Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.697
Peer-review started: September 14, 2020
First decision: November 20, 2020
Revised: November 26, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: January 26, 2021
Processing time: 128 Days and 11.3 Hours
Juvenile-onset primary open-angle glaucoma (JOAG), characterized by severe elevation of intraocular pressure and optic neuropathy prior to the age of 40, is a rare subtype of primary open-angle glaucoma. Several genetic mutations have been associated with JOAG.
The proband patient was a young male, diagnosed with primary open-angle glaucoma at the age of 27. The patient and his unaffected parents who have been excluded from classic genetic mutations for primary open-angle glaucoma were included to explore for other possible genetic variants through whole genome sequencing and bioinformatics analysis. In this trio, we found two heterozygous variants inherited from the parents in the proband: c.281G>A, p.Arg94His in OLFM2 and c.177C>G, p.Ile59Met in SIX6. Both genetic mutations are predicted through bioinformatics analysis to replace evolutionary conserved amino acids, therefore rendering a pathogenic effect on proteins. In contrast, very low frequencies for these genetic mutations were recorded in most common control databases.
This is the first report on coinherited mutations of OLFM2 and SIX6 in a JOAG family, which shows the complexity of JOAG inheritance. Large-scale clinical screening and molecular functional investigations on these coinherited mutations are imperative to improve our understanding of the development of JOAG.
Core Tip: This report describes a case of juvenile-onset primary open-angle glaucoma (JOAG) with a coinheritance of OLFM2 and SIX6 variants, which might contribute to the onset of JOAG. This finding may enrich the genetic spectrum of JOAG and prompt us to further investigate the functional effects of the two variants on JOAG.
- Citation: Yang X, Sun NN, Zhao ZN, He SX, Zhang M, Zhang DD, Yu XW, Zhang JM, Fan ZG. Coinheritance of OLFM2 and SIX6 variants in a Chinese family with juvenile-onset primary open-angle glaucoma: A case report. World J Clin Cases 2021; 9(3): 697-706
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/697.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.697
Primary open-angle glaucoma (POAG) is a group of polygenic heterogeneous diseases, with characteristic glaucomatous optic neuropathy and visual-field defects. POAG is the second most common cause of irreversible blindness worldwide[1]. Juvenile-onset primary open-angle glaucoma (JOAG) is a unique subtype of POAG, characterized by an early disease onset prior to 40-years-old, usually a significantly elevated intraocular pressure (IOP) and severe glaucomatous optic neuropathy[2]. JOAG progresses more rapidly in comparison with classic adult-onset POAG[3].
POAG is essentially a neurodegenerative disease in the elderly population[4], whose onset is determined by interactions between various environmental and genetic factors. JOAG, due to its early onset, is likely subsequent to a genetic mutation. Currently, several genes, including MYOC, OPTN, CYP1B1, OAS3, PITX2 and LTBP2 have been reported to be associated with JOAG[5-7] through traditional Mendel genetic analysis. However, clinically there are still many JOAG cases that cannot be attributed to these reported genetic mutations. Therefore, a strategy using whole genome sequencing (WGS) and de novo mutation trio analysis[8] can be used to explore novel genetic variants.
In this current study, we performed WGS on a trio (the proband and his unaffected parents) at Zhongshan Ophthalmic Center to identify potential disease-causing mutations. After excluding classic glaucoma-associated gene mutations, we surprisingly found two heterozygous gene variants that were predicted to be potentially pathogenic via bioinformatics analysis in the proband. To our knowledge, this is the first report on a JOAG family that has been associated with a coinheritance of OLFM2 (c.281G>A, p.Arg94His) and SIX6 (c.177C>G, p.Ile59Met) variants.
A 27-year-old male was admitted to our hospital due to elevated IOP of both eyes for nearly 3 months.
There was no relevant ocular or medical history.
The patient was previously diagnosed with myopia, and he was allergic to cefradine. No history of systemic hypertension, diabetes mellitus, coronary heart disease, stroke, smoking or obesity was reported.
There was no remarkable personal and family history.
His myopic refractive error at -3.00 diopter of both eyes was detected. The IOPs without prescription medications at the initial visit in Zhongshan Ophthalmic Center were 28.7 mmHg in the right eye and 31.4 mmHg in the left eye, which was recorded as the peak IOP. Best corrected visual acuity was 1.0 in each eye measured by the standard Snellen chart.
Routine blood examination, liver and kidney function and electrolyte test indicators were normal.
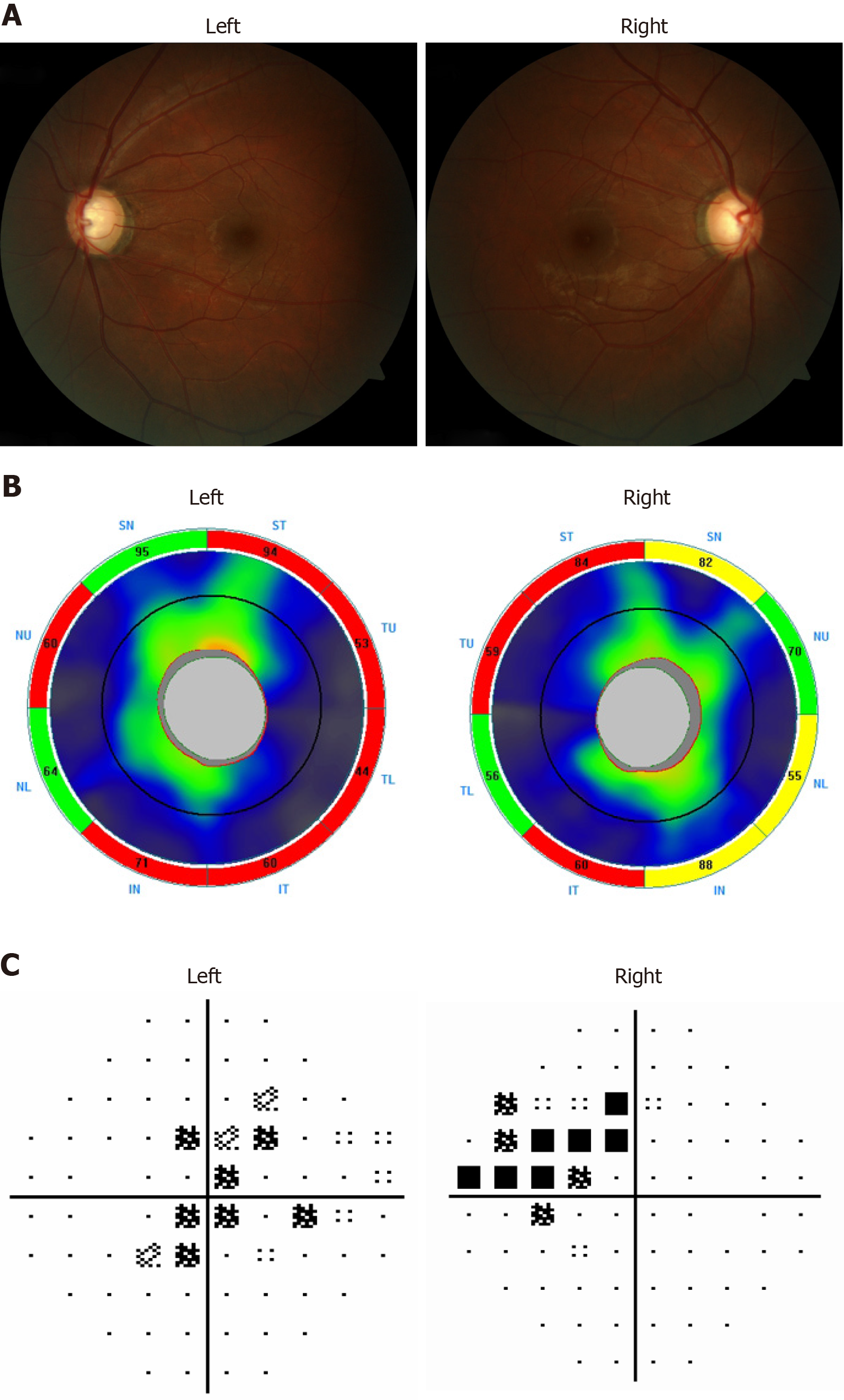
Fundus examination with color photos was accomplished with particular attention paid to make sure that image focus was exactly on the optic disk margins. The result showed vertical cup disc ratio of his right eye was 0.7 and his left eye was 0.8 with localized cupping in both eyes. A localized retinal nerve fiber layer thinning corresponding to the optic disc cupping was detected by Heidelberg Spectralis optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany) in each eye, with an average retinal nerve fiber layer thickness significantly reduced at 69 µm oculus dexter and 67 µm oculus sinister. Visual field defects were detected by Humphrey Visual Field Analyzer (Zeiss Meditech, Jena, Germany) in both eyes with the visual field mean deviation of -6.75 dB oculus dexter and -5.30 dB oculus sinister (Figure 1).
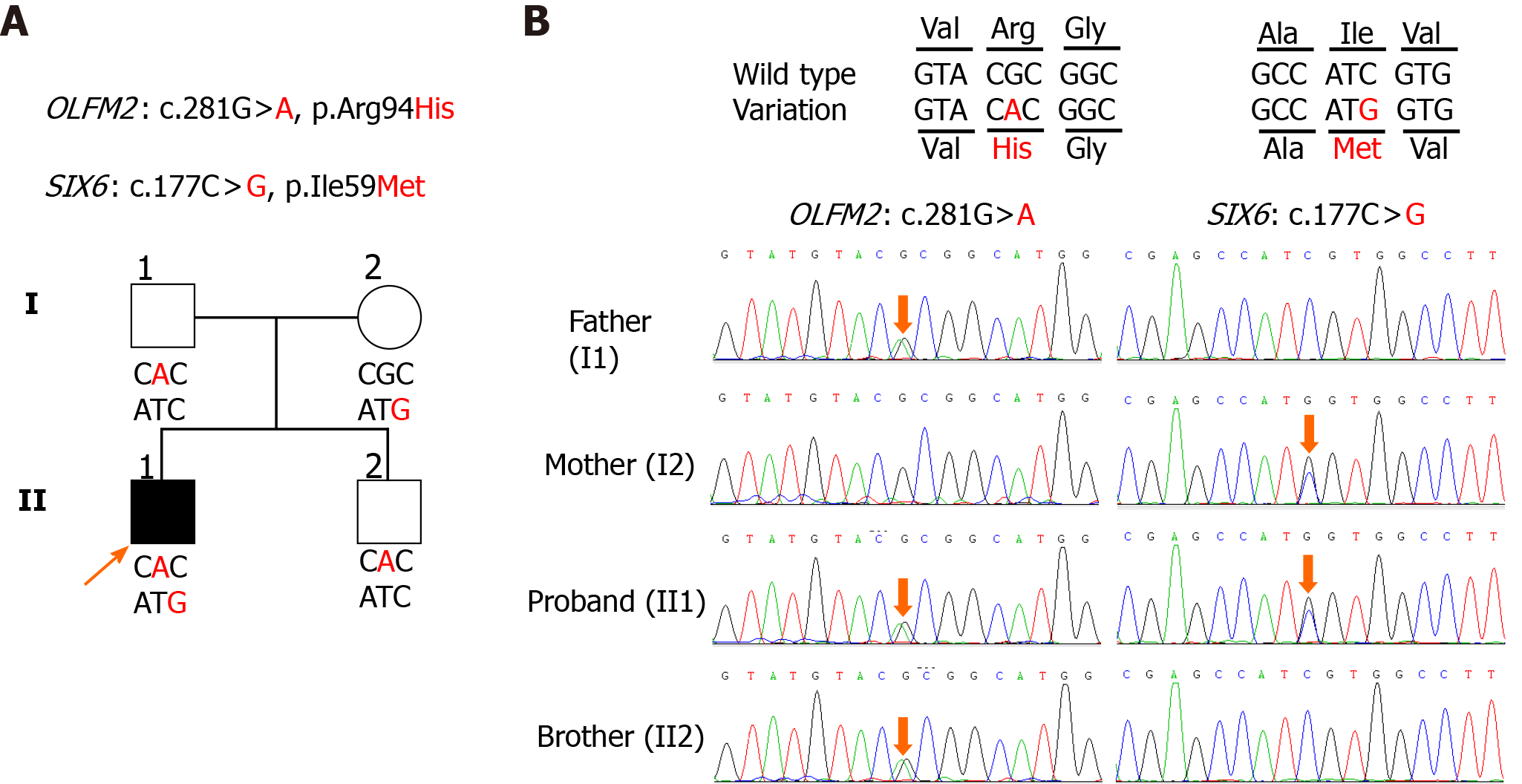
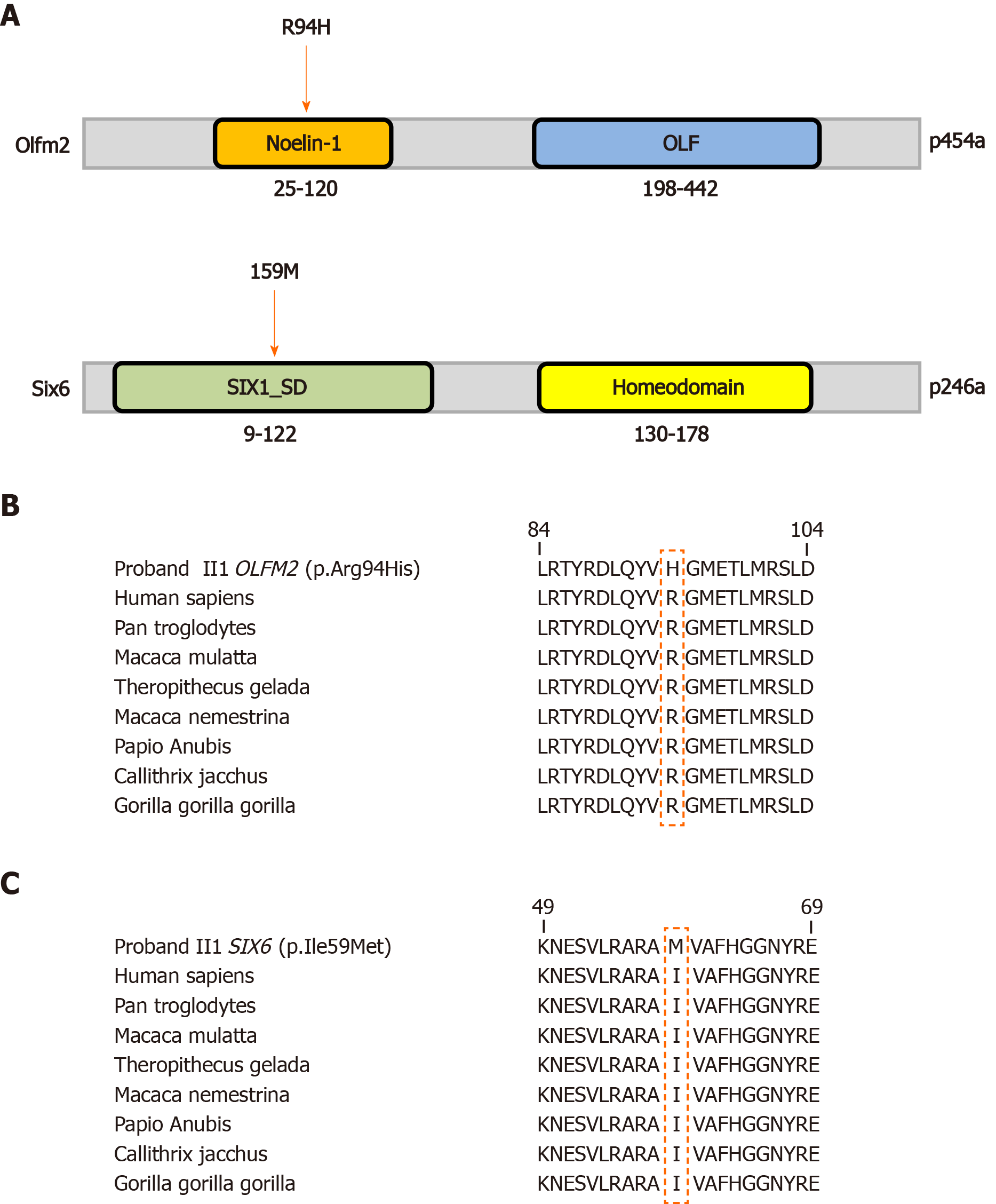
WGS: WGS followed the same protocol as previously reported[9]. The sequencing depth among participants ranged from 54.23X to 60.27X, with the percentage of coverage more than 10X at 91.53% to 92.20%. Mutations identified were analyzed using an autosomal recessive model as well as de novo dominant model. We have excluded mutations with a minor allele frequency more than 0.1% in either the Genome Aggregation Database or the Exome Aggregation Consortium. According to the data from the Human Phenotype Ontology, the names of the reference genes screened are listed in the Supplementary Table 1. The screening criteria for deleterious mutations were based on the American College of Medical Genetics guidelines[10]. In trio-based WGS, the single nucleotide polymorphism in some of the major gene loci (Supplementary Table 2) that were previously reported to affect JOAG phenotype, such as MYOC and LTBP2[6,7] as well as de novo dominant mutations were not detected. Importantly, it was found that the proband (II1) had coinherited mutations. The proband has a c.281G>A, p.Arg94His in OLFM2 (NM_058164.3) inherited from his father (I1) and a c.177C>G, p.Ile59Met in SIX6 (NM_007374.2) inherited from his mother (I2), which cosegregates with the phenotype in a recessive pattern (Figure 2). Interestingly, the OLFM2 mutation from the father is also transmitted to another sibling (II2), who is exempt from any glaucoma phenotypes. The OLFM2 mutation (c.281G>A, p.Arg94His) had a minor allele frequency of 0.005% in the Exome Aggregation Consortium and 0.004% in the Genome Aggregation Database, while the specific mutation was classified as deleterious in MutationTaster, mCAP and CADD. The SIX6 mutation (c.177C>G, p.Ile59Met) was absent in the Exome Aggregation Consortium and had a minor allele frequency of 0.0004% in the Genome Aggregation Database, which was classified as deleterious in MutationTaster, Fathmm and mCAP. Importantly, these mutations were found to be highly conserved across various species (Figure 3).
Sanger sequencing: Amplification using PCR followed by Sanger sequencing was used to confirm the identified mutations of OLFM2 and SIX6 in each family member. Sequencing was performed on an ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, United States). The reference genes were obtained from the Ensembl genome browser (in the public domain, http://www.ensembl.org). The primers used for PCR amplification are listed in Table 1.
| Gene | Sequence, 5’-3’ | Product size in bp |
| OLFM2 | F: CGTCGGTGTGGTGTTTGTAC | 372 |
| R: TGTGGCTCATATTGGACCCT | ||
| SIX6 | F: AATCCGCCTCATCAACAAGC | 825 |
| R: CAGAACGCAGGGCTCTTAAC |
JOAG in both eyes.
Since the diagnosis of glaucoma, the patient has been routinely treated with medication (brimonidine tartrate eye drops, three times a day; travatan, quaque nocte; azopt, twice a day) to control IOP for more than 3 months. At the age of 28, the patient received an express implant (Glaucoma Filtration Device Version P-200) in the right eye. One month later, his left eye was also treated with an express implant (Glaucoma Filtration Device Version P-200). Antibiotic eye drops (compound neomycin sulfate) and eye ointment (tobramycin dexamethasone) were used for anti-inflammation postoperatively.
The IOPs were well controlled at middle 10 s in both eyes postoperatively and during regular follow-up.
JOAG is a rare subtype of POAG that is closely associated with genetic mutations. In the present study, we have for the first time, reported a coinheritance of mutations in OLFM2 (c.281G>A, p.Arg94His) and SIX6 (c.177C>G, p.Ile59Met) in a patient with JOAG through WGS and bioinformatics analysis. The potentially pathogenic mutations in OLFM2 and SIX6, inherited from the father and the mother, respectively, might be responsible for the onset of JOAG in this proband. Digenic inheritance is the simplest form for genetically complex diseases. By contrast with the thousands of reports that mutations in single genes cause human diseases, there are many human disease phenotypes with evidence for digenic inheritance in some pedigrees. Therefore, whether the inherited pattern of the proband is consistent with digenic inheritance needs to be further verified through functional research. Our report of coinherited OLFM2 and SIX6 variants in a JOAG family indicates the complexity of JOAG inheritance and enriches our understanding of the mutation spectrum in JOAG.
OLFM2 encodes a secreted glycoprotein in the Noelin family of olfactomedin domain-containing proteins that regulate the timing of neuronal differentiation during development[11]. The olfactomedin domain-containing protein that has been studied most comprehensively is myocilin encoded by the MYOC gene. Through phylogenetic analysis in humans, it was suggested that MYOC may have evolved from OLFM2 through gene duplication followed by exon fusion, which may partially explain a 31% homology between MYOC and OLFM2[12]. MYOC is the first candidate gene mapped for POAG. Mutations in MYOC account for 4%-35% of adult-onset POAG and for more than 10% in JOAG[12-14]. Therefore, it is reasonable to speculate that certain mutations in OLFM2 may also have detrimental effects in the development of JOAG. Funayama et al[15] reported that the Arg144Gln (exon 4) mutation in OLFM2 might result in POAG in Japanese patients. They also found that the combination of single nucleotide polymorphisms in OLFM2/317G and OPTN/603A was significantly associated with IOP elevation. However, their data were derived from sporadic case analysis, and there was no genetic information based on family inheritance. The origin of disease-causing mutations was unknown.
Loss of retinal ganglion cells (RGCs) and their axons are the primary diagnostic pathology in glaucoma. We have long known that the retina and optic nerve are embryonically and structurally part of the brain. Numerous studies have attempted to establish connections between glaucoma and neurodegeneration in the brain[16,17]. Previously, it was reported that OLFM2 was mainly expressed in the olfactory bulb, cortex, piriform cortex, olfactory trabeculae and inferior and superior colliculus in the brain. Functional investigation using Olfm2-KOLacZ mice demonstrated that the loss of Olfm2 caused a reduction of synaptic proteins in the brain and the retina[18]. Although not systemically investigated, the impairment of synaptic connections between neurons within the retina and the brain can potentially affect structural and functional integrity of RGCs and their axons. Interestingly, it was shown that genetic mutant Olfm2 in mouse was associated with abnormal psychological phenotypes such as anxiety and reduced levels of exploratory and locomotor activities[18], while ours and various other studies have shown that psychological conditions such as anxiety are strongly connected with the incidence of POAG[19-21] likely through reduced retrograde axonal transport of neurotropic factors, such as brain-derived neurotrophic factor. Taken together, OLFM2 is likely a candidate gene for POAG/JOAG.
SIX6 is also known as OPTX2, which belongs to the SIX/Sine oculis homeobox family that covers 1 homeobox DNA-binding domain. Various reports on pathogenic mutations in SIX6 have been connected to POAG. The connection between single nucleotide polymorphisms (rs33912345, rs10483727 and rs12436579) in SIX6 and POAG was established by genome-wide association studies[22,23]. Another study including POAG patients with missense variant in SIX6 (rs33912345) showed that a smaller optic disc rim area, larger vertical cup-disc ratio and significantly decreased average nerve fiber layer thickness are detected in patients with homozygous mutations in contrast to those with the nonrisk allele[24,25]. It was found in another study that clinical features in POAG patients appear to be dependent on the SIX6 genotype[26].
In our study, we found two missense mutations in OLFM2 (c.281G>A, p.Arg94His) and SIX6 (c.177C>G, p.Ile59Met) by WGS in a trio. Interestingly, the proband with JOAG coinherited one mutation from each parent. Furthermore, we included another nine trios with a JOAG proband and unaffected parents to verify the deleterious mutations. However, we did not find any duplicate cases due to an insufficient number of trios. Nevertheless, combined with various functional studies on OLFM2 and SIX6 mutations, we still suspected the coinherited variants might contribute to occurrence of JOAG. Based on data from the FANTOM5 dataset[27,28], OLFM2 and SIX6 are both expressed in the human retina. In mice, OLFM2 is mainly detected in RGC[18]. In an in vitro disease model using the SIX6 risk allele (rs33912345; C>A; His141Asn) carrying glaucoma patient-specific induced pluripotent stem cells for functional RGC regeneration, it was demonstrated that the number of RGCs generated was decreased and the expression of RGC regulatory genes were suppressed by altered SIX6 function[29]. In Olfm2-KOLacZ mice, the primary wave at the visual cortex in visual evoked potential was found to be significantly reduced when compared with those in wild-type littermates. A slight reduction in the number of RGCs and their axons was also observed in the Olfm2-KOLacZ mice. In zebrafish, it was found that Olfm2-KO retarded nervous system development[30]. Additionally, loss of SIX6 in early development may cause small eye phenotype, under-development of the lens and reduced neural fiber layer around the optic nerve head[26]. There are clinical studies in which patients with anophthalmia and microphthalmia were found to have SIX6 mutations[31]. Significantly, mutations in OLFM2 can also result in anophthalmia, microphthalmia and coloboma[32], which altogether indicate that OLFM2 and SIX6 may have interactions working together in shaping eye development. We hypothesize that OLFM2 and SIX6 genes and their protein products may interact to regulate a critical pathway in ocular development, which if disturbed may lead to the pathogenesis of JOAG/POAG.
To date, there are only two reports on glaucoma that have shown clear evidence for digenic inheritance[33,34]. In primary congenital glaucoma, LTBP2 p.R299X homozygous mutation plus CYP1B1 p.E387K heterozygous mutation resulted in a severe clinical phenotype and poor prognosis[33]. Carriers of both the MYOC and CYP1B1 mutations lead to POAG at an earlier age (27-years-old) compared with carriers of only the MYOC mutation (51-years-old)[34]. These data indicate that coinherited mutations may play an important role in determining the age of disease onset. Under this situation, one genetic mutation may work as a “dominator” while the other functions as a “regulator”. Therefore, a conservative approach to counselling may be warranted, under the assumption that some SIX6 mutations may render susceptibility but are not sufficient to cause glaucoma. The existence of another genetic mutation, for example, OLFM2, may facilitate a pathogenic effect of SIX6 mutations. To investigate our hypothesis, functional work on the association between OLFM2 and SIX6 should be carried out in the future.
In summary, we identified a coinheritance of OLFM2 and SIX6 variants in a core family of JOAG through WGS, Sanger sequencing and bioinformatics analysis. This specific inheritance would not have been identified through traditional Mendelian inheritance studies or analysis with a gene chip-based test. WGS covering a great number of annotated exons was the best option for finding novel mutations. It should be emphasized that a WGS strategy on trios is crucial for further genetic studies in JOAG/POAG. Additionally, previous families investigated by Mendelian inheritance should be further explored with a WGS approach. JOAG/POAG is a group of polygenic heterogeneous diseases in which a single genetic mutation may not be sufficient to cause a clinical phenotype. Genetic mutations from digenic or polygenic inheritance may result in pathogenesis changes that are beyond physiological compensation. Large-scale clinical screening and molecular functional investigations into the association of this coinherited OLFM2 and SIX6 variants are imperative to enrich our understanding on the pathogenesis of JOAG/POAG.
The authors would like to thank all participants for providing blood samples and agreeing to participate in this research.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, Demir AB, Vunnam RR S-Editor: Chen XF L-Editor: Filipodia P-Editor: Li JH
| 1. | Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 918] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 2. | Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG). Semin Ophthalmol. 2008;23:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kwun Y, Lee EJ, Han JC, Kee C. Clinical Characteristics of Juvenile-onset Open Angle Glaucoma. Korean J Ophthalmol. 2016;30:127-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR. Primary open-angle glaucoma. Nat Rev Dis Primers. 2016;2:16067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 5. | Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085-12096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Huang C, Xie L, Wu Z, Cao Y, Zheng Y, Pang CP, Zhang M. Detection of mutations in MYOC, OPTN, NTF4, WDR36 and CYP1B1 in Chinese juvenile onset open-angle glaucoma using exome sequencing. Sci Rep. 2018;8:4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Saeedi O, Yousaf S, Tsai J, Palmer K, Riazuddin S, Ahmed ZM. Delineation of Novel Compound Heterozygous Variants in LTBP2 Associated with Juvenile Open Angle Glaucoma. Genes (Basel). 2018;9:527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Jiang YH, Yuen RK, Jin X, Wang M, Chen N, Wu X, Ju J, Mei J, Shi Y, He M, Wang G, Liang J, Wang Z, Cao D, Carter MT, Chrysler C, Drmic IE, Howe JL, Lau L, Marshall CR, Merico D, Nalpathamkalam T, Thiruvahindrapuram B, Thompson A, Uddin M, Walker S, Luo J, Anagnostou E, Zwaigenbaum L, Ring RH, Wang J, Lajonchere C, Wang J, Shih A, Szatmari P, Yang H, Dawson G, Li Y, Scherer SW. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet. 2013;93:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Guo C, Zhao Z, Chen D, He S, Sun N, Li Z, Liu J, Zhang D, Zhang J, Li J, Zhang M, Ge J, Liu X, Zhang X, Fan Z. Detection of Clinically Relevant Genetic Variants in Chinese Patients With Nanophthalmos by Trio-Based Whole-Genome Sequencing Study. Invest Ophthalmol Vis Sci. 2019;60:2904-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22543] [Article Influence: 2254.3] [Reference Citation Analysis (0)] |
| 11. | Davies GS, Cohen J. In-vitro study of the activity of ciprofloxacin alone and in combination against strains of Pseudomonas aeruginosa with multiple antibiotic resistance. J Antimicrob Chemother. 1985;16:713-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Sultana A, Nakaya N, Senatorov VV, Tomarev SI. Olfactomedin 2: expression in the eye and interaction with other olfactomedin domain-containing proteins. Invest Ophthalmol Vis Sci. 2011;52:2584-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Sears NC, Boese EA, Miller MA, Fingert JH. Mendelian genes in primary open angle glaucoma. Exp Eye Res. 2019;186:107702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Li Q, Liu A, Gu X, Su Z. Olfactomedin domain-containing proteins: evolution, functional divergence, expression patterns and damaging SNPs. Mol Genet Genomics. 2019;294:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Funayama T, Mashima Y, Ohtake Y, Ishikawa K, Fuse N, Yasuda N, Fukuchi T, Murakami A, Hotta Y, Shimada N; Glaucoma Gene Research Group. SNPs and interaction analyses of noelin 2, myocilin, and optineurin genes in Japanese patients with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:5368-5375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Mélik Parsadaniantz S, Réaux-le Goazigo A, Sapienza A, Habas C, Baudouin C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells. 2020;9:535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Williams PA, Marsh-Armstrong N, Howell GR; Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants. Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res. 2017;157:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Sultana A, Nakaya N, Dong L, Abu-Asab M, Qian H, Tomarev SI. Deletion of olfactomedin 2 induces changes in the AMPA receptor complex and impairs visual, olfactory, and motor functions in mice. Exp Neurol. 2014;261:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Zhang D, Fan Z, Gao X, Huang W, Yang Q, Li Z, Lin M, Xiao H, Ge J. Illness uncertainty, anxiety and depression in Chinese patients with glaucoma or cataract. Sci Rep. 2018;8:11671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Olson DJ, Le P, Lin FC, Fleischman D, Davis RM. The Association Between Glaucoma, Anxiety, and Depression in a Large Population. Am J Ophthalmol. 2017;183:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Rezapour J, Nickels S, Schuster AK, Michal M, Münzel T, Wild PS, Schmidtmann I, Lackner K, Schulz A, Pfeiffer N, Beutel ME. Prevalence of depression and anxiety among participants with glaucoma in a population-based cohort study: The Gutenberg Health Study. BMC Ophthalmol. 2018;18:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Rong SS, Lu SY, Matsushita K, Huang C, Leung CKS, Kawashima R, Usui S, Tam POS, Young AL, Tsujikawa M, Zhang M, Nishida K, Wiggs JL, Tham CC, Pang CP, Chen LJ. Association of the SIX6 locus with primary open angle glaucoma in southern Chinese and Japanese. Exp Eye Res. 2019;180:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kondkar AA, Azad TA, Almobarak FA, Kalantan H, Sultan T, Alsabaani NA, Al-Obeidan SA, Abu-Amero KK. Polymorphism rs10483727 in the SIX1/SIX6 Gene Locus Is a Risk Factor for Primary Open Angle Glaucoma in a Saudi Cohort. Genet Test Mol Biomarkers. 2018;22:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Khawaja AP, Chan MPY, Yip JLY, Broadway DC, Garway-Heath DF, Viswanathan AC, Luben R, Hayat S, Hauser MA, Wareham NJ, Khaw KT, Fortune B, Allingham RR, Foster PJ. A Common Glaucoma-risk Variant of SIX6 Alters Retinal Nerve Fiber Layer and Optic Disc Measures in a European Population: The EPIC-Norfolk Eye Study. J Glaucoma. 2018;27:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Yariz KO, Sakalar YB, Jin X, Hertz J, Sener EF, Akay H, Özbek MN, Farooq A, Goldberg J, Tekin M. A homozygous SIX6 mutation is associated with optic disc anomalies and macular atrophy and reduces retinal ganglion cell differentiation. Clin Genet. 2015;87:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Carnes MU, Liu YP, Allingham RR, Whigham BT, Havens S, Garrett ME, Qiao C; NEIGHBORHOOD Consortium Investigators; Katsanis N; Wiggs JL; Pasquale LR; Ashley-Koch A; Oh EC; Hauser MA. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS Genet. 2014;10:e1004372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Crisci V, Orso CA. [Involvement and paralysis of the ulnar nerve in elbow arthrosis]. Minerva Ortop. 1968;19:570-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 590] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 28. | Nishimura SL, Recht LD, Pasternak GW. Biochemical characterization of high-affinity 3H-opioid binding. Further evidence for Mu1 sites. Mol Pharmacol. 1984;25:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 168] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Teotia P, Van Hook MJ, Wichman CS, Allingham RR, Hauser MA, Ahmad I. Modeling Glaucoma: Retinal Ganglion Cells Generated from Induced Pluripotent Stem Cells of Patients with SIX6 Risk Allele Show Developmental Abnormalities. Stem Cells. 2017;35:2239-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Lee JA, Anholt RR, Cole GJ. Olfactomedin-2 mediates development of the anterior central nervous system and head structures in zebrafish. Mech Dev. 2008;125:167-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Aldahmesh MA, Khan AO, Hijazi H, Alkuraya FS. Homozygous truncation of SIX6 causes complex microphthalmia in humans. Clin Genet. 2013;84:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Holt R, Ugur Iseri SA, Wyatt AW, Bax DA, Gold Diaz D, Santos C, Broadgate S, Dunn R, Bruty J, Wallis Y, McMullan D, Ogilvie C, Gerrelli D, Zhang Y, Ragge N. Identification and functional characterisation of genetic variants in OLFM2 in children with developmental eye disorders. Hum Genet. 2017;136:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Azmanov DN, Dimitrova S, Florez L, Cherninkova S, Draganov D, Morar B, Saat R, Juan M, Arostegui JI, Ganguly S, Soodyall H, Chakrabarti S, Padh H, López-Nevot MA, Chernodrinska V, Anguelov B, Majumder P, Angelova L, Kaneva R, Mackey DA, Tournev I, Kalaydjieva L. LTBP2 and CYP1B1 mutations and associated ocular phenotypes in the Roma/Gypsy founder population. Eur J Hum Genet. 2011;19:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Héon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |