Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7825
Peer-review started: February 24, 2021
First decision: April 13, 2021
Revised: April 20, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: September 16, 2021
Processing time: 197 Days and 20.3 Hours
Aggressive malignant primary orbital tumors are extremely rare in newborns. The current cases further clarify the clinical features of malignant primary orbital tumors in neonates.
At the time of presentation at the Seventh Center of People’s Liberation Army General (PLAG) Hospital, the children were 1-, 2- and 5-mo-old, respectively, and included 2 boys and 1 girl. All three cases had unilateral proptosis at birth, and underwent mass excision and histopathologic examination. A peripheral primary neuroectodermal tumor, an aggressive infantile fibromatosis and an embryonic rhabdomyosarcoma were diagnosed, respectively. The first case underwent routine chemotherapy following surgery but died within three months due to worsening condition as the tumor spread throughout the body. The other two children were treated by surgery, and at the follow-up visits 6 mo and 1 year after surgery, respectively, the wound was completed healed, and they had normal growth and development without radiotherapy or chemotherapy. A review of highly uncommon orbital tumors in newborns is also provided.
Malignant primary tumors should be considered in the presence of unilateral proptosis in newborns.
Core Tip: The first case underwent routine chemotherapy following surgery but died within three months due to worsening condition as the tumor spread throughout the body. The other two children were treated by surgery, and at the follow-up visits 6 mo and 1 year after surgery, respectively, the wound was completed healed, and they had normal growth and development without radiotherapy or chemotherapy.
- Citation: Zhang Y, Li YY, Yu HY, Xie XL, Zhang HM, He F, Li HY. Rare neonatal malignant primary orbital tumors: Three case reports. World J Clin Cases 2021; 9(26): 7825-7832
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7825
Nonosseous, extraocular orbital lesions are rare in children and show a distinct histologic spectrum compared with those in adults. Rhabdomyosarcoma (RMS) is considered the most common soft tissue sarcoma in children[1]. Primary orbital RMS mostly affects individuals in the initial decade of life, with patient age averaging 6-8 years, although virtually all age groups are involved. Although the incidence of such tumors is very low, their diagnosis and treatment are of great concern to ophthalmologists.
Studies assessing primary malignant tumors in newborns are extremely uncommon. Case reports have described rare primary malignant tumors in newborns, including malignant orbital teratoma, congenital neuroblastoma, congenital orbital RMS, and desmoplastic small round cell tumor. It is difficult to detect any orbit abnormality by prenatal ultrasound, and massive proptosis at birth is the main symptom of primary orbital RMS[2]. Currently, its clinical characteristics, treatment and management remain unclear.
We report three neonatal cases with orbital proptosis who were diagnosed with aggressive orbital tumors, and underwent surgery and histopathological examination between October 2017 and October 2019. Their clinical characteristics, imaging findings, diagnostic pathologies, treatments and prognoses are described. The current report provides a clinical basis for the diagnosis and treatment of neonatal invasive orbital tumors.
Case 1: A 1-mo-old boy presented with an ocular mass.
Case 2: A 2-mo-old boy presented at the Seventh Center of the People’s Liberation Army General (PLAG) Hospital, Beijing, with a 2-mo history of swelling inferior to the right orbit without pain.
Case 3: A 5-mo-old girl presented at the Seventh Center of PLAG Hospital, Beijing, with a 5-mo history of proptosis to the right orbit without pain.
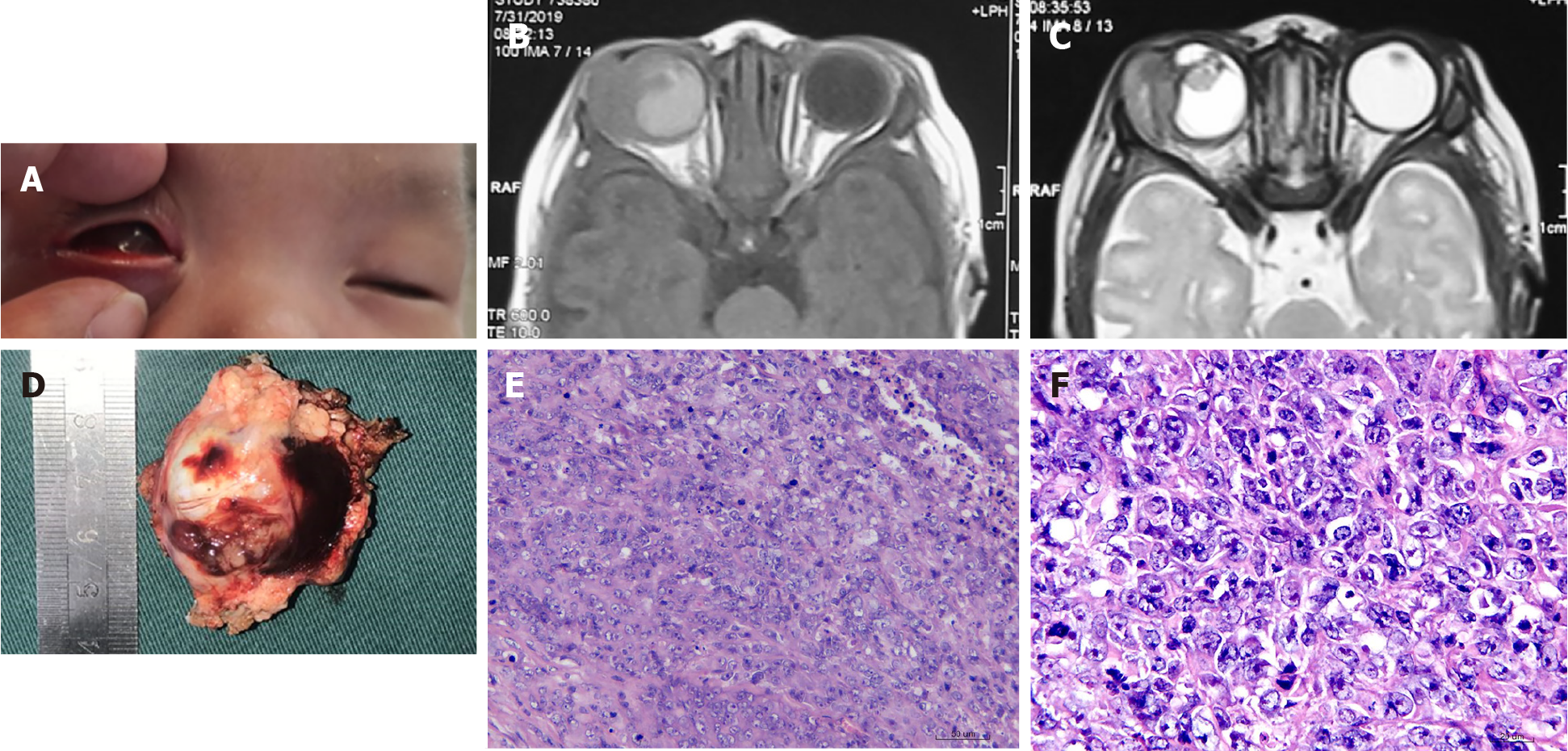
Case 1: His parents reported that he was born with a mass in his right eye, which grew rapidly (Figure 1A). Local tumor resection was performed at the local hospital 20 days after birth. His condition rapidly deteriorated after the operation with the lump recurring and growing rapidly. Therefore, the boy was transferred to the Ophthalmology Department of the Seventh Center of PLAG Hospital at 42 d of age.
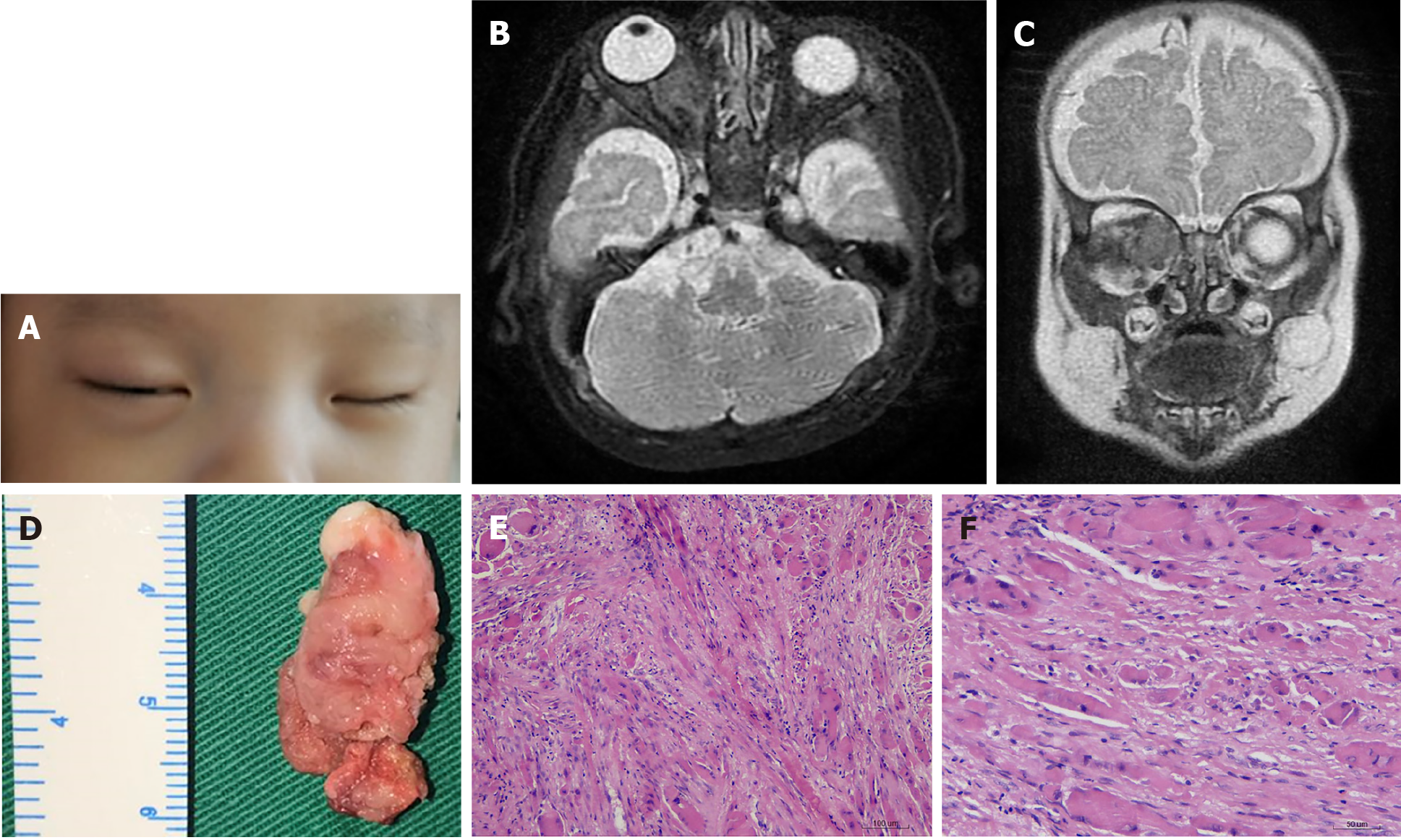
Case 2: His parents reported that he was born with eyelid skin redness, and right eye swelling with proptosis which then increased gradually (Figure 2A). A suspected diagnosis of orbital cellulitis was made at a local hospital but no treatment was performed.
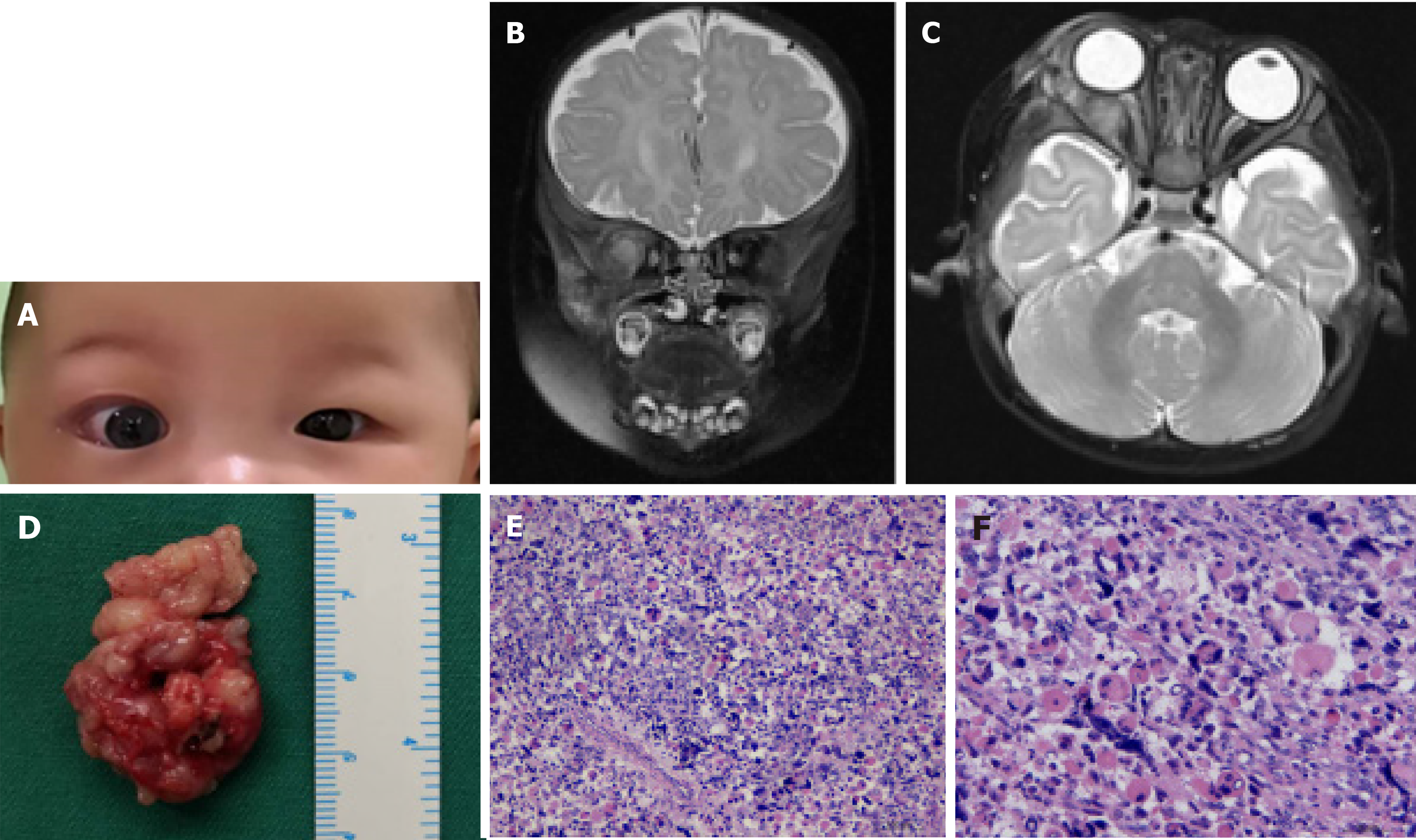
Case 3: Her parents reported that she was born with right eye proptosis, which increased gradually (Figure 3A).
Cases 1-3: The mother of each case had experienced a normal pregnancy.
Case 1-3: In each case, both parents of a non-consanguineous marriage were healthy.
Case 1: Ophthalmologic examination was performed. The right eye showed no apparent vision, with its outer eyelid swollen; proptosis and conjunctival hyperemia were present, and the cornea and the intraocular structure were unclear. Two enlarged lymph nodes were observed in front of the right ear and behind the neck. No other systemic abnormalities were detected.
Case 2: Ophthalmological and physical examinations showed proptosis of the right eye, incompletely closed eyelid, transparent cornea, a pupil diameter of 3 mm, and no relative afferent pupillary defect (RAPD), indicating normal intraocular pressure and retina.
Case 3: Ophthalmological analysis and physical examination showed right eye proptosis, incompletely closed eyelid, inverted lower eyelashes, conjunctival hyperemia, transparent cornea, a pupil diameter of 4 mm, positive RAPD, normal intraocular pressure and retina, and lighter disc color compared with that of the left eye. No lymphadenopathy or systemic abnormalities were detected.
Cases 1 and 3: Routine laboratory tests, including complete blood count and serum creatinine assessment, were within the normal ranges.
Case 2: Routine laboratory tests including complete blood count and serum creatinine assessment were within the normal ranges. No lymphadenopathy or systemic abnormalities were detected.
Case 1: Orbit magnetic resonance imaging (MRI; Figure 1B and C) showed that the right orbital tumor had a clear boundary and uneven signals. It was closely connected to the eyeball, whose structure was unclear, with abnormal signals. The lacrimal gland was unclear, and the optic nerve was not thickened; there was no obvious abnormality in the extraocular muscle. No abnormalities in intracranial structure were detected.
Case 2: Orbit MRI (Figure 2B and C) revealed an irregular mass in the right orbit (14 mm × 17 mm × 16 mm). The right optic nerve was compressed, with the extraocular muscle compressed and displaced, while the right orbital wall was destroyed and depressed. Doppler ultrasound showed an irregular rear right eyeball inner parenchyma mass with a clear boundary, slight blood supply, and the medial rectus showed unclear boundaries. No abnormalities were found in liver and spleen blood vessels.
Case 3: Orbit MRI revealed a mass in the right eye, with long T1 and slightly longer T2 signals. The lateral orbital wall was involved, and the mass grew into the muscle cone; the lacrimal gland and the external rectus muscle were unclear, and the optic nerve was compressed (Figure 3B and C). Doppler ultrasound showed an irregular hypoechoic area behind the right eyeball, with a discernible boundary; however, the boundary with the external rectus muscle was unclear, and the blood flow signal was visible in CDFI. No obvious thickening of the optic nerve was observed.
Histopathologic analysis revealed a full thickness tissue involvement eyeball tumor and surrounding striated muscle tissue, the cutting edge portion of the visible lesion (Figure 1D). Tumor cell atypia was found, with poorly differentiated cells (Figure 1E and F). Immunostaining showed actin (+), CD99 (+), vimentin (+), Ki-67 (70%-80%+), SMA (+), NeuN (+), Nestin (+) and Cyclin D1(+) tumor cells. Based on the clinical features, MRI findings, morphologic features, and IHC findings, the diagnosis of a peripheral primary neuroectodermal tumor was made. After surgery, routine chemotherapy was performed starting with the VACA (vindesine at 2 mg d1 + EPI at 75 mg/m2 + CTX at 1.2 g/m2 on days 1-2 + neomycin (6 μg/kg) on days 1-5) regimen in cycles 1, 3 and 5) and the VAC/IE (ifosfamide at 1.8 g/m2 on days 1-5 + VP-16 at 100 mg/m2 on days 1-5) regimen in cycles 2 and 4. Each chemotherapy cycle lasted 3 wk.
Histopathologic analysis revealed fibrous tissue hyperplasia and partial striated muscle atrophy (Figure 2E and F). Immunostaining for actin, β-catenin, CD34, Ki-67, SMA, Myo-D1, desmin, and vimentin was positive in tumor cells. Based on clinical features, MRI findings, morphologic features, and IHC findings, the diagnosis of aggressive infantile fibromatosis was made.
Mass excision was performed, and the tumor tissue in the orbit was removed, with dimensions of 40 mm × 25 mm (Figure 3D). Finally, the patient was transferred to the intensive care unit, where she experienced an uneventful recovery.
Orbital content evisceration was performed, and the patient was transferred to the intensive care unit, where he experienced an uneventful recovery.
Mass excision was performed, extracting off-white fusiform tumoral tissue that invaded the extraocular muscles and orbital bone wall, with dimensions of 30 mm × 15 mm × 15 mm (Figure 2D).
Histopathological analysis revealed a skeletal muscle scattered mass composed of spindle cells (Figure 3E and F). Immunostaining for actin, SMA, myogenin, p63 desmin, vimentin, Myo-D1, and CD99 was positive in tumor cells. Based on the morphological features and IHC findings, the diagnosis of embryonic RMS was made. After surgery, 7 cycles of chemotherapy were administered with vincristine at 1.5 mg/m2 on days 1, 8 and 15; actinomycin D at 0.02 mg/(kg·time) + intravenous saline infusion for 5 min on day 1; cyclophosphamide at 1.2 g/m2 intravenous infusion for 1 h on day 1, 2-mercaptoethylsulfonate at 360 mg/(m2·time) at 0, 3, 6, and 9 h + intravenous infusion of normal saline for 20 min to 30 min.
At the 3-mo follow-up visit, the infant died due to worsening condition, as the tumor spread throughout the body.
At 6-mo follow-up after surgery, without radiotherapy or chemotherapy, there was complete healing of the wound, and the infant’s growth and development were normal.
At the follow-up visit 1 year after surgery, complete healing of the wound was observed, and the infant’s growth and development were normal.
Neonatal malignant primary orbital tumors are extremely uncommon and hardly detected by prenatal ultrasound. Most neonatal malignant primary tumors reported to date are obviously occupying masses, with eyeballs as prominent sites. The three Chinese children in this study had mild to moderate eye protrusion, which is particularly important for distinguishing from other orbital tumors.
Benign tumors of the orbit include optic nerve sheath meningioma, hemangioma and teratoma, which have specific imaging features. Malignant tumors are granulosarcoma, glioma and primary orbital neuroblastoma.
Primitive neuroectodermal tumor (PNET) is a small round cell malignant tumor of neuroectodermal origin[3]. PNET is most common in young adults and adolescents, and primary orbital PNET is extremely rare in newborns. Most PNETs are located near the orbital wall, with a proclivity to arise in the lateral orbital wall. A previous case showed an intraconal location[4]. In these cases, masses were also located near the lateral orbital wall, and the lacrimal gland structure was possibly destroyed. In one patient, the tumor involved the eyeball, and grew around it. Histopathologically, these are small, round, dark blue tumors with a monotonous, highly cellular pattern, pseudo-rosette formation[3]. Immunohistochemical techniques are the most useful tool for the diagnosis of primary PNET. Neuron-specific enolase and CD99 are the immunohistochemical markers often detected in most cases, followed by the S100 protein, synaptophysin and vimentin.
Primary PNETs are very aggressive with rapid progression and poor prognosis, and bone invasion and extraorbital extension have been reported. In one of the current cases (case 1), the tumor grew rapidly, and lymph node metastasis had occurred before surgery. Surgery has been applied as the initial treatment option for primary orbital PNET in most cases, although some previous cases were treated with chemotherapy and radiotherapy without surgery, with reasonable results. Schmidt et al[5] reported a disease-free survival rate in primary PNET at 7.5-year follow-up of 45%. In addition, Kushner et al[6] documented a progression-free survival of localized tumors larger than 5 cm of 25% at 24 mo. A multimodality treatment approach was used for case 1, including surgery, radiotherapy, and chemotherapy. However, the patient survived only 3 mo after surgery. Deterioration of the disease may be related to extraorbital lymph node extension.
Aggressive fibromatosis in the head and neck region is uncommon, it is a monoclonal fibroblastic proliferation arising in musculoaponeurotic structures that is locally aggressive and has diffusely spreading margins. Aggressive fibromatosis has been associated with pregnancy, soft tissue trauma, and familial adenomatous polyposis[7]. Aggressive fibromatosis affecting the orbit is scarce in newborns. Case 2 had mild proptosis at birth, and the tumor grew rapidly thereafter. Fibromatosis has typical MRI features, appearing isointense to slightly hyperintense on T1 weighted images, intermediate between muscle and fat on T2 weighted images and enhanced after administration of a contrast agent. These typical findings were evident in case 2. The right orbital wall was destroyed and right optic nerve was compressed. This tumor invades and destroys surrounding tissues. Trisomy 8 and 20 have been detected in 56% of aggressive fibromatoses and 76% of fibrous bone lesions. However, gene sequencing was not performed in this study, and it is unclear whether such genetic anomalies were involved. Histopathological examination showed fibroblastic spindle cells with mild nuclear pleomorphism and a generous collagenous component, and ß-catenin, cyclin D1 and Ki-67 expression[8]. Patients with tumors amenable to surgery with good functional and cosmetic outcomes can be treated by resection alone if negative margins can be achieved. The rate of local recurrence after surgical resection ranges from 27% to 77%. Most recurrences occur within two years. Approximately 9% to 27% of aggressive fibromatoses are located in the head and neck. Due to the infant's age and physical condition, resection of the tumor was performed, with no recurrence 1-year later. Therefore, we recommend surgical resection as the preferred treatment option for such tumors in neonates, completely removing the masses to prevent recurrence.
RMS is the most common malignant tumor of the orbit in children, but rarely present at birth[9]. Some congenital syndromes are associated with RMS such as Li-Fraumeni syndrome (P53 gene mutation on chromosome 17p13), neurofibromatosis type 1, Noonan syndrome, Gorlin syndrome, Costello syndrome, hereditary retinoblastoma, and Beckwith-Wiedemann syndrome. The current patient (case 3) did not have any sign of the abovementioned syndromes, and no P53 mutation was detected. Orbital RMS is usually extraconal (37%-87%) or both intra and extraconal (13%-47%), and more commonly superonasal in location especially embryonal RMS. The mass is usually close to extraocular muscles, with no enlargement of the muscle belly. In the early stages, the tumor is well circumscribed, but the borders are irregular in later stages with pseudocapsular invasion. The tumor may show hemorrhage and cyst formation. These typical findings were evident in case 3, with the mass located in the intramuscular cone, also compressing the optic nerve. There was no damage to the orbital bone wall. Recurrence of orbital RMS is found in about 17% of cases at a median time of 18 mo, with 92% of these cases at local and 8% at distant sites[10]. Embryonal RMS has a 94% 5-year survival (vs alveolar 74%). In the current study, case 3 received radiotherapy and chemotherapy after surgery. The infant’s growth and development were normal at follow-up.
Neonatal malignant primary orbital tumors are extremely rare. Different to previous reports, early symptoms in these three Chinese neonatal tumors included mild proptosis, but the tumors grew rapidly. Early surgical resection is recommended to save lives and preserve visual function.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Alkatan HM, Al Marek F, Elkhamary S. Demographics of Pediatric Orbital Lesions: A Tertiary Eye Center Experience in Saudi Arabia. J Epidemiol Glob Health. 2019;9:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Prakash MV, Indira R, Radhakrishnan M, Leela G. Malignant orbital teratoma in a neonate: A clinicopathological case report. J Postgrad Med. 2017;63:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Tamer C, Oksuz H, Hakverdi S, Karazincir S, Balci A, Yaldiz M. Primary peripheral primitive neuroectodermal tumour of the orbit. Can J Ophthalmol. 2007;42:138-140. [PubMed] |
| 4. | Alyahya GA, Heegaard S, Fledelius HC, Rechnitzer C, Prause JU. Primitive neuroectodermal tumor of the orbit in a 5-year-old girl with microphthalmia. Graefes Arch Clin Exp Ophthalmol. 2000;238:801-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Schmidt D, Herrmann C, Jürgens H, Harms D. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer. 1991;68:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Kushner BH, Hajdu SI, Gulati SC, Erlandson RA, Exelby PR, Lieberman PH. Extracranial primitive neuroectodermal tumors. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1991;67:1825-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Zlotecki RA, Scarborough MT, Morris CG, Berrey BH, Lind DS, Enneking WF, Marcus RB Jr. External beam radiotherapy for primary and adjuvant management of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 2002;54:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Brueckl WM, Preuss JM, Wein A, Jung A, Brabletz T, Pflüger R, Wiest GH, Wolfl C, Kirchner T, Hahn EG, Hohenberger W, Günther K. Ki-67 expression and residual tumour (R) classification are associated with disease-free survival in desmoid tumour patients. Anticancer Res. 2001;21:3615-3620. [PubMed] |
| 9. | Rao AA, Naheedy JH, Chen JY, Robbins SL, Ramkumar HL. A clinical update and radiologic review of pediatric orbital and ocular tumors. J Oncol. 2013;2013:975908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sohaib SA, Moseley I, Wright JE. Orbital rhabdomyosarcoma--the radiological characteristics. Clin Radiol. 1998;53:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |