Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8035
Peer-review started: April 15, 2021
First decision: May 11, 2021
Revised: May 15, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: September 26, 2021
Processing time: 153 Days and 23.6 Hours
Myopia and high myopia are global public health concerns. Patients with high myopia account for 0.5%-5.0% of the global population.
To examine diopters, axial length (AL), higher-order aberrations, and other ocular parameters in Chinese children with myopia, to analyze the influence of structural parameters associated with myopia on visual quality, and to provide a theoretical basis for the prevention and treatment of childhood myopia and high myopia.
This study included 195 children aged 6–17 years with myopia. The AL was measured with an ultrasonic ophthalmic diagnostic instrument, and the aberrations, corneal curvature (minimum K1, maximum K2, and average Km), central corneal thickness, anterior chamber depth, and anterior chamber angle were measured using a Sirius three-dimensional anterior segment analyzer. Using a standard formula, the corneal radius of curvature R (337.3/Km) and AL/R values were obtained.
The diopter of high myopia compared with low-middle myopia was correlated with age and AL (r = -0.336, -0.405, P < 0.001), and AL of high myopia was negatively correlated with K1, K2, and Km (r = -0.673, -0.661, and -0.680, respectively; P < 0.001), and positively correlated with age and the anterior chamber depth (r = 0.214 and 0.275, respectively; P < 0.05). AL/R was more closely related to diopter than AL in children with myopia, and 94.4% of children with myopia had an AL/R of > 3.00.
The ocular structural parameters of children change because of different diopters. AL/R is more specific and sensitive than AL in evaluating the refractive status of myopia in children. An AL/R of > 3.00 may be used as a specific index of myopia in children. There are differences in AL/R between high myopia and low-middle myopia, which can be used for the classification of ametropia. The degree of myopia has a certain influence on higher-order aberrations.
Core Tip: We explored the relationship between changes in ocular structural parameters and the development of myopia to provide foundational evidence for the study of myopia in children.
- Citation: Li X, Hu Q, Wang QR, Feng ZQ, Yang F, Du CY. Analysis of ocular structural parameters and higher-order aberrations in Chinese children with myopia. World J Clin Cases 2021; 9(27): 8035-8043
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8035.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8035
Myopia and high myopia are global public health concerns. Patients with high myopia account for 0.5%-5.0% of the global population. Relevant studies predicted that by 2050, the number of global myopia and high-myopia patients will have reached 5 billion and 900 million, respectively[1]. China has a high incidence of myopia. The prevalence of high myopia in children is 6.5%–38.4%, with a trend toward onset at a relatively young age[2].
High myopia is often accompanied by axial length (AL) prolongation and fundus changes, such as a temporal arc spot, pigmented epithelium thinning, leopard pattern fundus, Fuch spot, and retinal choroid atrophy, among others, alongside progressive vision loss, which can be complicated by amblyopia, glaucoma, cataract, vitreous opacity, retinal detachment, and other ophthalmic diseases[3], affecting patients’ quality of life.
It remains unclear whether there is a correlation between the changes in ocular structural parameters and the development of myopia, and whether these changes play an important role in the occurrence and development of myopia. The present study aimed to observe the changes in the ocular structural parameters of myopia and to explore the progression of high myopia. These results may contribute to discussions on the ongoing myopic shift in childhood.
A total of 195 participants (100 men and 95 women) were recruited from the optometry center of the First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China from July to September 2019. Children aged between 6 and 17 years (average age, 11.93 ± 2.52 years) with myopia, single eye or double eye equivalent spherical of ≥ -0.50 diopters and cylinder of 0 to -1.50 diopters, and corrected vision of ≥ 5.0 (LogMAR = 0) were included. According to the diopter, the participants were divided into three groups: 139 eyes in low-myopia group: (0 to –3.00 diopters), 59 in middle-myopia group (within -3.00 and -6.00 diopters), and 157 in high-myopia group (≥ -6.00 diopters). Moreover, children with glaucoma, cataract, fundus hemorrhage, keratoconus, and systemic immune diseases were excluded, and no rigid contact lens wearing history was found in recent months and no soft contact lens wearing history in 2 wk. This clinical study was approved by the ethics committee of the First Affiliated Hospital of Harbin Medical University, and informed consent was obtained from all participants.
Each participant underwent an eye examination conducted by an ophthalmologist that included a cycloplegic objective refraction assessment performed with an autorefractor (Topcon, Japan) 30 min after cycloplegia (tropicamide phenylephrine eye drops; one drop, 10 min apart), and a subjective refraction assessment performed with a comprehensive refractometer (Topcon, Japan) to obtain sphericity and cylinder values. Subsequently, the spherical equivalent value was calculated (spherical equivalent = sphericity +1/2 cylinder). The AL was measured using an ultrasonic ophthalmic diagnostic instrument (Quantel Medical, France). Measurements were taken three times in each eye and the mean value of the AL was used for statistical analysis.
A Sirius three-dimensional anterior segment analyzer (CSO, Italy) was used to take pictures in a dark environment. The participants were asked to place their mandible on the jaw support, and the forehead was placed on the frontal support. The patient was instructed to open his or her eyes after blinking and focus immediately; measurements were taken automatically to obtain effective images and to avoid the cornea being blocked by eyelashes or eyelids, and tear film rupture. The measure
SPSS 22.0 was used to process the data, and descriptive statistics are expressed as the mean ± SD. One-way ANOVA and LSD were used to examine the differences among the three groups. The Pearson correlation coefficient was used to quantify the association between the ocular parameters of interest. Multiple linear stepwise regression analysis was used to evaluate the associations among AL factors of high myopia, and the receiver operating characteristic (ROC) curve was used to analyze the reliability of AL and AL/R values in evaluating myopia. P values of < 0.05 were considered statistically significant.
One-way ANOVA was used to compare ocular structural parameters among the low-, middle-, and high-myopia groups. There were significant differences in the spherical equivalent, AL, AL/R, and K2 values (P < 0.001); however, there was no significant difference in R, K1, Km, CCT, ACD, or ACA values. The LSD was used to analyze multiple intergroup comparisons, revealing no significant difference in the spherical equivalent, AL, or AL/R values among the groups (P < 0.001). The values of K2 and ACD differed between the low- and high-myopia groups; however, middle myopia showed no significant association with low and high myopia in K2 and ACD (Table 1).
| Low myopia (mean ± SD) | Middle myopia (mean ± SD) | High myopia (mean ± SD) | F | P value | |
| Spherical equivalent (D) | -1.69 ± 0.70 | -3.86 ± 0.69 | -7.55 ± 1.12 | 1572.765 | < 0.001 |
| AL (mm) | 23.83 ± 0.75 | 24.63 ± 0.69 | 26.00 ± 0.94 | 259.188 | < 0.001 |
| R (mm) | 7.77 ± 0.25 | 7.73 ± 0.24 | 7.74 ± 0.27 | 1.809 | 0.165 |
| AL/R | 3.07 ± 0.07 | 3.19 ± 0.08 | 3.37 ± 0.10 | 468.348 | < 0.001 |
| K1 (D) | 42.95 ± 1.36 | 43.17 ± 1.27 | 43.07 ± 1.61 | 0.503 | 0.605 |
| K2 (D) | 43.89 ± 1.49 | 44.21 ± 1.37 | 44.46 ± 1.74 | 4.925 | < 0.001 |
| Km (D) | 43.43 ± 1.42 | 43.69 ± 1.31 | 43.77 ± 1.67 | 1.933 | 0.146 |
| CCT (μm) | 561.52 ± 32.11 | 559.15 ± 23.58 | 552.85 ± 36.26 | 2.684 | 0.070 |
| ACD (mm) | 3.30 ± 0.17 | 3.33 ± 0.15 | 3.34 ± 0.17 | 2.450 | 0.088 |
| ACA (°) | 60.73 ± 5.84 | 59.19 ± 6.69 | 59.89 ± 7.27 | 1.253 | 0.287 |
One-way ANOVA was used to compare the higher-order aberrations among the low-, middle-, and high-myopia groups. There was a significant difference in the spherical aberration values (P < 0.05), but not in the total higher-order aberration, coma, and trefoil aberration values (P > 0.05). The LSD was used for multiple intergroup comparisons of spherical aberration values, revealing that middle myopia was significantly associated with low and high myopia (P < 0.001), but there was no difference between low and high myopia (P > 0.05) (Table 2).
| Total higher-order aberration (μm) | Spherical aberration (μm) | Coma aberration (μm) | Trefoil aberration (μm) | |
| Low myopia | 0.24 ± 0.12 | 0.06 ± 0.04 | 0.14 ± 0.07 | 0.13 ± 0.11 |
| Middle myopia | 0.24 ± 0.09 | 0.09 ± 0.04 | 0.13 ± 0.07 | 0.12 ± 0.07 |
| High myopia | 0.25 ± 0.13 | 0.07 ± 0.04 | 0.13 ± 0.06 | 0.14 ± 0.10 |
| F | 0.115 | 6.735 | 0.536 | 0.650 |
| P value | 0.891 | < 0.001 | 0.585 | 0.523 |
Pearson correlation analysis was used to examine the relationship between diopter and the ocular structural parameters (Al, K1, K2, km, CCT, ACA, and ACD) in the three groups. Spherical equivalent values were not associated with either CCT or ACA values in low, middle, or high myopia (P > 0.05) (Table 3).
| Age | AL (mm) | K1 (D) | K2 (D) | Km (D) | ACD (mm) | CCT (mm) | ACA (°) | |
| Low myopia | ||||||||
| r | -0.153 | -0.123 | -0.251 | -0.163 | -0.204 | 0.193 | 0.075 | -0.008 |
| P value | 0.073 | 0.148 | 0.003a | 0.055 | 0.016a | 0.023a | 0.378 | 0.923 |
| Middle myopia | ||||||||
| r | -0.199 | -0.388 | -0.040 | -0.137 | -0.093 | -0.242 | 0.017 | -0.065 |
| P value | 0.131 | 0.002a | 0.764 | 0.302 | 0.484 | 0.065 | 0.899 | 0.625 |
| High myopia | ||||||||
| r | -0.336 | -0.405 | -0.035 | -0.094 | -0.069 | 0.046 | 0.062 | 0.001 |
| P value | < 0.001 | < 0.001 | 0.662 | 0.240 | 0.387 | 0.565 | 0.438 | 0.986 |
In the low-myopia group, the absolute value of the spherical equivalent was positively correlated with K1 and km, negatively correlated with ACD, and not correlated with age or AL; thus, with the increase in the spherical equivalent absolute value, corneal curvature became steeper and ACD became shallower. In the middle-myopia group, the absolute value of the spherical equivalent was positively correlated with AL, but not with age, K1, K2, Km, or ACD. In the high-myopia group, the absolute value of the spherical equivalent was positively correlated with age and AL (P < 0.001), but not with K1, K2, Km, or ACD.
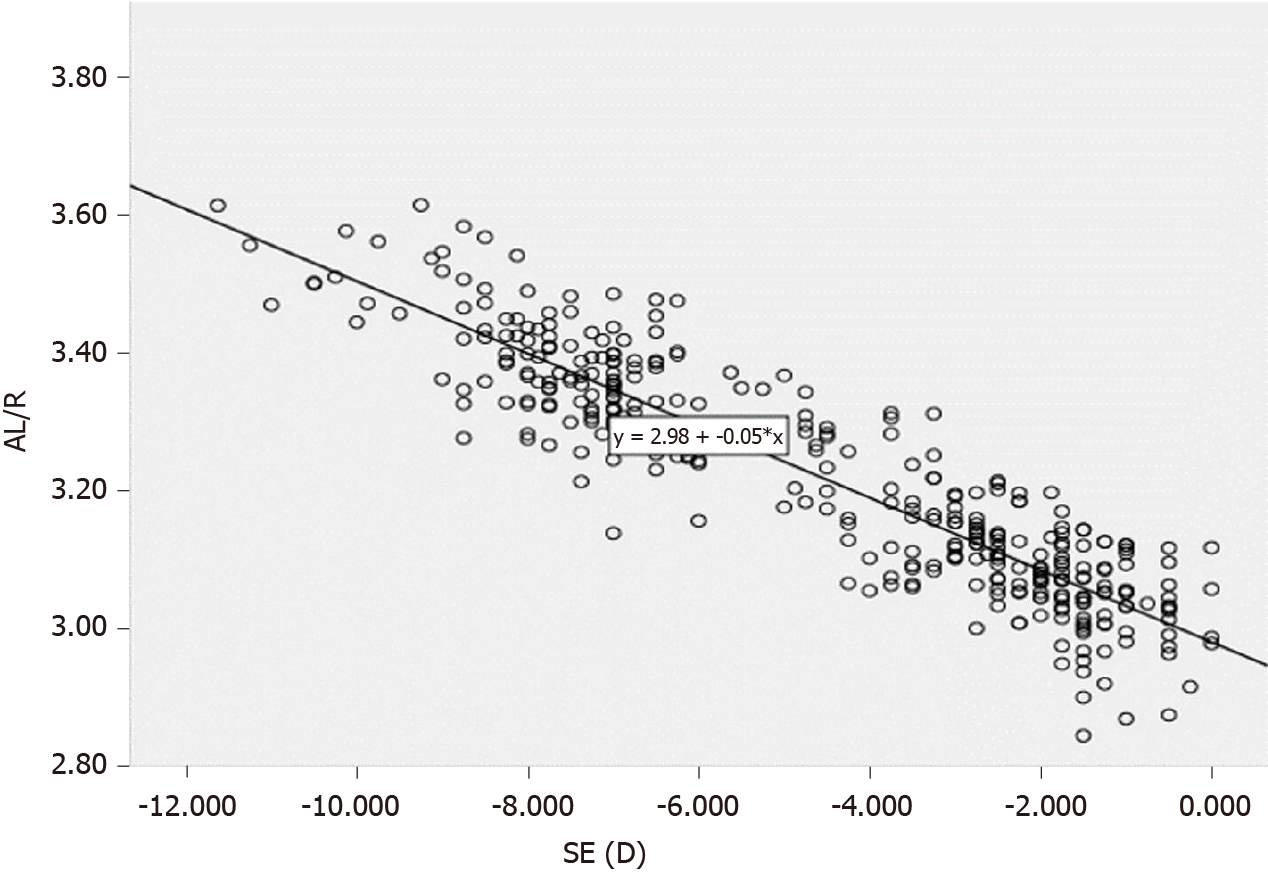
There was a significant correlation between the spherical equivalent value and AL/R (r = -0.898, P < 0.001), showing that with an increase in myopia, the AL/R also increased (Figure 1). In addition, an AL/R of > 3.00 was observed in 94.4% of children with myopia, and the correlation coefficients (r) of AL/R with AL and R were 0.771 and 0.312 (P < 0.001), respectively.
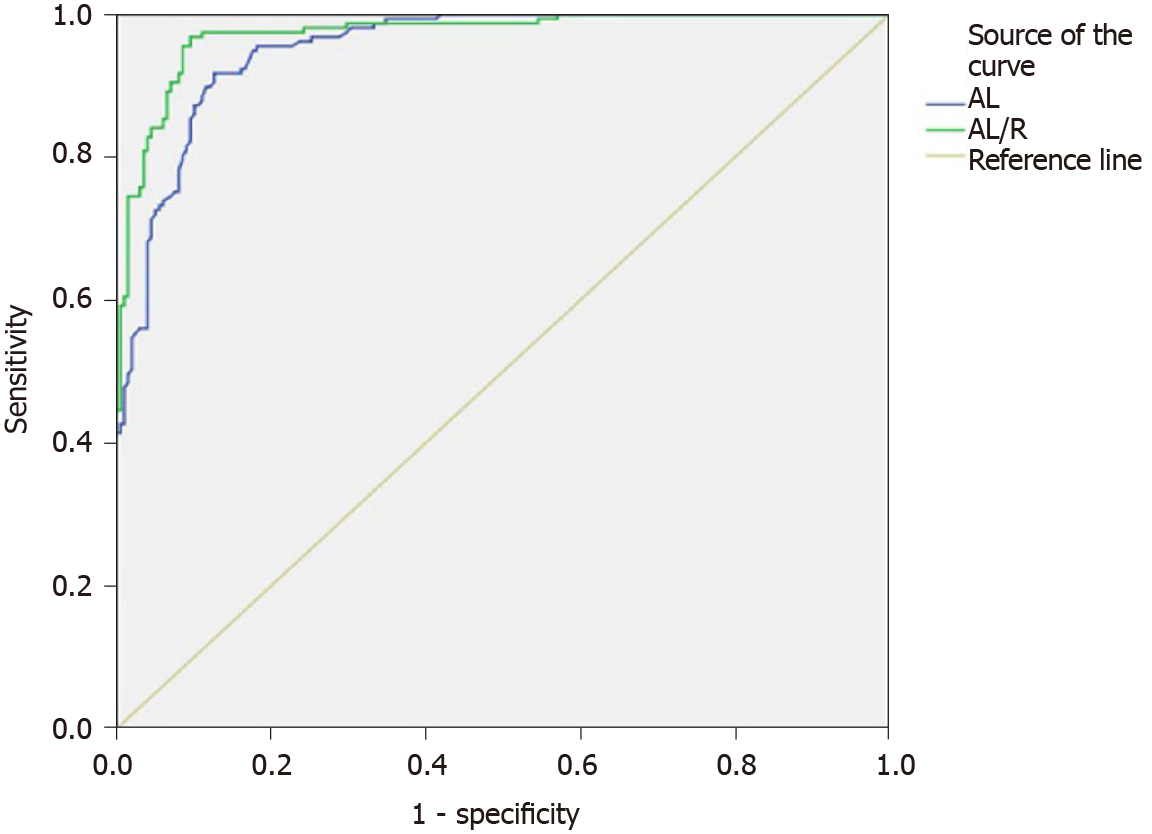
The low-myopia and middle-myopia groups were combined (low-middle myopia) to examine the difference of AL/R between the high-myopia and low-middle-myopia groups. The ROC curve analysis revealed an area under the AL/R curve that was larger than that under the AL curve, suggesting that AL/R may be more reliable than AL in evaluating the degree of myopia. In addition, the cutoff value of AL/R was 3.22 and that of AL was 24.91 mm for high myopia and low-middle myopia, respectively (Figure 2).
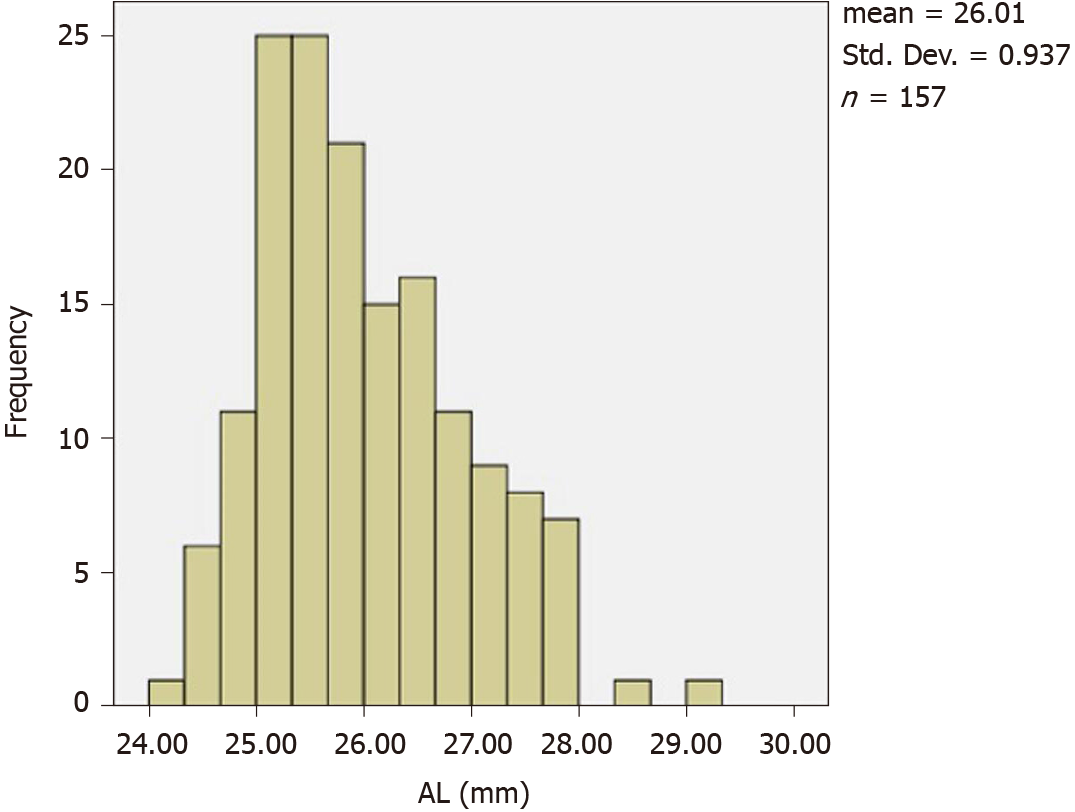
The average AL of 87 high-myopia children was 26.04 ± 0.95 mm (Figure 3). There was a significant correlation between diopter and AL in the high-myopia group. Therefore, we examined factors influencing AL in the high-myopia group using Pearson correlation analysis. The results showed that AL correlated with age, the spherical equivalent, K1, K2, km, and ACD values (P < 0.05), but not with CCT or ACA values (P > 0.05). Factors with P-values of < 0.05, including age, spherical equivalent, K1, K2, km, and ACD, were included in multiple stepwise linear regression analysis. The following regression equation was obtained AL = -0.395 × Km - 0.342 × spherical equivalent + 1.132 × ACD + 0.077 × Age + 35.925 (F = 106.193, R2 = 0.730, P < 0.001). The results showed that with an increase in age, the AL gradually increased, corneal curvature flattened, and ACD increased.
The progression of middle and high myopia is mainly related to axial growth, which may determine the degree to which myopia increases. Rozema et al[4] conducted a large sample survey of 1302 Singaporean children aged 6-9 years, showing that rapid growth in AL preceded the occurrence of myopia. At the early stage of axial growth, the lens changes to compensate for the decrease in the total eye diopter. However, myopia appears when the lens diopter decreases to a level at which it can no longer compensate for axial growth; meanwhile, rapid axial growth is compensated by the decrease in the lens diopter at the early stage of myopia; the lens diopter tends to stabilize at 12 years of age. Thus, axial growth after 12 years of age is associated with the worsening of myopia, as the lens can no longer compensate for axial growth. Muthu Krishnan et al[5] found that corneas with higher curvature tend to be thinner, but our study did not find a correlation between corneal curvature and CCT, which is of great significance for adult refractive surgery.
Second, in children with high myopia, eye diopters were related to age. Specifically, with increasing age, the degree of myopia increased. This result may be related to an increase in eye use and AL. Kearney et al[6] conducted a study on individuals aged 5-20 years and found that in patients with emmetropia, body growth and axial growth may be related. Body growth seemed to be stable among the participants with myopia, while eye axial growth proceeded at a faster rate, which indicated that normal eye growth was out of balance. Although axial compression plays an important role in the development of high myopia, the mechanism of axial growth remains unclear. Studies have shown that environmental factors such as a large amount of close work may activate the expression of the AL gene, which leads to an increase in AL and in myopia[7].
Studies have shown that AL/R has a closer relationship with diopter than with AL or corneal curvature radius and that it may predict the degree of myopia more effectively. An objective tool for risk assessment of high myopia is required; AL plays a major role in AL/R[8]. The correlation coefficients of AL/R with AL and R in our study were 0.771 and -0.312, respectively (P < 0.001), which confirmed this observation. We found that there were significant differences in AL/R among low-, middle-, and high-myopia groups, and that there was a significant correlation between AL/R and SE in myopia in children (r = -0.898, P < 0.001); thus, AL/R increases with increasing myopia. A comparison between the high-myopia and low-middle-myopia groups using the ROC curve analysis showed that the AL/R values were larger than the area under the AL curve (0.972 > 0.953), indicating that AL/R was more reliable and effective than AL for evaluating myopia. The cutoff value of AL/R for high myopia and low-middle myopia was 3.22, and the cutoff value for AL was 24.91 mm.
This study employed a Sirius Scheimpflug-Placido three-dimensional anterior segment analyzer combined with a rotating Scheimpflug camera and Placido disc technology. Anterior and posterior corneal topography, wavefront aberration, and corneal thickness measurements were obtained in one scan[9]. This technique is simple and fast and helps prevent subjective errors associated with younger patients’ age. To date, no consistent conclusion has been reached regarding the correlation between higher-order aberrations and refractive error. Previous studies have shown no significant correlation between higher-order aberrations and ametropia[10,11]. However, Cheng et al[12] evaluated higher-order aberrations in patients with pathological myopia and found that high myopia was associated with a greater rate of higher-order aberrations than emmetropia due to the increase in intraocular aberrations. There was no correlation between diopter and higher-order aberrations (P > 0.05) in our study, and higher-order aberrations were negatively correlated with AL (P < 0.05). These results show that higher-order aberrations change with changes in the ocular structural parameters. We speculate that axial growth plays an important role in the reduction of higher-order aberrations. Axial growth may be a compensatory mechanism to reduce higher-order aberrations and thus promote the development of myopia.
The spherical aberration of middle myopia was highest in our study and was different from that of low myopia and high myopia, but there was no difference between low myopia and high myopia on this parameter. Little et al[13] found that when the pupil diameter was 5 mm, the spherical aberration was significantly correlated with AL, and spherical aberration decreased with increasing ocular AL. Anbar et al[14] found that the spherical aberration of hyperopia was lowest among other diopters, showing that there was no linear relationship between spherical aberration and diopter. There was no correlation between spherical aberration and AL in our study (P > 0.05). The difference in these results may be due to the differences in age, pupil diameter, and diopter distribution in the study samples, and aberration measuring instruments used. Coma and trefoil aberrations are higher-order aberrations that affect visual quality. Zhao et al[15] suggested that the third-order aberration was the main factor affecting visual quality at low-middle frequencies, and that Z33 (trefoil), Z31 (horizontal coma), and Z5-1 were the main factors affecting visual sensitivity. There was no difference in coma and trefoil aberration values among the three groups[16,17]. Peripheral lens flattening during the regulation process may lead to a decrease in spherical aberrations[16,17]. Parallel light from an infinite distance is refracted into a spherical wavefront in emmetropia, which appropriately converges to a precise focus on the retina. However, ciliary muscle regulation may be destroyed by coma and spherical aberrations in patients with myopia[18], thus affecting visual quality.
AL is the main determinant of eyeball size and one of the basic anatomical parameters of eyeball optics. At present, AL remains the main index used to evaluate diopters. In our research, the average AL of 87 children with high myopia was 26.04 ± 0.95 mm, which was much higher than that of 6364 children aged 4-18 years in Shandong Province (with an average AL of 23.45 ± 1.20 mm)[19].
We used multiple linear regression analysis to identify factors affecting the AL, and obtained the following equation: AL = -0.395 × km - 0.342 × SE + 1.132 × ACD + 0.077 × Age + 35.925 (F = 106.193, R2 = 0.730, P < 0.001). There was a negative correlation between AL and km, indicating that the corneal curvature became flat with increasing ocular axial compression. Olsen et al[20] found a significant negative correlation between AL and corneal refractive power. Lee et al[21] also found that the corneal curvature in the high myopia group decreased significantly. Thus, the decrease in corneal refractive power may partially compensate for the increase in refractive power in high myopia caused by AL prolongation, which is consistent with our results.
Moreover, there was a positive correlation between AL and ACD. The average size of the ACD in the low-, middle-, and high-myopia groups was 3.30, 3.33, and 3.34 mm, respectively. Statistical analysis showed that ACD differed only between the low-myopia and high-myopia groups (P < 0.05). There was no difference between the low- and middle-myopia groups, which may be due to the small size of the middle-myopia group. Therefore, we speculate that an increase in ACD may be caused by excessive AL prolongation. Correlation analysis has shown that axial activity was positively associated with age, which was related to increases in participant height, weight, and degree of eye use, and a decrease in outdoor activities. Pärssinen et al[22] has shown that the longer the baseline AL, the greater the AL increase with age.
Ocular structural parameters in children change because of different diopters. AL/R is more specific and sensitive than AL in evaluating the refractive status of myopia in children. The AL/R of > 3.00 may be used as a specific index of myopia in children. There are differences in AL/R between high myopia and low-middle myopia, which can be used for the classification of ametropia. The degree of myopia has some influence on higher-order aberrations.
Patients with high myopia account for 0.5%-5.0% of the global population. Relevant studies predicted that by 2050, the global number of myopia and high-myopia patients will have reached 5 billion and 900 million, respectively.
An association between changes in ocular structural parameters and the development of myopia and their role in the development of myopia require elucidation.
To explore the progression of high myopia.
This study included 195 children aged 6–17 years with myopia who underwent eye examination conducted by an ophthalmologist.
The diopter of high myopia was correlated with age and axial length (AL), and AL of high myopia was negatively correlated with K1, K2, and Km, and positively correlated with age and anterior chamber depth.
There are differences in AL/R values between high myopia and low-middle myopia, which can be used for the classification of ametropia. The degree of myopia has some influence on higher-order aberrations.
Changes in ocular structure parameters are closely related to the progression of myopia.
Manuscript source: Unsolicited manuscript
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schuster AK S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Leveziel N, Yu Y, Reynolds R, Tai A, Meng W, Caillaux V, Calvas P, Rosner B, Malecaze F, Souied EH, Seddon JM. Genetic factors for choroidal neovascularization associated with high myopia. Invest Ophthalmol Vis Sci. 2012;53:5004-5009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Kumar A, Chawla R, Kumawat D, Pillay G. Insight into high myopia and the macula. Indian J Ophthalmol. 2017;65:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Chong EW, Mehta JS. High myopia and cataract surgery. Curr Opin Ophthalmol. 2016;27:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Rozema J, Dankert S, Iribarren R, Lanca C, Saw SM. Axial Growth and Lens Power Loss at Myopia Onset in Singaporean Children. Invest Ophthalmol Vis Sci. 2019;60:3091-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Muthu Krishnan V, Jayalatha K, Vijayakumar C. Correlation of Central Corneal Thickness and Keratometry with Refraction and Axial Length: A Prospective Analytic Study. Cureus. 2019;11:e3917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Kearney S, Strang NC, Cagnolati B, Gray LS. Change in body height, axial length and refractive status over a four-year period in caucasian children and young adults. J Optom. 2020;13:128-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Tideman JW, Snabel MC, Tedja MS, van Rijn GA, Wong KT, Kuijpers RW, Vingerling JR, Hofman A, Buitendijk GH, Keunen JE, Boon CJ, Geerards AJ, Luyten GP, Verhoeven VJ, Klaver CC. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016;134:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 8. | You QS, Peng XY, Xu L, Chen CX, Wang YX, Jonas JB. Myopic maculopathy imaged by optical coherence tomography: the beijing eye study. Ophthalmology. 2014;121:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Edwards MH, Brown B. IOP in myopic children: the relationship between increases in IOP and the development of myopia. Ophthalmic Physiol Opt. 1996;16:243-246. [PubMed] |
| 10. | Bayhan HA, Aslan Bayhan S, Muhafız E, Can I. Repeatability of aberrometric measurements in normal and keratoconus eyes using a new Scheimpflug-Placido topographer. J Cataract Refract Surg. 2014;40:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Cheng X, Bradley A, Hong X, Thibos LN. Relationship between refractive error and monochromatic aberrations of the eye. Optom Vis Sci. 2003;80:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Little JA, McCullough SJ, Breslin KM, Saunders KJ. Higher order ocular aberrations and their relation to refractive error and ocular biometry in children. Invest Ophthalmol Vis Sci. 2014;55:4791-4800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Anbar M, Mohamed Mostafa E, Elhawary AM, Awny I, Farouk MM, Mounir A. Evaluation of Corneal Higher-Order Aberrations by Scheimpflug-Placido Topography in Patients with Different Refractive Errors: A Retrospective Observational Study. J Ophthalmol. 2019;2019:5640356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Zhao PF, Li SM, Lu J, Song HM, Zhang J, Zhou YH, Wang NL. Effects of higher-order aberrations on contrast sensitivity in normal eyes of a large myopic population. Int J Ophthalmol. 2017;10:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zhou XY, Wang L, Zhou XT, Yu ZQ. Wavefront aberration changes caused by a gradient of increasing accommodation stimuli. Eye (Lond). 2015;29:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Fritzsch M, Dawczynski J, Jurkutat S, Vollandt R, Strobel J. [Monochromatic aberration in accommodation. Dynamic wavefront analysis]. Ophthalmologe. 2011;108:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ke B, Mao X, Jiang H, He J, Liu C, Li M, Yuan Y, Wang J. The Relationship Between High-Order Aberration and Anterior Ocular Biometry During Accommodation in Young Healthy Adults. Invest Ophthalmol Vis Sci. 2017;58:5628-5635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Lu TL, Wu JF, Ye X, Hu YY, Wu H, Sun W, Guo DD, Wang XR, Bi HS, Jonas JB. Axial Length and Associated Factors in Children: The Shandong Children Eye Study. Ophthalmologica. 2016;235:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: the Reykjavik Eye Study. Acta Ophthalmol Scand. 2007;85:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Lee MW, Lee SE, Lim HB, Kim JY. Longitudinal changes in axial length in high myopia: a 4-year prospective study. Br J Ophthalmol. 2020;104:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Pärssinen O, Kauppinen M. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019;97:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |