyed, it leads to the accumulation of incorrectly folded proteins in the ER, which leads to severe ER stress (ERS)[81,82]. ERS is a new pathway that is different from the mitochondrial pathway and can independently lead to apoptosis. ERS plays an important role in the occurrence and development of the liver injury induced by AP[83].
The treatment of AP includes close monitoring of vital signs, fluid resuscitation, nutrition, pain management, prevention of secondary infections, and management of local complications[84]. The treatment of LIAAP is closely related to the treatment of AP because the degree of liver damage depends on the severity of the pancreatitis. According to the mechanism of LIAAP, treatment methods include anti-inflammatory and antioxidant therapies, the improvement of the microcirculation, the inhibition of apoptosis, the promotion of liver cell regeneration, and supportive therapy.
In the rat model, sodium butyrate can inhibit the activation of NF-κB in the liver, reduce the expression of the HMGB1 gene, reduce the level of the HMGB1 protein, and ultimately reduce the lethal outcome of SAP rats[33]. Antioxidants, such as melatonin, ascorbic acid, and N-acetyl cysteine, reduce the damage to the pancreas and liver during AP by restoring the activity of tissue antioxidant enzymes[57]. Recombinant human soluble thrombomodulin and prostaglandin E1 reduce liver and pancreas damage by maintaining the microcirculation, which improves the prognosis of SAP[85,86]. Nilotinib reduces pancreatic and liver damage through antioxidant and anti-inflammatory effects[87]. Heme oxygenase-1 can prevent pancreatic and liver damage through antioxidation, the maintenance of the microcirculation, and anti-inflammatory mechanisms[88]. When SAP is complicated by liver and kidney injuries, the level of vascular endothelial cell growth factor (VEGF) is increased. The administration of recombinant VEGF to an SAP rat model can effectively improve the liver and kidney function and significantly inhibit cell apoptosis in the liver and kidney[89]. Somatostatin can inhibit the overexpression of serum TNF-α mRNA in AP rats and reduce the pancreatic and liver tissue damage[90]. EP significantly reduces serum ALT and liver necrosis in the SAP mouse model through anti-inflammatory and antioxidant effects[91].
Because of the influence of multiple factors in the course of AP, the combined use of the above drugs may improve the prognosis. In addition to supportive therapy, treatment of the pancreas and the extrapancreatic organs is also a promising treatment method, but further research is needed.
The clinical manifestations of LIAAP are usually the presence of abnormal serum biochemical indexes and abnormal liver perfusion. There are many reasons for AP complications and secondary liver injury. These factors cooperate and cause cascade reactions, which lead to the liver injury. Treatment of the liver injury can alleviate the severity of AP. Finally, a new mechanism of LIAAP may be found through proteomics and metabonomics, and further related studies are needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tantau AI, Uhlmann D S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yu HG
| 1. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 324] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Multiple organ dysfunction associated with severe acute pancreatitis. Crit Care Med. 2002;30:1274-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Xiao B, Zhang XM, Jiang ZQ, Tang W, Huang XH, Yang L, Feng ZS. Fatty liver in acute pancreatitis: characteristics in magnetic resonance imaging. J Comput Assist Tomogr. 2012;36:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Hou S, Tang X, Cui H, Liu C, Bai X, Shi L, Shi Y. Fatty liver disease is associated with the severity of acute pancreatitis:A systematic review and meta-analysis. Int J Surg. 2019;65:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 6. | Shi L, Zhang D, Zhang J. Albumin-bilirubin score is associated with in-hospital mortality in critically ill patients with acute pancreatitis. Eur J Gastroenterol Hepatol. 2020;32:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Xu X, Ai F, Huang M. Deceased serum bilirubin and albumin levels in the assessment of severity and mortality in patients with acute pancreatitis. Int J Med Sci. 2020;17:2685-2695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | McCord KW, Webb CB. Hepatic dysfunction. Vet Clin North Am Small Anim Pract. 2011;41:745-758, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Güngör B, Cağlayan K, Polat C, Seren D, Erzurumlu K, Malazgirt Z. The predictivity of serum biochemical markers in acute biliary pancreatitis. ISRN Gastroenterol. 2011;2011:279607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Li S, Zhang Y, Li M, Xie C, Wu H. Serum albumin, a good indicator of persistent organ failure in acute pancreatitis. BMC Gastroenterol. 2017;17:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 11. | Liu C, Zhou X, Ling L, Chen S, Zhou J. Prediction of mortality and organ failure based on coagulation and fibrinolysis markers in patients with acute pancreatitis: A retrospective study. Medicine (Baltimore). 2019;98:e15648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Mathuram Thiyagarajan U, Ponnuswamy A, Thomas R. Predictivity of Biochemical Markers on Aetiology and Length of Hospitalisation in Acute Pancreatitis. Cureus. 2020;12:e11989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Xu C, Qiao Z, Lu Y, Zhang D, Jia Z, Zhuang X, Shi Y, Xu T, Xing L, Shen J. Influence of Fatty Liver on the Severity and Clinical Outcome in Acute Pancreatitis. PLoS One. 2015;10:e0142278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Mikolasevic I, Orlic L, Poropat G, Jakopcic I, Stimac D, Klanac A, Carovic F, Milic S. Nonalcoholic fatty liver and the severity of acute pancreatitis. Eur J Intern Med. 2017;38:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yoon SB, Lee IS, Choi MH, Lee K, Ham H, Oh HJ, Park SH, Lim CH, Choi MG. Impact of Fatty Liver on Acute Pancreatitis Severity. Gastroenterol Res Pract. 2017;2017:4532320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Wu D, Zhang M, Xu S, Wu K, Wang N, Wang Y, Wu J, Lu G, Gong W, Ding Y, Xiao W. Nonalcoholic Fatty Liver Disease Aggravated the Severity of Acute Pancreatitis in Patients. Biomed Res Int. 2019;2019:9583790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Oğul H, Kantarcı M, Genç B, Pirimoğlu B, Cullu N, Kızrak Y, Yılmaz O, Karabulut N. Perfusion CT imaging of the liver: review of clinical applications. Diagn Interv Radiol. 2014;20:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tutcu S, Serter S, Kaya Y, Kara E, Neşe N, Pekindil G, Coşkun T. Hepatic perfusion changes in an experimental model of acute pancreatitis: evaluation by perfusion CT. Eur J Radiol. 2010;75:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Pieńkowska J, Gwoździewicz K, Skrobisz K, Czarnowska-Cubała M, Kozak O, Hać S, Studniarek M, Szurowska E. Can Disturbed Liver Perfusion Revealed in p-CT on the First Day of Acute Pancreatitis Provide Information about the Expected Severity of the Disease? Gastroenterol Res Pract. 2019;2019:6590729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Meyers MA, Oliphant M, Berne AS, Feldberg MA. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Fujiwara T, Takehara Y, Ichijo K, Tooyama N, Kodaira N, Yamamoto H, Watahiki H. Anterior extension of acute pancreatitis: CT findings. J Comput Assist Tomogr. 1995;19:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Arita T, Matsunaga N, Takano K, Hara A, Fujita T, Honjo K. Hepatic perfusion abnormalities in acute pancreatitis: CT appearance and clinical importance. Abdom Imaging. 1999;24:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Siegelman SS, Copeland BE, Saba GP, Cameron JL, Sanders RC, Zerhouni EA. CT of fluid collections associated with pancreatitis. AJR Am J Roentgenol. 1980;134:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Koyasu S, Isoda H, Tsuji Y, Yamamoto H, Matsueda K, Watanabe Y, Chiba T, Togashi K. Hepatic arterial perfusion increases in the early stage of severe acute pancreatitis patients: evaluation by perfusion computed tomography. Eur J Radiol. 2012;81:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Juvonen PO, Tenhunen JJ, Heino AA, Merasto M, Paajanen HE, Alhava EM, Takala JA. Splanchnic tissue perfusion in acute experimental pancreatitis. Scand J Gastroenterol. 1999;34:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Tong C, Xu X, Liu C, Zhang T, Qu K. Assessment of liver volume variation to evaluate liver function. Front Med. 2012;6:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1975] [Article Influence: 179.5] [Reference Citation Analysis (1)] |
| 28. | Murr MM, Yang J, Fier A, Kaylor P, Mastorides S, Norman JG. Pancreatic elastase induces liver injury by activating cytokine production within Kupffer cells via nuclear factor-Kappa B. J Gastrointest Surg. 2002;6:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Zhang XP, Zhang L, Yang P, Zhang RP, Cheng QH. Protective effects of baicalin and octreotide on multiple organ injury in severe acute pancreatitis. Dig Dis Sci. 2008;53:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Suzuki M, Shimizu T, Kudo T, Shoji H, Ohtsuka Y, Yamashiro Y. Octreotide prevents L-asparaginase-induced pancreatic injury in rats. Exp Hematol. 2008;36:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Zhang XP, Wang L, Zhang J. Study progress on mechanism of severe acute pancreatitis complicated with hepatic injury. J Zhejiang Univ Sci B. 2007;8:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Yuan BS, Zhu RM, Braddock M, Zhang XH, Shi W, Zheng MH. Interleukin-18: a pro-inflammatory cytokine that plays an important role in acute pancreatitis. Expert Opin Ther Targets. 2007;11:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Zhang T, Xia M, Zhan Q, Zhou Q, Lu G, An F. Sodium Butyrate Reduces Organ Injuries in Mice with Severe Acute Pancreatitis Through Inhibiting HMGB1 Expression. Dig Dis Sci. 2015;60:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Therapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitis. Pancreas. 2012;41:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 37. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Lisman T, Porte RJ. Activation and regulation of hemostasis in acute liver failure and acute pancreatitis. Semin Thromb Hemost. 2010;36:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Trapnell JE. Pathophysiology of acute pancreatitis. World J Surg. 1981;5:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Butenas S. Tissue factor structure and function. Scientifica (Cairo). 2012;2012:964862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Carmeliet P, Collen D. Tissue factor. Int J Biochem Cell Biol. 1998;30:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Ou ZB, Miao CM, Ye MX, Xing DP, He K, Li PZ, Zhu RT, Gong JP. Investigation for role of tissue factor and blood coagulation system in severe acute pancreatitis and associated liver injury. Biomed Pharmacother. 2017;85:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Selzner N, Liu H, Boehnert MU, Adeyi OA, Shalev I, Bartczak AM, Xue-Zhong M, Manuel J, Rotstein OD, McGilvray ID, Grant DR, Phillips MJ, Levy GA, Selzner M. FGL2/fibroleukin mediates hepatic reperfusion injury by induction of sinusoidal endothelial cell and hepatocyte apoptosis in mice. J Hepatol. 2012;56:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Chen T, Ye X, Huang Z, Chen R, Zhuge X, Chen X, Du Y. Fgl2 prothrombinase is involved in severe acute pancreatitis-associated liver injury. Hepatogastroenterology. 2012;59:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2265] [Article Influence: 283.1] [Reference Citation Analysis (0)] |
| 47. | Wu ZT, Qi XM, Sheng JJ, Ma LL, Ni X, Ren J, Huang CG, Pan GY. Timosaponin A3 induces hepatotoxicity in rats through inducing oxidative stress and down-regulating bile acid transporters. Acta Pharmacol Sin. 2014;35:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Barcelos RP, Souza MA, Amaral GP, Stefanello ST, Bresciani G, Fighera MR, Soares FA, Barbosa NV. Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci. 2014;96:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Sid B, Verrax J, Calderon PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic Res. 2013;47:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO. Ameliorative effect of supplementation with L-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte cultures. Cytotechnology. 2012;64:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Uruñuela A, Sevillano S, de la Mano AM, Manso MA, Orfao A, de Dios I. Time-course of oxygen free radical production in acinar cells during acute pancreatitis induced by pancreatic duct obstruction. Biochim Biophys Acta. 2002;1588:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Yamamoto Y, Niki E, Eguchi J, Kamiya Y, Shimasaki H. Oxidation of biological membranes and its inhibition. Free radical chain oxidation of erythrocyte ghost membranes by oxygen. Biochim Biophys Acta. 1985;819:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Krüger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328-G338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Wang G, Sun B, Gao Y, Meng QH, Jiang HC. The effect of emodin-assisted early enteral nutrition on severe acute pancreatitis and secondary hepatic injury. Mediators Inflamm. 2007;2007:29638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Dabrowski A, Gabryelewicz A, Wereszczyńska-Siemiatkowska U, Chyczewski L. Oxygen-derived free radicals in cerulein-induced acute pancreatitis. Scand J Gastroenterol. 1988;23:1245-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1681] [Cited by in RCA: 2107] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 60. | Wang J, Dong R, Zheng S. Roles of the inflammasome in the gutliver axis (Review). Mol Med Rep. 2019;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 61. | Yang LJ, Wan R, Shen JQ, Shen J, Wang XP. Effect of L-cysteine on remote organ injury in rats with severe acute pancreatitis induced by bile-pancreatic duct obstruction. Hepatobiliary Pancreat Dis Int. 2013;12:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Chen F, Zhou YJ. Hepatic effect of NAC on sevear acute pancteatise of rats. Asian Pac J Trop Med. 2014;7:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Lv P, Fan LJ, Li HY, Meng QS, Liu J. Protective Effect of Thalidomide on Liver Injury in Rats with Acute Pancreatitis via Inhibition of Oxidative Stress. Ann Clin Lab Sci. 2015;45:508-514. [PubMed] |
| 64. | Bakır M, Geyikoglu F, Colak S, Turkez H, Bakır TO, Hosseinigouzdagani M. The carvacrol ameliorates acute pancreatitis-induced liver injury via antioxidant response. Cytotechnology. 2016;68:1131-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Windsor JA, Fearon KC, Ross JA, Barclay GR, Smyth E, Poxton I, Garden OJ, Carter DC. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg. 1993;80:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Kazantsev GB, Hecht DW, Rao R, Fedorak IJ, Gattuso P, Thompson K, Djuricin G, Prinz RA. Plasmid labeling confirms bacterial translocation in pancreatitis. Am J Surg. 1994;167:201-6; discussion 206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Wang Q, Zhang X, Lei S, Wang Y, Zhuang Y, Chen Y, Zeng C, Zhang H, Liu C, Wang G. RNA sequence analysis reveals pathways and candidate genes associated with liver injury in a rat pancreatitis model. Pancreatology. 2018;18:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Role of toll-like receptor 4 in the pathophysiology of severe acute pancreatitis in mice. Surg Today. 2007;37:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Peng Y, Sigua CA, Rideout D, Murr MM. Deletion of toll-like receptor-4 downregulates protein kinase C-zeta and attenuates liver injury in experimental pancreatitis. Surgery. 2008;143:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1190-G1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1488] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 72. | Wei S, Huang Q, Li J, Liu Z, You H, Chen Y, Gong J. Taurine attenuates liver injury by downregulating phosphorylated p38 MAPK of Kupffer cells in rats with severe acute pancreatitis. Inflammation. 2012;35:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Peng Y, Gallagher SF, Landmann R, Haines K, Murr MM. The role of p65 NF-kappaB/RelA in pancreatitis-induced Kupffer cell apoptosis. J Gastrointest Surg. 2006;10:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Leonard WJ, Lin JX. Cytokine receptor signaling pathways. J Allergy Clin Immunol. 2000;105:877-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Li M, Zhang X, Wang B, Xu X, Wu X, Guo M, Wang F. Effect of JAK2/STAT3 signaling pathway on liver injury associated with severe acute pancreatitis in rats. Exp Ther Med. 2018;16:2013-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 76. | Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 526] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 77. | Segalés J, Perdiguero E, Muñoz-Cánoves P. Regulation of Muscle Stem Cell Functions: A Focus on the p38 MAPK Signaling Pathway. Front Cell Dev Biol. 2016;4:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 78. | Twait E, Williard DE, Samuel I. Dominant negative p38 mitogen-activated protein kinase expression inhibits NF-kappaB activation in AR42J cells. Pancreatology. 2010;10:119-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Xu FL, You HB, Li XH, Chen XF, Liu ZJ, Gong JP. Glycine attenuates endotoxin-induced liver injury by downregulating TLR4 signaling in Kupffer cells. Am J Surg. 2008;196:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Gallagher SF, Yang J, Baksh K, Haines K, Carpenter H, Epling-Burnette PK, Peng Y, Norman J, Murr MM. Acute pancreatitis induces FasL gene expression and apoptosis in the liver. J Surg Res. 2004;122:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 795] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 82. | Wang X, Eno CO, Altman BJ, Zhu Y, Zhao G, Olberding KE, Rathmell JC, Li C. ER stress modulates cellular metabolism. Biochem J. 2011;435:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Hong YP, Deng WH, Guo WY, Shi Q, Zhao L, You YD, Mei FC, Zhou Y, Wang CY, Chen C, Yu J, Wang WX. Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G838-G847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 85. | Matsumoto M, Kamei K, Chikugo T, Matsumoto I, Kawaguchi K, Takeyama Y. Efficacy of Recombinant Human-Soluble Thrombomodulin for Severe Acute Pancreatitis in a Rat Experimental Model. Pancreas. 2020;49:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 86. | Takahashi H, Imamura M, Mikami Y, Yamauchi H. Exacerbation of acute pancreatitis in the presence of chronic liver injury in rats, with special reference to therapeutic efficacy of prostaglandin E1. Pancreas. 1999;19:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Nader MA, Wagih HM. Nilotinib, a tyrosine kinase inhibitor exhibits protection against acute pancreatitis-induced lung and liver damage in rats. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Zhang FH, Sun YH, Fan KL, Dong XB, Han N, Zhao H, Kong L. Protective effects of heme oxygenase-1 against severe acute pancreatitis via inhibition of tumor necrosis factor-α and augmentation of interleukin-10. BMC Gastroenterol. 2017;17:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, Kuroda Y. Vascular endothelial growth factor increases in serum and protects against the organ injuries in severe acute pancreatitis. J Surg Res. 2006;134:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Zhang XP, Zhang J, Ren Z, Feng GH, Zhu W, Cai Y, Yang QJ, Ju TF, Xie Q, Yuan WQ. Study on protecting effects of baicalin and octreotide on hepatic injury in rats with severe acute pancreatitis. World J Gastroenterol. 2008;14:6551-6559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Yang R, Shaufl AL, Killeen ME, Fink MP. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J Surg Res. 2009;153:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |