Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8789
Peer-review started: February 26, 2021
First decision: July 8, 2021
Revised: July 19, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 16, 2021
Processing time: 231 Days and 6.9 Hours
ACTA2 gene is a specific gene that encodes actin α2. Multisystem smooth muscle dysfunction syndrome (MSMDS) is a multisystem disease characterized by aortic and cerebrovascular lesions caused by ACTA2 gene mutations. There have been many reports of cardiac, pulmonary and cerebrovascular lesions caused by MSMDS; however, few studies have focused on seizures caused by MSMDS.
Our patient was a girl aged 7 years and 8 mo with recurrent cough, asthma and seizures for 7 years. She was diagnosed with severe pneumonia, congenital heart disease, cardiac insufficiency, and malnutrition in the local hospital. Cardiac ultrasonography revealed congenital heart disease, patent ductus arteriosus (with a diameter of 0.68 cm), left coronary arteriectasis, patent oval foramen (0.12 cm), tricuspid and pulmonary regurgitation, and pulmonary hypertension. Cerebral magnetic resonance imaging and magnetic resonance angiography indicated stiffness in the brain vessels, together with multiple aberrant signaling shadows in bilateral paraventricular regions. A heterozygous mutation (c.536G>A) was identified in the ACTA2 gene, resulting in generation of p.R179H. Finally, the girl was diagnosed with MSMDS combined with epilepsy. The patient had 4 episodes of seizures before treatment, and no onset of seizure was reported after oral administration of sodium valproate for 1 year.
MSMDS has a variety of clinical manifestations and unique cranial imaging features. Cerebrovascular injury and white matter injury may lead to seizures. Gene detection can confirm the diagnosis and prevent missed diagnosis or misdiagnosis.
Core Tip: Multisystem smooth muscle dysfunction syndrome (MSMDS) is a disease caused by ACTA2 gene mutation. We report a case of MSMDS complicated with epilepsy. Since birth, the child developed several system dysfunctions, including dyspnea, congenital heart disease, and malnutrition. Brain magnetic resonance imaging (MRI) and magnetic resonance angiography showed cerebrovascular stiffness. It was accompanied by multiple abnormal signaling shadows around the bilateral ventricles, which may have been the focus of the seizures. Reviewing the literature and imaging reports, head MRI shows that abnormal signals and vascular malformations should be paid more attention to, which may lead to seizures in older patients.
- Citation: Yang WX, Zhang HH, Hu JN, Zhao L, Li YY, Shao XL. ACTA2 mutation is responsible for multisystemic smooth muscle dysfunction syndrome with seizures: A case report and review of literature. World J Clin Cases 2021; 9(29): 8789-8796
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8789.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8789
Multisystem smooth muscle dysfunction syndrome (MSMDS), initially reported by Milewicz et al[1] in 2008, is a serious genetic disease caused by mutations in the ACTA2 gene. It is characterized by aortic and cerebrovascular diseases, persistent ductus arteriosus, congenital mydriasis and organ dysfunction dependent on smooth muscle function, including the bladder and intestinal tract[2]. The diagnosis and treatment are still a challenge as few cases induced by ACTA2 mutation are available. In this case report, we present a patient with MSMDS induced by ACTA2 mutation combined with seizures. We summarize the clinical manifestations, laboratory test findings and molecular features. At the same time, we searched PubMed for related cases from 1980 to 2020 by using the keywords “multi-system smooth muscle dysfunction” and “ACTA2”, and summarized the clinical characteristics, laboratory results and molecular characteristics of these cases.
A girl aged 7 years and 8 mo came to our department for repeated cough, shortness of breath for 7 years and one convulsion. There were four convulsions within 2 d after admission. No fever, vomiting, diarrhea and other symptoms were found.
The patient had convulsions shortly after waking up in the morning, for no obvious reason. They were characterized by generalized tonic–clonic seizures and relieved spontaneously within 2 min.
The patient had a paroxysmal cough after catching a cold, combined with cyanosis in the mouth and lips, and shortness of breath since 8 mo of age. Chest radiography indicated increased pulmonary markings in both lungs. Cardiac ultrasonography indicated congenital heart disease, patent ductus arteriosus (PDA), patent oval foramen and pulmonary hypertension. She was admitted to the local hospital several times for the treatment of severe pneumonia, congenital heart disease, heart insufficiency, and malnutrition. These conditions showed remission after symptomatic treatment.
The child was born by cesarean section at 38 wk. Her parents and one of her brothers were healthy.
After admission, the patient’s temperature was 37.0°C; pulse, 112 bpm; respiration, 29 breaths/min; blood pressure, 98/60 mmHg; body weight, 18 kg; height, 118 cm; and head circumference, 50 cm. She was conscious, but presented with an appearance of malnutrition. The subcutaneous fat was thin. No swelling was noticed in the tonsils. No cyanosis was identified in the mouth and lips. No obvious edema was observed. her language, intelligence and movement showed slight delay. Congenital mydriasis was noticed. The pupils showed a diameter of 5 mm, and were no longer sensitive to light reflex. The heart rate was 112 bpm. The cardiac sound was loud, P2 showed accentuation. Persistent machinery murmur (III/6) was identified in the left sternal border. The pulmonary respiration in both lungs was coarse, without dry or moist rales. The abdomen was soft, and the liver was palpable under the ribs (1.0 cm). The boundary was sharp, and the texture was soft. No tenderness was felt. For nervous system examination, the neck was soft, and the pathological signs were negative. The myodynamia and muscular tension were normal, and the tendon reflex was normal. Appetite, sleeping, urination and defecation were normal.
Blood analysis revealed leukocytosis 8.58 × 109/L with the neutrophils as the major cells (68%). The hematocrit and platelet count were normal. The level of procalcitonin increased slightly (1.42 ng/mL). Serum C-reactive protein was 33.84 mg/L (normal range < 8 mg/L). Stool occult blood test was positive. Electrocardiography showed sinus rhythm and axis deviation to the right. Chest X-ray showed thickened texture in both lungs, together with patchy blurred shadows and enlarged heart shadow. There was obvious protrusion in the pulmonary artery segment, plump edge of the right heart, and left heart margin beyond the midline of the clavicle. There were no abnormalities in liver and renal function determination, cardiac enzymes, electrolytes, blood glucose and organic acid. The score based on the Wechsler Intelligence Scale was 75.
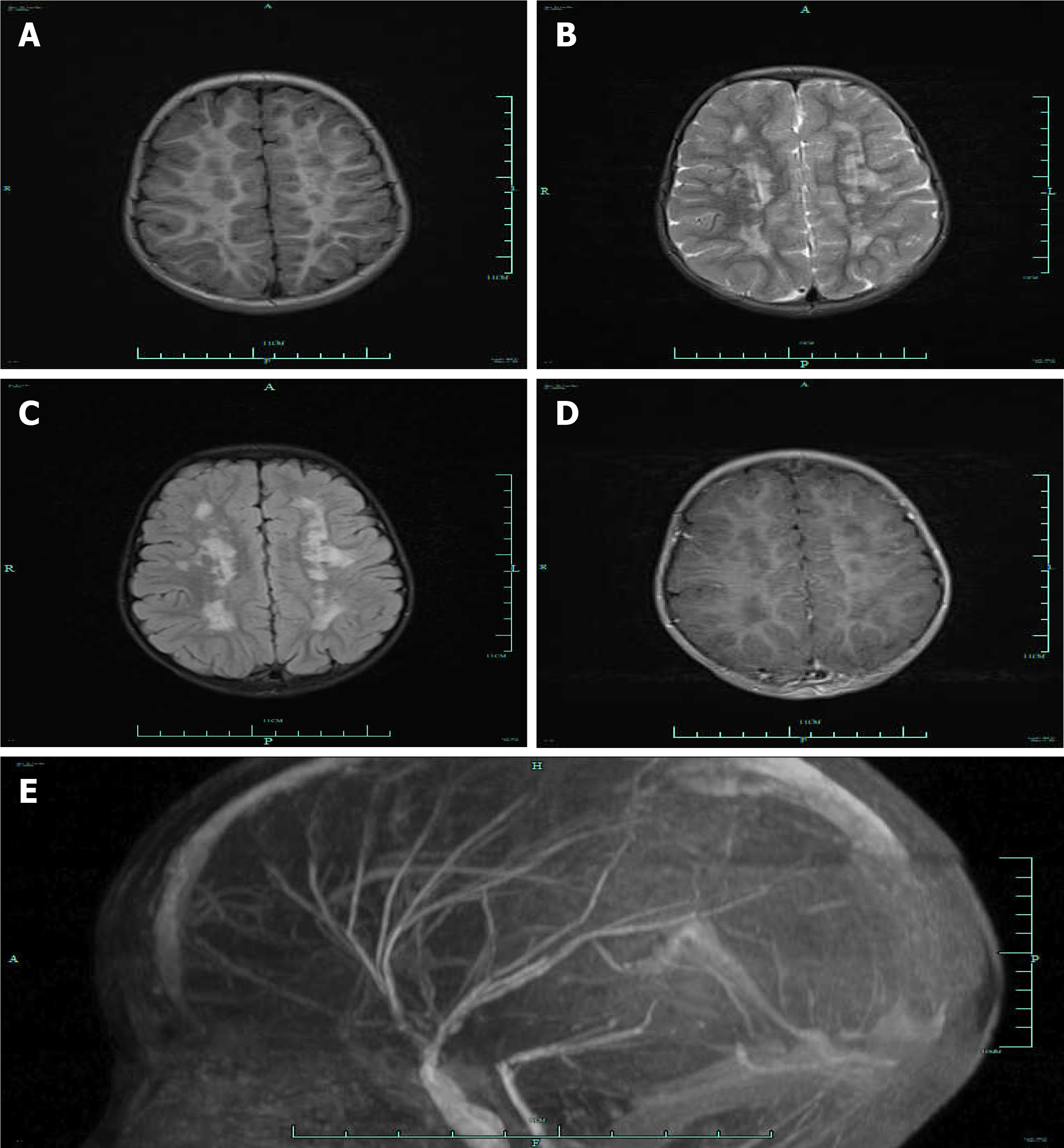
The video electroencephalogram findings showed background activity of 6–7 c/s in consciousness. The bilateral activity was symmetric, with no obvious spike/sharp wave. No paradoxical discharge was noticed in the presence of flash stimulation. Cardiac ultrasonography revealed congenital heart disease: PDA with a diameter of 0.68 cm, left coronary arteriectasis, patent oval foramen with a diameter of 0.12 cm, tricuspid and pulmonary regurgitation, and pulmonary hypertension. Cranial magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) indicated stiffness in the brain vessels, together with multiple aberrant signaling shadows in bilateral paraventricular regions (Figure 1).
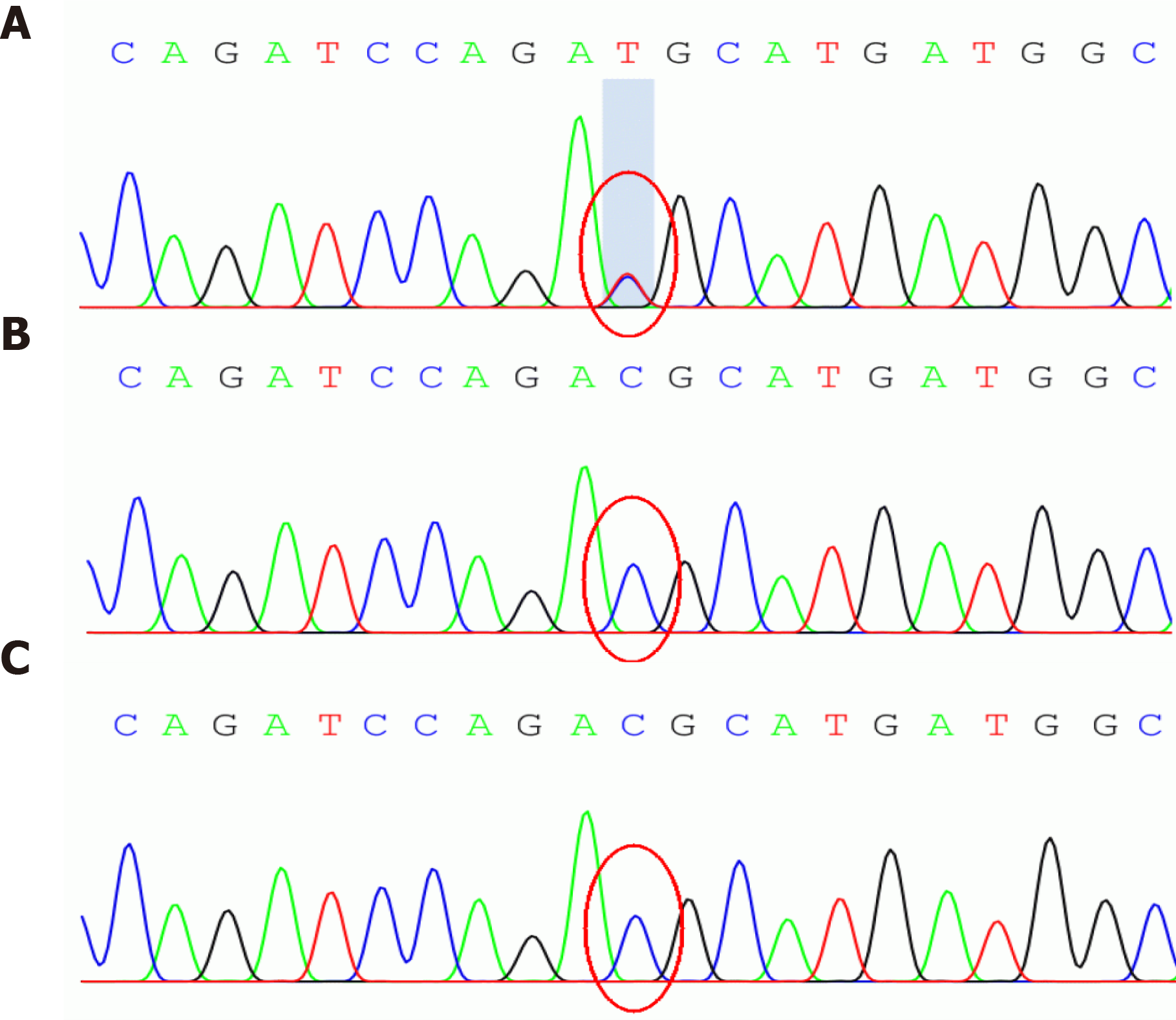
For the gene sequencing, venous blood samples (3 mL) were collected from the patient and her parents using a tube containing EDTA after obtaining informed consent. The study protocols were approved by the Ethical Committee of our hospital (approval No. 2016066). The pathogenic gene was detected by whole exon sequencing, and verified by Sanger technique. A heterozygous mutation (c.536G>A) was identified in the ACTA2 gene, which resulted in generation of p.R179H (Figure 2, Table 1). No mutations were identified in the ACTA2 gene in her parents.
| Item | Results |
| Nucleotide changes | c.536G>A |
| NM No. | NM_001613.2 |
| Homozygous/heterozygous mutation | Heterozygous mutation |
| Amino acid changes | p.R179H |
| Minor allele frequency | N/A |
| Pathogenicity | Pathogenic mutation |
| Disease/phenotype | Multi-systemic smooth muscle dysfunction syndrome |
| Genetic type | Autosomal dominant |
| Mutation source | Newly identified |
MSMDS.
To date, there is no standardized treatment for MSMDS. For children with MSMDS, we reviewed and screened the conventional treatment strategies for related symptoms, and administered the following treatments to alleviate the patient’s conditions. Sildenafil was utilized to decrease pulmonary hypertension[3]. Fructose diphosphate sodium was used to nourish the cardiac muscles[4]. Oral administration of sodium valproate was given for the treatment of epilepsy[5].
The patient was followed up every 3 mo after discharge. Recurrent coughing and purplish relapse could usually be improved after anti-infective treatment, and she has not shown seizures until now, through oral administration of sodium valproate (5 mL, bid).
We searched “multiple system smooth muscle dysfunction” and “ACTA2” in PubMed and Medline, and reviewed the clinical symptoms and imaging of the previous cases. Besides our case, we searched PubMed for a total of 19 published articles involving 37 MSMDS patients. Details of these cases are summarized in Table 2[1,2,6-22]. According to the analysis of these patients, the youngest was age 3 d[8] and the oldest 41 years[12]. The main clinical manifestations were congenital fixed mydriasis and PDA. Thirty-seven patients had congenital pupil dilation and 35 had PDA. Four patients had convulsions. Twenty-five patients had abnormal signals of white matter on MRI findings. Thirty-seven patients underwent gene sequencing analysis. In total, 28 cases had Arg179His mutation, five Arg179Cys mutation, and two each Arg179Leu and Asn117Lys mutation.
| Mutation type | Arg179His1 | Arg179Cys | Arg179Leu | Asn117Lys | |
| Total number of patients (unit: example) | 28 | 5 | 2 | 2 | |
| Clinical features | |||||
| Visual system symptoms | |||||
| Congenital mydriasis | 28 | 5 | 2 | 2 | |
| Retinal vessels twists and turns | 13 | 1 | 1 | 0 | |
| Cardiovascular system | |||||
| Patent ductus arteriosus | 26 | 5 | 2 | 2 | |
| Pulmonary hypertension | 12 | 2 | 0 | 0 | |
| Thoracic aortic aneurysm | 9 | 0 | 0 | 0 | |
| Pulmonary artery dilatation | 10 | 1 | 0 | 0 | |
| Nervous system | |||||
| Underdevelopment | 11 | 1 | 0 | 0 | |
| Cerebral infarction or hemiplegia | 5 | 1 | 0 | 1 | |
| White matter lesion | 21 | 3 | 0 | 1 | |
| Manifestations of moyamoya-like disease | 19 | 4 | 1 | 0 | |
| Epileptic seizure | 4 | 1 | 0 | 0 | |
| Respiratory system | |||||
| Dyspnea | 14 | 3 | 1 | 0 | |
| Asthma | 3 | 0 | 0 | 0 | |
| Digestive system | |||||
| Intestinal malrotation | 5 | 2 | 0 | 0 | |
| Poor intestinal peristalsis | 5 | 1 | 0 | 0 |
ACTA2 gene is located in the long arm of chromosome 10q23.31, which encodes the expression of actin α2. MSMDS is a serious disease caused by ACTA2 mutation, which is characterized by familial thoracic aortic aneurysm and cerebrovascular lesions. Milewicz et al[1] summarized the clinical symptoms of the disease in 2010: (1) Visual system: congenital nonreactive mydriatic fixation; (2) Cardiovascular system: PDA, pulmonary artery dilatation or hypertension, thoracic aortic aneurysm; (3) Nervous system: cerebral infarction, hemiplegia, motor/mental delay; (4) Respiratory system: shortness of breath, recurrent respiratory infection, bronchial asthma; (5) Digestive system: intestinal malrotation or intestinal dyskinesia; and (6) Other systematic manifestations: hypotonic bladder, congenital absence of abdominal muscle.
To date, the clinical pedigree of neurological manifestations of ACTA2 mutations is not well described. The main symptoms are motor and/or mental delay, cerebral infarction and/or hemiplegia[11]. In the literature review, we found three patients with neurological epilepsy besides our case. The main imaging manifestations were cerebrovascular abnormalities and white matter signaling changes. The specificity of cerebrovascular disease was mainly epidural artery dilatation, intradural artery stiffness, large artery and distal microvascular malformation[10,15,22]. In our case, initial MRI showed that the blood vessels in the brain were stiff, and the white matter showed multiple signals. No changes in gray matter were found. With the increase of age, further attention should be paid to the occurrence of gray matter infarction. For the four convulsions in our case, we speculate that the possible mechanism is as follows: (1) Cerebrovascular rigidity and occlusion led to low regional cerebral blood flow and ischemic penumbra, in which surviving neurons repeatedly produced epileptic discharges; (2) Cerebrovascular lesions led to the loss of small vascular smooth muscle cells, thickening, stenosis and hardness of vascular wall, decrease of vasomotor activity, change of blood–brain barrier permeability and decrease of neuronal response threshold. It triggered increase in the excitability of neurons and albumin exudation. In the presence of albumin absorbed by astrocytes, the ability to buffer extracellular K+ and reuptake extracellular glutamate was affected, which eventually triggered the changes in neuronal microenvironment and epileptic electricity generation[23]; and (3) The change in signaling in the white matter. The white matter is an important part of the central nervous system and the gathering place of nerve fibers in the brain, which undertake the functions of neural information sharing and information communication in various brain areas. The pathological changes in cerebral vessels cause ischemia and hypoxia in the white matter, which promotes the death of nerve cells. This facilitates new synaptic connections between neurons to form a new abnormal neural network, leading to seizures.
MSMDS caused by ACTA2 mutation showed different clinical symptoms. Seizures may be one of the neurological manifestations in the evolution of the disease. The disease is characterized by multiple system involvement with no obvious specificity. Its diagnosis is still a challenge. Cranial MRI and MRA examinations are recommended in children with convulsions, which have important diagnostic value for the diagnosis of the disease. Gene sequencing is crucial to evaluate the patient population in order to provide accurate prognosis and genetic counseling. Pediatricians should be familiar with this rare disease and its prognosis.
The authors thanks for the English revision of the article from Dr Kin Man in Royal Free Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sikiric P, Zavras N S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Milewicz DM, Østergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, Bradley TJ, Olney AH, Adès L, Maher JF, Guo D, Buja LM, Kim D, Hyland JC, Regalado ES. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437-2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 2. | Brodsky MC, Turan KE, Khanna CL, Patton A, Kirmani S. Congenital mydriasis and prune belly syndrome in a child with an ACTA2 mutation. J AAPOS. 2014;18:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bhogal S, Khraisha O, Al Madani M, Treece J, Baumrucker SJ, Paul TK. Sildenafil for Pulmonary Arterial Hypertension. Am J Ther. 2019;26:e520-e526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Bai YT, Shi QB, Li Y. Clinical Study of Fructose Sodium Diphosphate in the Treatment of Acute Myocardial Infarction. Zhongguo Yaofang. 2017;28:1076-1079. |
| 5. | Nevitt SJ, Marson AG, Weston J, Tudur Smith C. Sodium valproate vs phenytoin monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2018;8:CD001769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Al-Mohaissen M, Allanson JE, O'Connor MD, Veinot JP, Brandys TM, Maharajh G, Dennie CJ, Beauchesne LM. Brachial artery occlusion in a young adult with an ACTA2 thoracic aortic aneurysm. Vasc Med. 2012;17:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Amans MR, Stout C, Fox C, Narvid J, Hetts SW, Cooke DL, Higashida RT, Dowd CF, McSwain H, Halbach VV. Cerebral arteriopathy associated with Arg179His ACTA2 mutation. J Neurointerv Surg. 2014;6:e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 8. | Logeswaran T, Friedburg C, Hofmann K, Akintuerk H, Biskup S, Graef M, Rad A, Weber A, Neubauer BA, Schranz D, Bouvagnet P, Lorenz B, Hahn A. Two patients with the heterozygous R189H mutation in ACTA2 and Complex congenital heart defects expands the cardiac phenotype of multisystemic smooth muscle dysfunction syndrome. Am J Med Genet A. 2017;173:2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Chen SN, Wang YQ, Hao CL, Lu YH, Jiang WJ, Gao CY, Wu M. Multisystem smooth muscle dysfunction syndrome in a Chinese girl: A case report and review of the literature. World J Clin Cases. 2019;7:4355-4365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 10. | Yetman AT, Starr LJ, Bleyl SB, Meyers L, Delaney JW. Progressive Aortic Dilation Associated With ACTA2 Mutations Presenting in Infancy. Pediatrics. 2015;136:e262-e266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | de Grazia J, Delgado I, Sanchez-Montanez A, Boronat S, Del Campo M, Vazquez E. Cerebral arteriopathy associated with heterozygous Arg179Cys mutation in the ACTA2 gene: Report in 2 newborn siblings. Brain Dev. 2017;39:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Mc Glacken-Byrne AB, Prentice D, Roshandel D, Brown MR, Tuch P, Yau KS, Sivadorai P, Davis MR, Laing NG, Chen FK. High-resolution iris and retinal imaging in multisystemic smooth muscle dysfunction syndrome due to a novel Asn117Lys substitution in ACTA2: a case report. BMC Ophthalmol. 2020;20:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Meuwissen ME, Lequin MH, Bindels-de Heus K, Bruggenwirth HT, Knapen MF, Dalinghaus M, de Coo R, van Bever Y, Winkelman BH, Mancini GM. ACTA2 mutation with childhood cardiovascular, autonomic and brain anomalies and severe outcome. Am J Med Genet A. 2013;161A:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 14. | Moller HU, Fledelius HC, Milewicz DM, Regalado ES, Ostergaard JR. Eye features in three Danish patients with multisystemic smooth muscle dysfunction syndrome. Br J Ophthalmol. 2012;96:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Moosa AN, Traboulsi EI, Reid J, Prieto L, Moran R, Friedman NR. Neonatal stroke and progressive leukoencephalopathy in a child with an ACTA2 mutation. J Child Neurol. 2013;28:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Moreno CA, Metze K, Lomazi EA, Bertola DR, Barbosa RH, Cosentino V, Sobreira N, Cavalcanti DP. Visceral myopathy: Clinical and molecular survey of a cohort of seven new patients and state of the art of overlapping phenotypes. Am J Med Genet A. 2016;170:2965-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Munot P, Saunders DE, Milewicz DM, Regalado ES, Ostergaard JR, Braun KP, Kerr T, Lichtenbelt KD, Philip S, Rittey C, Jacques TS, Cox TC, Ganesan V. A novel distinctive cerebrovascular phenotype is associated with heterozygous Arg179 ACTA2 mutations. Brain. 2012;135:2506-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Prabhu S, Fox S, Mattke A, Armes JE, Alphonso N. Extracorporeal Life Support in Multisystem Smooth Muscle Dysfunction Syndrome. World J Pediatr Congenit Heart Surg. 2017;8:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Richer J, Milewicz DM, Gow R, de Nanassy J, Maharajh G, Miller E, Oppenheimer L, Weiler G, O'Connor M. R179H mutation in ACTA2 expanding the phenotype to include prune-belly sequence and skin manifestations. Am J Med Genet A. 2012;158A:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Zhou YL, Zhang YY, Cheng BL, Xu D, Tang LF, Chen ZM. [Multisystemic smooth muscle dysfunction syndrome in children: a case report and literature review]. Zhonghua Er Ke Za Zhi. 2017;55:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Roulez FM, Faes F, Delbeke P, Van Bogaert P, Rodesch G, De Zaeytijd J, Depasse F, Coucke PJ, Meire FM. Congenital fixed dilated pupils due to ACTA2- multisystemic smooth muscle dysfunction syndrome. J Neuroophthalmol. 2014;34:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Kanamori K, Sakaguchi Y, Tsuda K, Ihara S, Miyama S. Refractory cerebral infarction in a child with an ACTA2 mutation. Brain Dev. 2021;43:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 23. | Yang H, Rajah G, Guo A, Wang Y, Wang Q. Pathogenesis of epileptic seizures and epilepsy after stroke. Neurol Res. 2018;40:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |