Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7762
Peer-review started: April 13, 2021
First decision: May 11, 2021
Revised: May 16, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: September 16, 2021
Processing time: 149 Days and 21.7 Hours
Postoperative ileus is a frequent postoperative complication, especially after abdominal surgery. Sympathetic excitation is the primary factor for postoperative ileus. Sympathetic activation becomes increased by surgical stress, postoperative pain, and inflammation. Dexmedetomidine (DEX) can inhibit sympathetic nerve activity, inflammation, and pain.
To observe whether DEX promotes bowel movements in patients after laparoscopic nephrectomy.
One hundred and twenty patients undergoing laparoscopic nephrectomy were assigned to three groups: C (normal saline infusion), D1 (DEX 0.02 µg/kg/h), and D2 (DEX 0.04 µg/kg/h). The primary outcomes were the recorded times to first flatus, defecation, and eating after surgery. The secondary outcomes were postoperative pain, assessed using the numerical rating scale (NRS), adverse effects, and the duration of the postoperative hospital stay.
The times to first flatus, defecation, and eating in groups D1 and D2 were significantly shorter than those in group C (P < 0.01). The NRS scores at 8 h and 24 h after surgery were significantly lower in groups D1 and D2 than in group C (P < 0.05). No adverse effects were observed (P > 0.05).
Postoperative infusion of DEX at 0.04 µg/kg/h facilitates bowel movements in patients undergoing laparoscopic nephrectomy.
Core Tip: Postoperative ileus (POI) is a perplexing problem for clinical surgeons. In this study, laparoscopic nephrectomy was chosen to investigate postoperative gastrointestinal function recovery, avoiding damage to the gut itself. Based on the reported effects of DEX, the authors hypothesized that DEX could promote postoperative gastrointestinal function.
- Citation: Huang SS, Song FX, Yang SZ, Hu S, Zhao LY, Wang SQ, Wu Q, Liu X, Qi F. Impact of intravenous dexmedetomidine on postoperative bowel movement recovery after laparoscopic nephrectomy: A consort-prospective, randomized, controlled trial. World J Clin Cases 2021; 9(26): 7762-7771
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7762.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7762
Postoperative ileus (POI) is a perplexing problem for clinical surgeons. It occurs not only after abdominal surgery but also after any surgery that requires general anesthesia[1,2]. POI is defined as the dysfunction of gastrointestinal motility after surgery, characterized by a decrease in, or stagnation of, intestinal peristalsis. Common clinical manifestations include abdominal pain, abdominal distention, nausea, vomiting, delayed flatus, delayed defecation, and inability to consume orally[3-5]. POI is an uncomfortable experience, enhances the possibility of postoperative complications, prolongs hospital stay[4], and increases the economic burden[6,7]. Postoperative gastrointestinal function recovery is of great concern. There is currently an urgent need to improve postoperative recovery of gastrointestinal function.
The mechanism of POI varies, including autonomic regulation, inflammatory response, gastrointestinal hormones, and postoperative use of opioid drugs. Surgical gut damage destroys the intestinal barrier, stimulates the sympathetic and parasympathetic nervous system, and enhances the release of inflammatory factors[1,5,8,9]. These factors precipitate the occurrence of POIs. The current use of laparoscopic techniques can reduce incision size and surgical trauma, enabling careful manipulation[10-12]. Thus, the influence of the surgical procedure itself has decreased. Some studies have shown that intraoperative use of short-acting opioids or postoperative use of opioid receptor antagonists can ensure postoperative analgesia and eliminate the impact of intraoperative use of opioids on POI. Adjuvant epidural analgesia, intraoperative restriction of fluid intake, reduction of intraoperative blood loss, and early oral administration of nutrients after surgery can promote POI recovery[13]. However, POI remains a medical problem during clinical surgery; therefore, a more effective and noninvasive method is required.
Dexmedetomidine (DEX), as a highly selective α-2 adrenergic receptor agonist, has the effects of synergetic analgesia, sedation, inhibition of sympathetic hyperactivity, and reduced release of inflammatory mediators with little respiratory inhibition[13,14]. Previous studies on POI were all based on gastrointestinal tract surgery. Hence, in the present study, laparoscopic nephrectomy was chosen to investigate postoperative gastrointestinal function recovery, avoiding damage to the gut itself. Based on the reported effects of DEX, we hypothesized that DEX could promote postoperative gastrointestinal function.
This randomized, double-blinded, controlled trial was approved by the Institutional Medical Ethics Committee of Qilu Hospital of Shandong University. It was registered at chictr.org (ChiCTR-IPR-15007628) and is in accordance with the CONSORT guidelines. We chose patients who were treated by laparoscopic nephrectomy under general anesthesia at Qilu Hospital and did not have the following conditions: Body mass index greater than 32 kg/m2 or less than 18 kg/m2; age older than 75 or younger than 18 years; presence of bradycardia [basal heart rate (HR) less than 60 bpm] or other cardiac arrhythmia; presence of clinically significant dysfunction, including cardio
The patients who met the enrollment criteria provided informed consent for participating in the trial. Then, according to a computer-generated randomization table, participants were randomly assigned to one of the three groups: C (normal saline infusion), D1 (DEX 0.02 µg/kg/h), and D2 (DEX 0.04 µg/kg/h). On the day of surgery, the drugs and patient-controlled analgesia (PCA) were prepared by an anesthetist who was blinded to the group assignment. Furthermore, the associated doctors and nurses were blinded to group assignment.
Patients were premedicated with atropine 0.5 mg by intramuscular injection in the ward. Before anesthesia induction, each patient was monitored for electrocardiography, noninvasive blood pressure measurements, pulse oximetry saturation (SpO2), and end-tidal carbon dioxide (EtCO2) using an automated system (Philips IntelliVue MP50; Philips Company, Beijing, China). HR, SpO2, and mean blood pressure (MBP) were monitored every 5 min.
After obtaining a baseline measurement of HR and MBP, groups D1 and D2 received 0.5% DEX, and group C received 0.9% normal saline for 10 min. We used propofol, rocuronium, and sufentanil for sequential induction. The laryngeal mask airway (LMA) was intubated after positive pressure mask ventilation for 5 min. An arterial cannula was required to monitor invasive arterial blood pressure in the left radial artery. Anesthetic depth was monitored using a bispectral index (BIS) monitor, and sevoflurane was administered to maintain the depth of anesthesia (BIS scores in the range of 40 to 60). Controlled ventilation was performed with 100% oxygen, and EtCO2 was maintained at 35–40 mmHg. We inserted a temperature probe through the nasal cavity and maintained the body temperature at 36–37 °C. We started to infuse the test drugs (groups D1 and D2 received the DEX infusion at rates of 0.2 μg/kg/h and 0.4 µg/kg/h, respectively, while group C received saline instead of DEX) after the establishment of pneumoperitoneum and suspended them for 30 min before the end of surgery. Rocuronium was administered intermittently to maintain satisfactory muscle relaxation.
If more than a 20% fluctuation in the MBP baseline level was detected, vasoactive drugs (noradrenaline 5-10 µg or nitroglycerin 50-100 µg) were used to maintain hemodynamic stability. If the HR decreased to less than 45 bpm, atropine 0.5 mg was administered. Conversely, if the HR was greater than 100 bpm, esmolol 0.5 mg/kg was administered to decrease the HR. When the laparoscope was withdrawn, palonosetron (0.25 mg) was intravenously administered to prevent postoperative nausea and vomiting (PONV). When spontaneous breathing appeared at the end of the surgery, neostigmine 0.04 mg/kg and atropine 0.02 mg/kg were administered to antagonize neuromuscular blockade before LMA extubation. If the SpO2 was greater than 90% without oxygen for at least 5 min, patients could be sent back to the ward.
At the end of the surgery, a PCA pump was started (group C with sufentanil 0.02 µg/kg/h; group D1 with both sufentanil and DEX 0.02 µg/kg/h; group D2 with sufentanil 0.02 µg/kg/h and DEX 0.04 µg/kg/h). The PCA was programmed to deliver at a constant speed of 2 mL/h, and an additional dose (0.5 mL) was administered with a lockout time of 10 min.
Regarding postoperative bowel movements, patients were given abdominal massage, miso soup, or both if the time to flatus was more than 48 h. Intravenous nutrition was provided if the time to flatus was more than 72 h.
The primary outcome measures were the times to first flatus and defecation, and the duration of postoperative hospital stay. The secondary outcome measures were postoperative pain scores, both at rest and during movement, and adverse effects.
HR, MBP, and SpO2 were collected at the following six time points: Entering the operating room (T0), 5 min after finishing the baseline test drug infusion (T1), 5 min after pneumoperitoneum establishment (T2), 1 h after pneumoperitoneum estab
Statistical analyses were performed using SPSS software (version 21.0, SPSS Inc. Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the distribution of the variables. Levene’s test was used to compare the homogeneity of variance among the three groups. Normally distributed data are expressed as the mean and standard deviation, whereas data with a skewed distribution are expressed as the median and number (n). Percentages (%) are used to represent categorical data. Parameters such as age, operation time, anesthesia time, time to first flatus and defecation, MBP, and HR among these groups were compared using two-way analysis of variance. The Mann-Whitney test was used to evaluate the NRS scores among the three groups, and adverse reactions were analyzed using the χ2 test. Multiple comparisons were performed using the LSD post-hoc test. Statistical significance was set at P < 0.05.
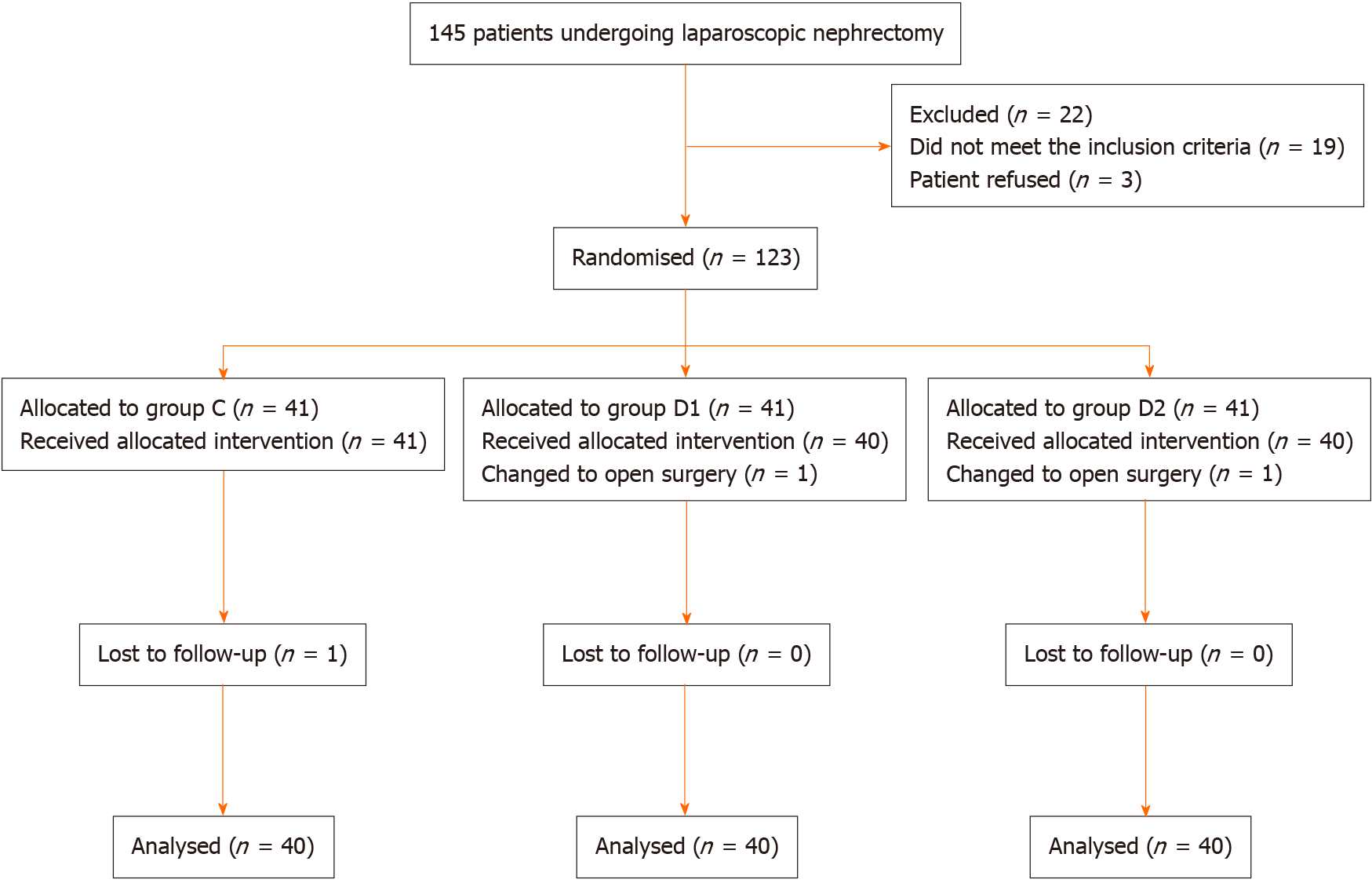
A total of 123 patients were randomly distributed into three groups. Among the patients, two were eliminated due to conversion to open nephrectomy (one from group D1 and one from group D2). In addition, one patient was excluded after surgery because of incomplete clinical data (from group C) (Figure 1). The baseline characteristics and demographics of the patients were comparable among the three groups (Table 1).
| Group C (n = 40) | Group D1 (n = 40) | Group D2 (n = 40) | P value | |
| Sex, F/M | 11/29 | 12/28 | 10/30 | 0.8820 |
| Age, yr | 52.73 ± 10.68 | 52.33 ± 7.99 | 51.73 ± 8.58 | 0.886 |
| BMI, kg/m2 | 25.58 ± 2.70 | 26.15 ± 1.70 | 25.95 ± 2.04 | 0.505 |
| Hypertension, Yes/No | 11/29 | 12/28 | 15/25 | 0.606 |
| DM, Yes/No | 6/34 | 5/35 | 5/35 | 0.930 |
| ASA, I/II | 10/30 | 7/33 | 6/34 | 0.497 |
| Duration of anaesthesia, min | 148.95 ± 49.54 | 145.90 ± 47.24 | 144.60 ± 50.24 | 0.920 |
| Duration of surgery, min | 128.48 ± 46.83 | 130.90 ± 47.13 | 127.33 ± 50.18 | 0.944 |
| Dosage of sufentanil during surgery, μg | 32.58 ± 7.20 | 31.63 ± 3.28 | 32.75 ± 3.91 | 0.570 |
| Dosage of sufentanil after surgery (8 h), mL | 16.35 ± 0.51 | 16.15 ± 0.43 | 16.29 ± 0.53 | 0.180 |
| Dosage of sufentanil after surgery (24 h), mL | 48.41 ± 0.59 | 48.26 ± 0.76 | 48.30 ± 0.60 | 0.566 |
| Postoperative stay in hospital, d | 8.60 ± 1.72 | 8.38 ± 1.35 | 8.43 ± 1.68 | 0.803 |
The times to first flatus and defecation after surgery in groups D1 (41.50 ± 8.24 h and 73.33 ± 19.19 h, respectively) and D2 (38.66 ± 7.60 h and 71.33 ± 19.70 h, respectively) were significantly shorter than those in group C (51.31 ± 11.78 h and 92.80 ± 25.51 h, respectively, P < 0.05; Table 2). The time to eating after surgery in groups D1 (44.50 ± 8.94 h) and D2 (42.29 ± 7.75 h) was shorter than that in group C (54.78 ± 11.58 h) (P < 0.05; Table 2).
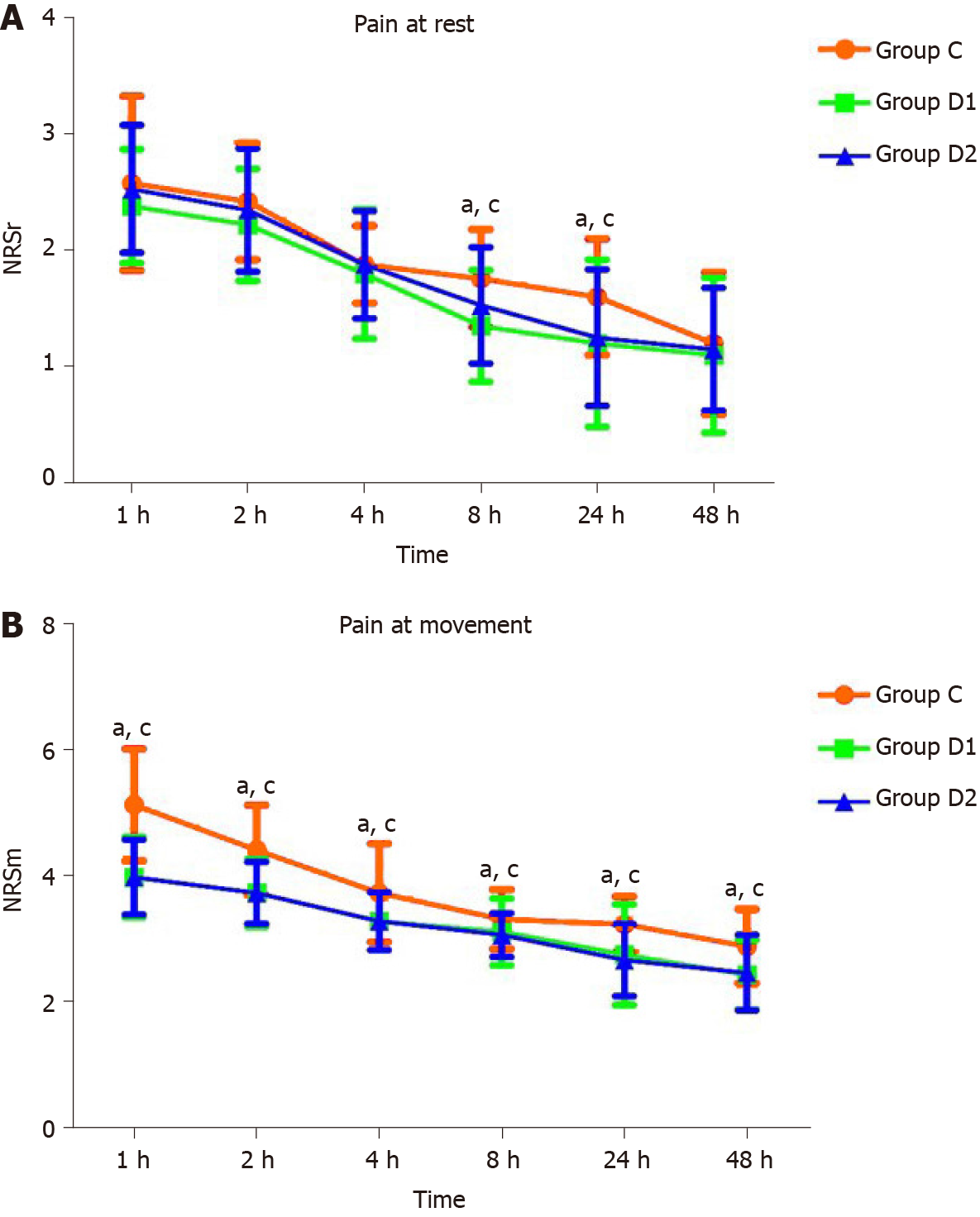
MBP at T1 in groups D1 and D2 was significantly lower than that in group C. MBP was lower than the baseline at T2, T3, T4, and T5 in group C, and at T1 and T4 in groups D1 and D2 (P < 0.05, Table 3). HR at T1 and T2 in group D1 was significantly lower than that in group C. HR was lower than the baseline at T2, T3, and T4 in group C, and at T1, T2, T3, and T4 in groups D1 and D2 (P < 0.05; Table 3). The NRS scores at rest or with movement at 8 h and 24 h after surgery were significantly lower in groups D1 and D2 than in group C (P < 0.05; Figure 2).
| Time point | Group C (n = 40) | Group D1 (n = 40) | Group D2 (n = 40) | P value |
| MBP, mm Hg | ||||
| T0 | 103.80 ± 11.34 | 99.73 ± 11.28 | 99.53 ± 9.91 | 0.144 |
| T1 | 101.53 ± 9.45 | 88.88 ± 13.07a,c | 87.65 ± 12.71a,c | 0.001 |
| T2 | 98.90 ± 9.81a | 98.88 ± 11.65 | 97.85 ± 12.53 | 0.895 |
| T3 | 97.40 ± 10.11a | 96.38 ± 7.78 | 95.88 ± 8.72 | 0.739 |
| T4 | 98.88 ± 8.33a | 94.58 ± 9.62a,c | 93.83 ± 8.54a,c | 0.025 |
| T5 | 105.45 ± 13.24a | 102.50 ± 13.55 | 102.38 ± 12.14 | 0.490 |
| HR, bpm | ||||
| T0 | 75.93 ± 11.18 | 71.43 ± 9.48 | 74.30 ± 8.70 | 0.122 |
| T1 | 74.78 ± 10.22 | 63.56 ± 10.96a,c | 65.88 ± 8.56a,c | 0.001 |
| T2 | 64.88 ± 7.74a | 59.80 ± 10.02a,c | 63.48 ± 6.93a | 0.022 |
| T3 | 66.43 ± 11.53a | 62.45 ± 9.01a | 65.53 ± 7.97a | 0.158 |
| T4 | 67.30 ± 10.47a | 66.05 ± 11.08a | 67.55 ± 9.85a | 0.791 |
| T5 | 75.80 ± 9.32 | 73.05 ± 9.86 | 72.90 ± 6.20 | 0.242 |
Adverse effects were not significantly different among the three groups (P > 0.05; Table 4).
| Group C (n = 40) | Group D1 (n = 40) | Group D2 (n = 40) | P values | |
| Abdominal massage/simo soup | 5 (12.5) | 3 (7.5) | 2 (5) | 0.466 |
| Intravenous nutrition | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Nausea and vomiting | 8 (20) | 7 (17.5) | 8 (20) | 0.948 |
| Severe abdominal pain and distention | 6 (15) | 5 (12.5) | 4 (10) | 0.796 |
| Drowsiness | 1 (2.5) | 2 (5) | 4 (10) | 0.346 |
| Serious respiratory depression | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Delirium | 0 (0) | 0 (0) | 0 (0) | 1.000 |
The perioperative use of 0.04 µg/kg/h DEX enhanced the recovery of postoperative gastrointestinal function in our study.
Experts agree that patients with POI present with several related symptoms, such as abdominal pain and distension, nausea, vomiting, absence of normal bowel sounds, intolerance of oral intake, and difficulty in defecation[15]. We did not observe the time at which bowel sounds occurred in our study because such data is subjective.
POI occurs temporarily after surgery and is not caused by mechanical reasons[15]. The mechanism of POI is complicated and involves many factors, in particular, the regulation of sympathetic and parasympathetic nerves, the inflammatory response, and postoperative use of opioid drugs. In this study, we observed the influence of DEX on the postoperative outcomes of nephrectomy to avoid gut damage.
Gastrointestinal peristalsis mainly depends on parasympathetic stimulation and is inhibited by sympathetic stimulation. Sympathetic hyperactivity is considered one of the main causes of postoperative intestinal paralysis[3,5]. Surgery, pain, gut damage, CO2 used to establish pneumoperitoneum, and other factors directly or indirectly activate the sympathetic nerves and inhibit postoperative gastrointestinal function recovery. Activated sympathetic nerves increase the release of catecholamines, which inhibit postoperative gastrointestinal function by restricting intestinal smooth muscle contraction[16,17]. DEX is a highly selective α2-adrenoceptor agonist that acts on α2-adrenoceptors in the central nervous system to reduce the upregulation of sympathetic nerve activation and decrease catecholamine release[18]. Consequently, we firmly believe that treatment with DEX could inhibit sympatholytic excitation, reduce catecholamine activation, excite the parasympathetic nerves, and facilitate posto
Surgical stress and gut damage activate the intestinal immune system, causing the release of inflammatory factors. Inflammatory factors released due to intestinal injury increase intestinal permeability and damage the intestinal lining. White blood cells can easily migrate to the muscle layer, and inflammatory substances can inhibit smooth muscle contraction and weaken gastrointestinal peristalsis[19]. DEX treatment enhances postoperative intestinal function, as it increases the efferent activity of the parasympathetic nerves, the release of acetylcholine, and the expression level of α7 nicotinic acetylcholine receptor (α7nAchR), and reduces the release of some inflammatory transmitters[20,21]. DEX was also found to play an anti-inflammatory role through α7nAchR, reducing postoperative intestinal inflammation and promoting the recovery of intestinal function[22]. Better gastrointestinal function was observed after the use of DEX in our study, which verified our hypothesis.
Visualizing laparoscopic surgery and pneumoperitoneum induction can lead to sympathetic nerve activation[16,23]. In addition, CO2 pneumoperitoneum can induce hypercarbia, which can directly or indirectly stimulate the sympathetic nervous system and cause elevated levels of catecholamines[16,24]. These factors lead to greater excitability of sympathetic nerves than parasympathetic nerves. Subsequently, gastrointestinal function is inhibited, and POI occurs. DEX, a highly selective α-2 adrenergic receptor agonist, acts on α2-adrenoceptors in the central nervous system to reduce sympathetic nerve activation and decrease catecholamine secretion[24,25]. DEX has been proven to attenuate sympathetic nerve activation induced by pneumoperitoneum and surgical stress[26], and to decrease the inflammatory response[20] to facilitate postoperative bowel movements. Groups D1 and D2 had significantly shorter times to flatus and defecation in our study than group C. Although group D2 had shorter times to flatus and defecation than group D1, and the difference was not significant for patients in the clinic, the observed 1-h difference is still important. This study provided evidence for the relief of postoperative gastrointestinal function in patients undergoing endoscopic surgery.
Although opioids are a priority for postoperative pain, they are unfavorable because they inhibit gastrointestinal motility and aggravate POI[27-29]. The perioperative use of DEX has been previously reported to relieve postoperative pain and reduce the total volume of opioids required[30,31]. The total volume of opioid drugs used in our study did not differ among the three groups, which may be due to a reduced level of postoperative pain experienced following laparoscopic surgery compared with that associated with open surgery. However, the use of DEX still significantly relieved postoperative pain with rest and movement at 8 h and 24 h after surgery in our study. Effective pain relief contributed to the alleviation of POI and allowed patients who received DEX to resume activity earlier postoperatively than those who did not. We hypothesized that DEX accelerated gastrointestinal function to relieve postoperative pain.
The blood vessels contracted when DEX was administered as a bolus, and hyper
DEX produces sedation with minimal respiratory inhibition[37]. No respiratory inhibition was observed in the present study. There were no significant differences in easily arousable drowsiness, PONV, or postoperative delirium among the groups.
Our study had some limitations. First, DEX was administered at a rate of 0.5 mg/kg for 10 min before the induction of anesthesia and then at a rate of 0.2 to 0.4 μg/kg/h during the operation. However, we were unable to determine the effect of plasma DEX concentrations on intraoperative hemodynamics because we did not measure the serum concentrations of DEX at any time point. Finally, laparoscopic nephrectomy was performed using two different surgical methods: Transabdominal and retroperitoneal. Therefore, different surgical techniques might have had different effects on postoperative analgesia and recovery of gastrointestinal function.
Perioperative DEX infusion at 0.04 µg/kg/h resulted in better and faster recovery of gastrointestinal function and a more favorable analgesic effect without additional adverse effects in patients who underwent laparoscopic nephrectomy.
Postoperative ileus (POI) is a perplexing problem for clinical surgeons. POI occurs not only after abdominal surgery, but also after any other surgery that requires general anesthesia.
Regarding enhanced recovery after surgery, postoperative gastrointestinal function recovery is of great concern. Currently, there is an urgent need to improve posto
This study aimed to observe whether dexmedetomidine (DEX) promotes bowel movements in patients after laparoscopic nephrectomy
A total of 120 patients who underwent laparoscopic nephrectomy were assigned into three groups: C (normal saline infusion), D1 (DEX 0.02 µg/kg/h), and D2 (DEX 0.04 µg/kg/h).
Mean blood pressure (MBP) at T1 in groups D1 and D2 was significantly lower than that in group C. MBP was lower than the baseline at T2, T3, T4, and T5 in group C, and at T1 and T4 in groups D1 and D2.
Perioperative DEX infusion at 0.04 µg/kg/h resulted in better and faster recovery of gastrointestinal function and a more favorable analgesic effect without additional adverse effects in patients who underwent laparoscopic nephrectomy.
This study suggests a new method for postoperative intestinal function recovery.
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujisawa M, Heidenreich A S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Deng WW, Lan M, Peng AF, Chen T, Li ZQ, Liu ZL, Liu JM. The risk factors for postoperative ileus following posterior thoraco-lumbar spinal fusion surgery. Clin Neurol Neurosurg. 2019;184:105411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Petca A, Borislavschi A, Dumitrascu MC, Sandru F, Geoarsa M, Petca RC. Postoperative Ileus Complicated with Incomplete Evisceration after Hysterectomy for Benign Pathology. Chirurgia (Bucur). 2020;115:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87:1480-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Tevis SE, Carchman EH, Foley EF, Harms BA, Heise CP, Kennedy GD. Postoperative Ileus--More than Just Prolonged Length of Stay? J Gastrointest Surg. 2015;19:1684-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, Meurette G. Postoperative ileus: Pathophysiology, incidence, and prevention. J Visc Surg. 2016;153:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 6. | Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. 2009;15:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. J Am Coll Surg. 2010;210:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Chapuis PH, Bokey L, Keshava A, Rickard MJ, Stewart P, Young CJ, Dent OF. Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg. 2013;257:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr. 2015;34:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 10. | Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol. 2014;49:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Wormser C, Runge JJ. Advances in Laparoscopic Surgery. Vet Clin North Am Small Anim Pract. 2016;46:63-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Best LM, Mughal M, Gurusamy KS. Laparoscopic vs open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016;3:CD011389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1195] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Zhao X, Wang Y. Dexmedetomidine: a review of applications for cardiac surgery during perioperative period. J Anesth. 2015;29:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Gero D, Gié O, Hübner M, Demartines N, Hahnloser D. Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbecks Arch Surg. 2017;402:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Xie Y, Xue Y, Wang B, Jin X. Effects of ultrasound-guided stellate ganglion block on autonomic nervous function during CO2-pneumoperitoneum: A randomized double-blind control trial. J Clin Anesth. 2016;32:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Rasmussen JP, Dauchot PJ, DePalma RG, Sorensen B, Regula G, Anton AH, Gravenstein JS. Cardiac function and hypercarbia. Arch Surg. 1978;113:1196-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 426] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. 2001;182:3S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Wehner S, Straesser S, Vilz TO, Pantelis D, Sielecki T, de la Cruz VF, Hirner A, Kalff JC. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology. 2009;136:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Yu T, Liu D, Gao M, Yang P, Zhang M, Song F, Zhang X, Liu Y. Dexmedetomidine prevents septic myocardial dysfunction in rats via activation of α7nAChR and PI3K/Akt- mediated autophagy. Biomed Pharmacother. 2019;120:109231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Rohloff M, Cicic A, Christensen C, Maatman TK, Lindberg J, Maatman TJ. Reduction in postoperative ileus rates utilizing lower pressure pneumoperitoneum in robotic-assisted radical prostatectomy. J Robot Surg. 2019;13:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Cioccari L, Luethi N, Bailey M, Shehabi Y, Howe B, Messmer AS, Proimos HK, Peck L, Young H, Eastwood GM, Merz TM, Takala J, Jakob SM, Bellomo R; ANZICS Clinical Trials Group and the SPICE III Investigators. The effect of dexmedetomidine on vasopressor requirements in patients with septic shock: a subgroup analysis of the Sedation Practice in Intensive Care Evaluation [SPICE III] Trial. Crit Care. 2020;24:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Peng Y, Zhu H, Chen H, Zhu Z, Zhou H, Zhang S, Gao L, Shi L, Li X, Luo Z. Dexmedetomidine attenuates acute paroxysmal sympathetic hyperactivity. Oncotarget. 2017;8:69012-69019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Kim NY, Han DW, Koh JC, Rha KH, Hong JH, Park JM, Kim SY. Effect of Dexmedetomidine on Heart Rate-Corrected QT and Tpeak-Tend Intervals During Robot-Assisted Laparoscopic Prostatectomy With Steep Trendelenburg Position: A Prospective, Randomized, Double-Blinded, Controlled Study. Medicine (Baltimore). 2016;95:e3645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66:2321-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Gelman D, Gelmanas A, Urbanaitė D, Tamošiūnas R, Sadauskas S, Bilskienė D, Naudžiūnas A, Širvinskas E, Benetis R, Macas A. Role of Multimodal Analgesia in the Evolving Enhanced Recovery after Surgery Pathways. Medicina (Kaunas). 2018;54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | de Boer HD, Detriche O, Forget P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Abdel Hamid MHE. Intravenous Dexmedetomidine Infusion Compared with that of Fentanyl in Patients Undergoing Arthroscopic Shoulder Surgery under General Anesthesia. Anesth Essays Res. 2017;11:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Sharma R, Gupta R, Choudhary R, Singh Bajwa SJ. Postoperative Analgesia with Intravenous Paracetamol and Dexmedetomidine in Laparoscopic Cholecystectomy Surgeries: A Prospective Randomized Comparative Study. Int J Appl Basic Med Res. 2017;7:218-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 34. | Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 35. | Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth. 2001;87:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Kabukçu HK, Sahin N, Temel Y, Titiz TA. Hemodynamics in coronary artery bypass surgery: effects of intraoperative dexmedetomidine administration. Anaesthesist. 2011;60:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |