Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5909
Peer-review started: March 30, 2021
First decision: April 28, 2021
Revised: May 4, 2021
Accepted: June 7, 2021
Article in press: June 7, 2021
Published online: July 26, 2021
Processing time: 112 Days and 18.6 Hours
Experimental evidence has indicated the benefits of statins for the treatment of postoperative delirium. Previously, clinical trials did not reach definite conclu
To evaluate whether perioperative rosuvastatin treatment reduces the incidence of delirium and improves clinical outcomes.
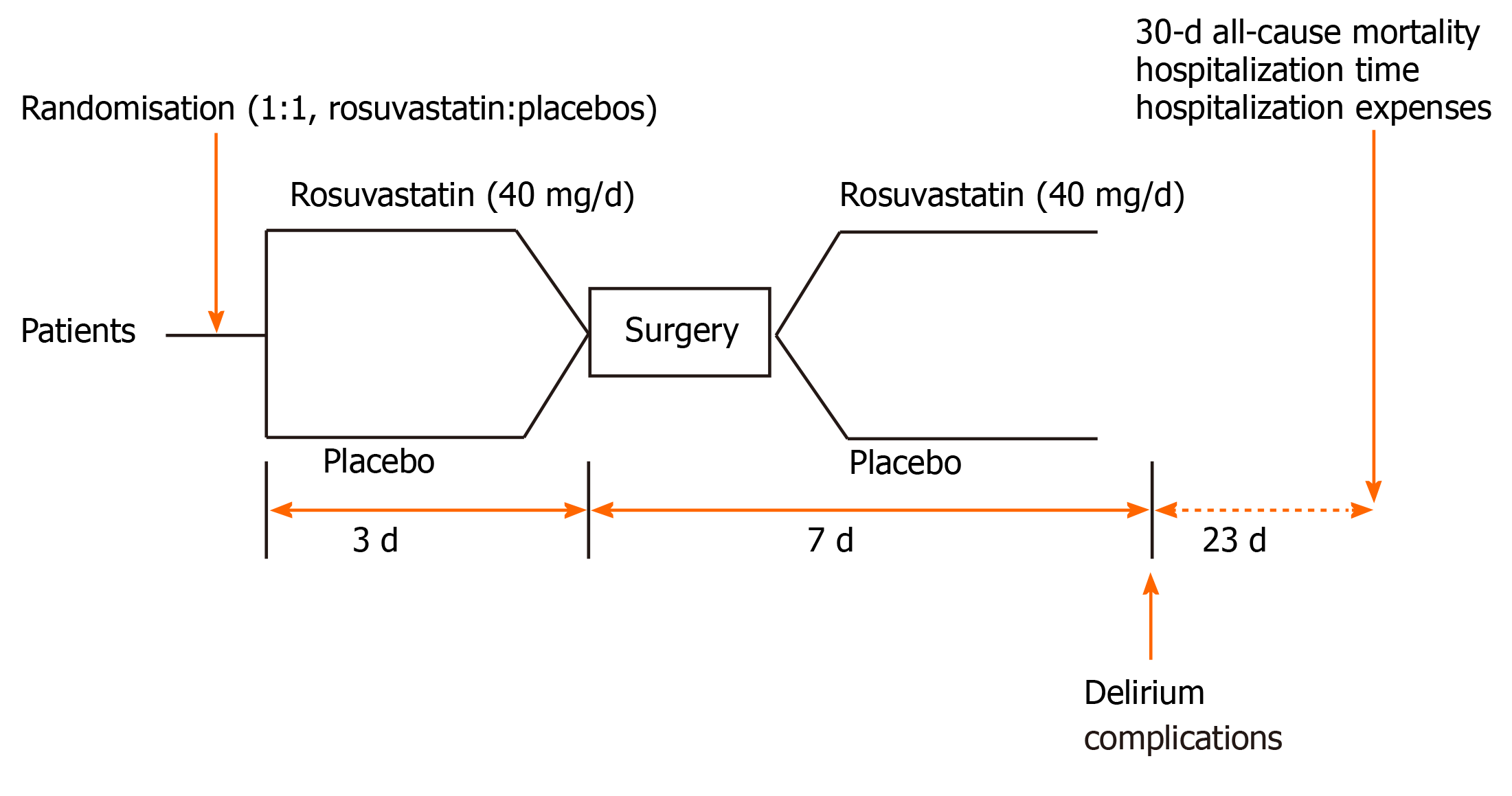
This randomized, double-blind, and placebo-controlled trial was conducted in a single center in Jiangsu, China. This study enrolled patients aged greater than 60 years who received general anesthesia during elective operations and provided informed consent. A computer-generated randomization sequence (in a 1:1 ratio) was used to randomly assign patients to receive either rosuvastatin (40 mg/d) or placebo. Participants, care providers, and investigators were all masked to group assignments. The primary endpoint was the incidence of delirium, which was assessed twice daily with the Confusion Assessment Method during the first 7 postoperative days. Analyses were performed on intention-to-treat and safety populations.
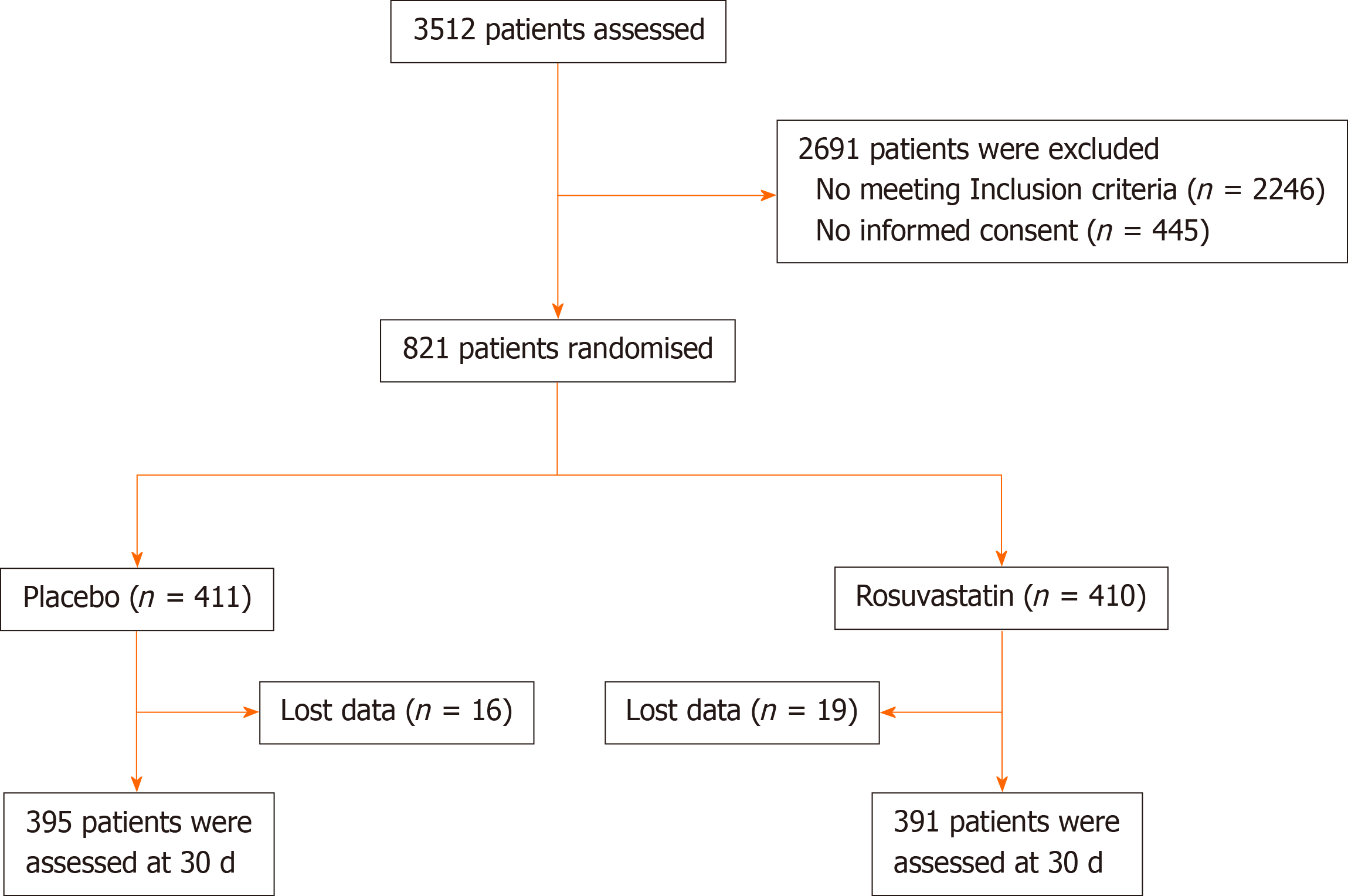
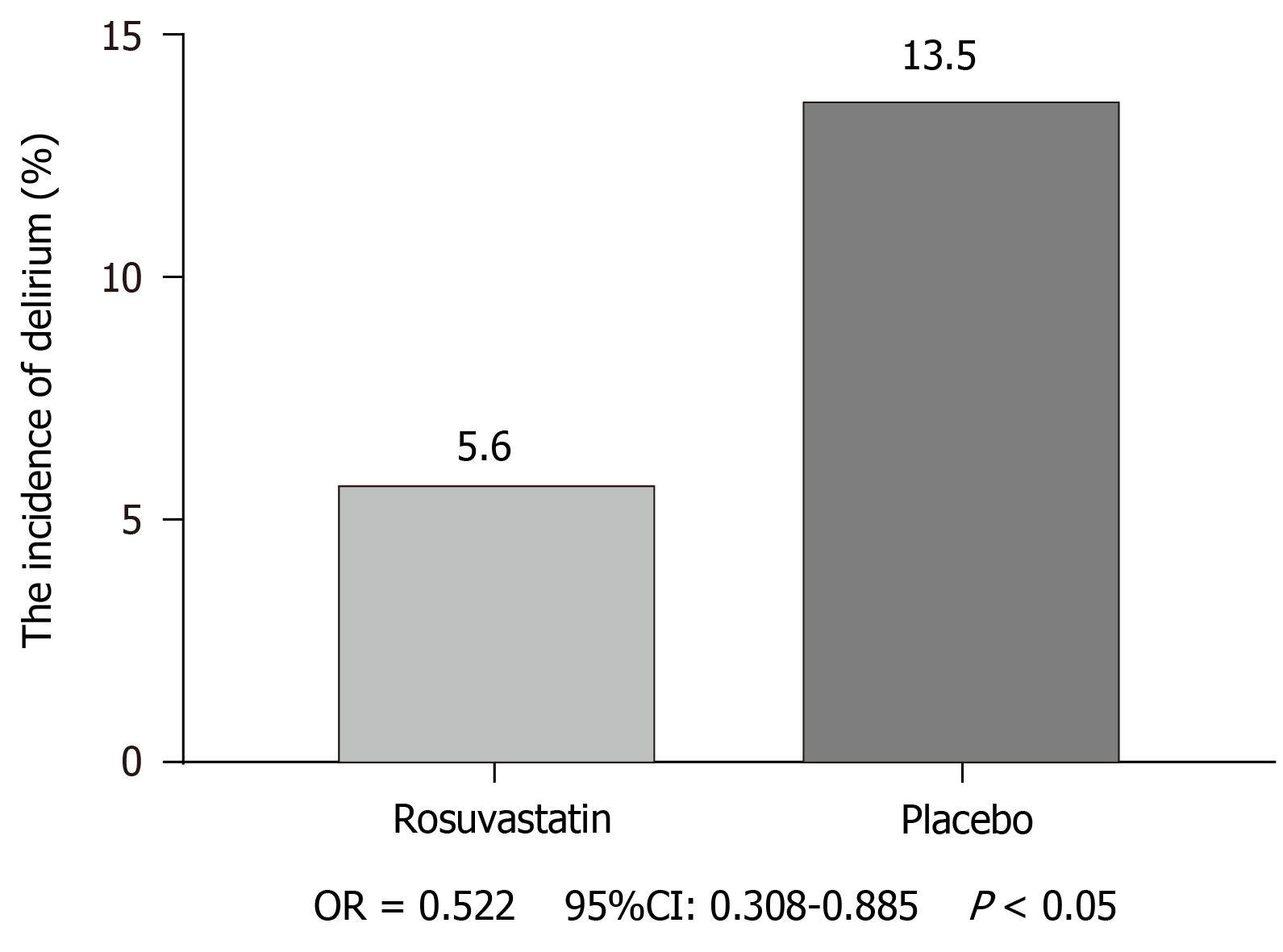
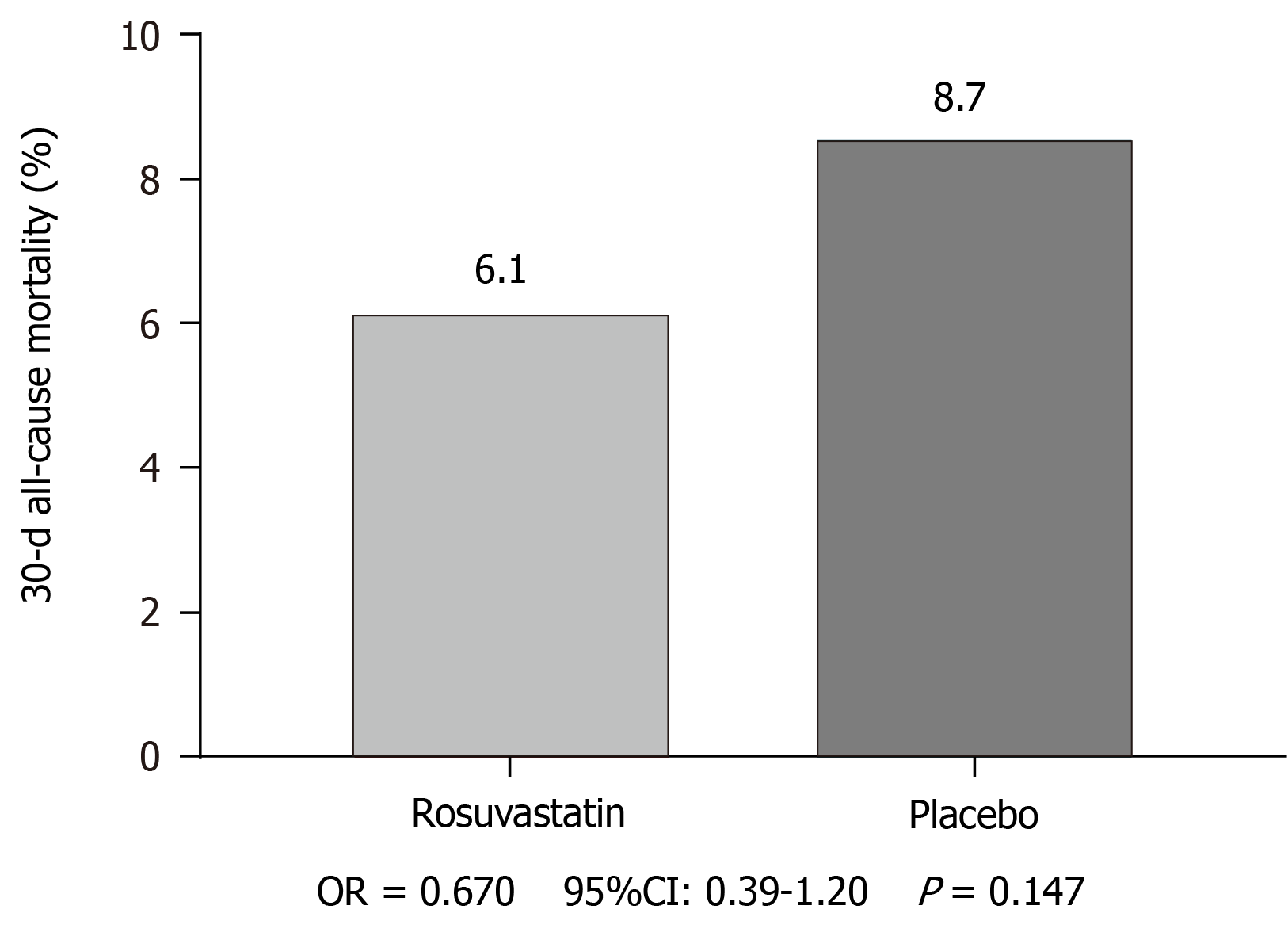
Between January 1, 2017 and January 1, 2020, 3512 patients were assessed. A total of 821 patients were randomly assigned to receive either placebo (n = 411) or rosuvastatin (n = 410). The incidence of postoperative delirium was significantly lower in the rosuvastatin group [23 (5.6%) of 410 patients] than in the placebo group {42 (13.5%) of 411 patients [odds ratios (OR) = 0.522, 95% confidence interval (CI): 0.308-0.885; P < 0.05]}. No significant difference in 30-d all-cause mortality (6.1% vs 8.7%, OR = 0.67, 95%CI: 0.39-1.2, P = 0.147) was observed between the two groups. Rosuvastatin decreased the hospitalization time (13.8 ± 2.5 vs 14.2 ± 2.8, P = 0.03) and hospitalization expenses (9.3 ± 2.5 vs 9.8 ± 2.9, P = 0.007). No significant differences in abnormal liver enzymes (9.0% vs 7.1%, OR = 1.307, 95%CI: 0.787-2.169, P = 0.30) or rhabdomyolysis (0.73% vs 0.24%, OR = 3.020, 95%CI: 0.31-29.2, P = 0.37) were observed between the two groups.
The current study suggests that perioperative rosuvastatin treatment reduces the incidence of delirium after an elective operation under general anesthesia. However, the evidence does not reveal that rosuvastatin improves clinical out
Core Tip: Postoperative delirium is a severe clinical syndrome, which may induce long-term cognitive impairment and death/disability. This randomized, double-blind, and placebo-controlled trial indicated that perioperative rosuvastatin treatment can significantly decrease the incidence of delirium after an elective operation under general anesthesia, and reduce the hospitalization time and hospitalization expenses, but did not improve the clinical outcomes.
- Citation: Xu XQ, Luo JZ, Li XY, Tang HQ, Lu WH. Effects of perioperative rosuvastatin on postoperative delirium in elderly patients: A randomized, double-blind, and placebo-controlled trial. World J Clin Cases 2021; 9(21): 5909-5920
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5909.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5909
Delirium is a severe clinical syndrome characterized by a temporary organic mental disorder, acute brain dysfunction, changes in cognition, and disturbances in orientation, which may induce long-term cognitive impairment, death/disability, and an increased length of hospital stay and costs[1]. Inouye et al[2] reported that the incidence of postoperative delirium was 11%-51% after surgery, and the rate is further increased in elderly patients. In the United States, more than 2.6 million elderly patients experience delirium each year, accounting for an estimated annual health care expenditure of more than $164 billion. Additionally, delirium or postoperative delirium may increase the long-term risk of dementia and mortality[3]. In the past 20 years, the treatment of delirium in elderly patients has become a substantial challenge; haloperidol and ziprasidone are the main drugs used to treat delirium, but recent studies have indicated that haloperidol and ziprasidone do not prevent delirium or reduce the duration of delirium[4,5]. Avidan et al[6] reported that ketamine, a widely used anti-delirium drug, also does not decrease delirium in elderly patients after major surgery, and on the contrary, it might cause harm by inducing negative experiences. Currently, specific treatments or drugs for delirium are unavailable.
Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are widely used to treat cardiovascular disorders as cholesterol-lowering medications. According to recent studies, statins exert pleiotropic effects, including anti-inflammatory and antioxidative stress effects, inhibit platelet aggregation, and promote neuroprotection[7-11]. As the neuroprotection-related molecular mechanism is similar to the molecular mechanism of delirium, statins may prevent delirium. A retrospective, single-center study that included 1132 vascular surgical patients confirmed that preoperative statins significantly decrease the incidence of posto
This randomized, double-blind, and parallel-arm placebo-controlled trial was conducted at a single center in Jiangsu, China between January 1, 2017 and January 1, 2020, and 3512 patients were assessed. Anhui Medical University Affiliated with Wuxi Clinical College (904th Hospital of PLA) was the study center. The study was registered in chictr.org.cn with number ChiCTR-IPR-17011984 (registration date: July 13, 2017). The study was designed to assess the superiority of the intervention. The study protocol was approved by the Clinical Research Ethics Committees of Anhui Medical University Affiliated with Wuxi Clinical College (YXLL-2017-02). The study protocol received Ethics Committee approval from all participating centers. Written informed consent was obtained from patients whose competence was established by their accurate orientation for time, place, and person, as well as an understanding of the recruiter’s description of the trial or otherwise from their next of kin or legal representative.
All patients were randomly assigned (1:1) to receive 40 mg/d rosuvastatin or placebo within 7 d after surgery and 3 d before surgery (Figure 1). Rosuvastatin or placebo was administered orally. Delirium was assessed twice daily with the Confusion Assessment Method (CAM) during the first 7 postoperative days. The final follow-up was 30 d after surgery.
Eligible patients were greater than 60 years old who underwent joint replacement surgery in the ICU. The inclusion criteria were: (1) Aged more than 60 years; (2) Able to be randomized and receive rosuvastatin or placebo within 10 d during the perioperative period; and (3) Received general anesthesia and were admitted to the ICU. The exclusion criteria were: (1) Likely unsalvageable patients identified at admission; (2) High cholesterol levels combined with diabetes; (3) Brain injury or neurosurgery; (4) Severe sinus bradycardia; (5) Neurological disease; (6) Abnormal liver enzymes, and patients with rhabdomyolysis and myopathy; (7) Patients with a history of mental illness and epilepsy; (8) Patients with severe lung disease and multiple organ dysfunction; and (9) Other reasons identified by the researchers.
The patients were randomized into the rosuvastatin group (40 mg/d, 10 d) or the placebo group using fixed randomization schemes with random numbers (in a 1:1 ratio) according to a computer system created. An independent statistician who was blinded in the trial finished this process. All drugs were identical in appearance and packaged in identical medical envelopes, and the medication was given by the nurse according to the randomization sequence. To ensure patient safety, the group allocation could be unblinded with two on-call experts or pharmacist if severe adverse events or any unexpected deterioration in the patient’s clinical status occurred, and all situations need to be documented in the case report forms. Patients and all investiga
No premedication was administered. All patients underwent a standard preoperative evaluation and were assigned an American Society of Anesthesiologists classification based on their medical comorbidities. All patients received the same general anesthesia protocol. Intravenous (IV) midazolam (1-2 mg) and fentanyl (50-100 mcg) were administered for preoperative sedation, and anesthesia was maintained with 3 to 6 mcg/kg IV fentanyl. All patients were routinely monitored with electrocardiography, noninvasive blood pressure, pulse oximetry (SpO2), and bispectral index. Radial arterial pressure and central venous pressures were monitored as necessary.
After surgery, the patients were admitted to the ICU, and all patients received the same management. Patients in the treatment group received 40 mg/d rosuvastatin, while the placebo group received an equal amount of starch. All study drugs (rosuvastatin and placebo) were provided by Zhejiang Jingxin Medicine Co, Ltd. (Zhejiang, China).
All investigators assessing the outcomes and collecting the data were blinded to treatment allocation. All investigators need to be trained before the study and not participate in the treatment of patients. The incidence of delirium at the first 7 d after surgery was the primary endpoint. The first postoperative evaluation of delirium was performed approximately 24 h after surgery twice daily[16,17]. Delirium was assessed using the CAM and the CAM for the ICU (CAM-ICU). Both the CAM and CAM-ICU detect four features of delirium: (1) Acute onset of mental status changes or a fluctuating course; (2) Inattention; (3) Disorganized thinking; and (4) An altered level of consciousness. A patient must display features 1 and 2, with either feature 3 or 4 to be diagnosed with delirium[18]. If patients died or discharged within 7 d after surgery, then the last delirium assessment was missing[16]. The secondary endpoints included all-cause 30-d mortality, length of stay in the ICU, the occurrence of nondelirium postoperative complications, and hospital costs.
The most common adverse events include abnormal liver enzyme levels, rhabdomyolysis, and myopathy. We evaluated all related adverse events and groups were unblinded if necessary.
The sample size was according to the previous study published in 2014; we set a type I error of 0.05, and 80% power, then 739 patients were required to detect a difference[19]. Further assuming a 10% loss to follow-up, 845 patients were enrolled. Clinical data of all patients were collected by specialized nurses and stored in a database. This study described the incidence and relative risk reduction of dichotomous variables for the rosuvastatin-treated group relative to the placebo group, with the corresponding 95% confidence interval (CI). Normally distributed continuous data are presented as the mean ± SD and were analyzed using the unpaired t-test. Non-normally distributed data were compared by an independent-samples Mann-Whitney U test. Categorical data were compared using the χ2 test, or continuity correction χ2 test. We calculated mean differences or risk ratios with two-sided 95%CIs. P < 0.05 indicated statistical significance. Statistical analyses were performed using IBM SPSS Statistics (version 18, SPSS, Chicago, IL, United States). This study did not perform an interim analysis. The Clinical Research Ethics Committee from the 904th Hospital of Joint Logistic Support Force of PLA was involved in overseeing the data.
Between January 1, 2017 and January 1, 2020, 3512 patients were assessed. Eight hundred and twenty-one patients were randomly assigned to receive either placebo (n = 411) or rosuvastatin (n = 410) (Figure 2). The two groups were similar in baseline characteristics without significant differences (Table 1). During the study period, no patients withdrew consent. Thirty-five patients (16 patients in the placebo group and 19 patients in the rosuvastatin group) were lost to follow-up or had clinical data collected at 30 d. In the final intention-to-treat analysis, 786 patients were included (Figure 2). The last randomized patient attended the final follow-up visit on October 31, 2020.
| Group | Place group | Rosuvastatin group | P value |
| Number of patients | 411 | 410 | |
| Age (mean ± SD) | 66.5 ± 5.3 | 66.3 ± 5.1 | 0.664 |
| Gender, n (%) | 0.243 | ||
| Male | 186(45.3) | 169 (41.2) | |
| Female | 225 (54.7) | 241 (58.8) | |
| History of hypertension, n (%) | 0.623 | ||
| Yes | 109 (26.5) | 115 (28.0) | |
| No | 302 (73.5) | 295 (72) | |
| History of diabetes, n (%) | 0.558 | ||
| Yes | 88 (21.4) | 81 (19.8) | |
| No | 323 (78.6) | 329 (80.2) | |
| Nicotine use, n (%) | 0.518 | ||
| Yes | 72 (17.5) | 79 (19.3) | |
| No | 339 (82.5) | 331 (80.7) | |
| Type of surgery, n (%) | > 0.05 | ||
| Thoracic operation | 77 (18.7) | 69 (16.8) | |
| Abdominal operation | 93 (22.6) | 105 (25.6) | |
| Orthopedic operation | 158 (38.4) | 142 (34.6) | |
| Gynecological operation | 44 (10.7) | 58 (14.4) | |
| Others | 39 (9.6) | 36 (8.6) | |
| Duration of anesthesia (min) | 114.0 ± 42.7 | 115.5 ± 42.2 | 0.625 |
| Blood transfusion during surgery, n (%) | 0.660 | ||
| Yes | 48 (11.7) | 52 (12.7) | |
| No | 363 (88.3) | 358 (87.3) | |
| Intraoperative medication, n (%) | > 0.05 | ||
| Midazolam | |||
| Fentanyl | 411 (100) | 410 (100) | |
| Propofol | 411 (100) | 410 (100) | |
| Atropine | 75 (18.2) | 79 (19.3) | |
| Postoperative analgesics (7 d), n (%) | > 0.05 | ||
| Diclofenac sodium | 104 (25.3) | 99 (24.1) | |
| Morphine | 85 (20.7) | 91 (22.2) | |
| Midazolam | 41 (10.0) | 57 (13.9) | |
| No | 181 (44.0) | 163 (39.8) |
A total of 3512 patients were enrolled in the present trial, of whom 821 were eligible for this study: 410 (50%) were assigned to the rosuvastatin group, and 411 (50%) were assigned to the placebo group. Patient demographics and baseline characteristics were not significantly different between the two groups. The general anesthetic medication, anesthesia time, and operative time were also similar between the two groups. Postoperative medication and management were also similar (Table 1).
The incidence of postoperative delirium was 23 (5.6%) of 410 patients in the rosuvastatin group, and 42 (13.5%) of 411 patients experienced delirium in the placebo group [odds ratios (OR) = 0.522, 95%CI: 0.308-0.885, P < 0.05; Figure 3].
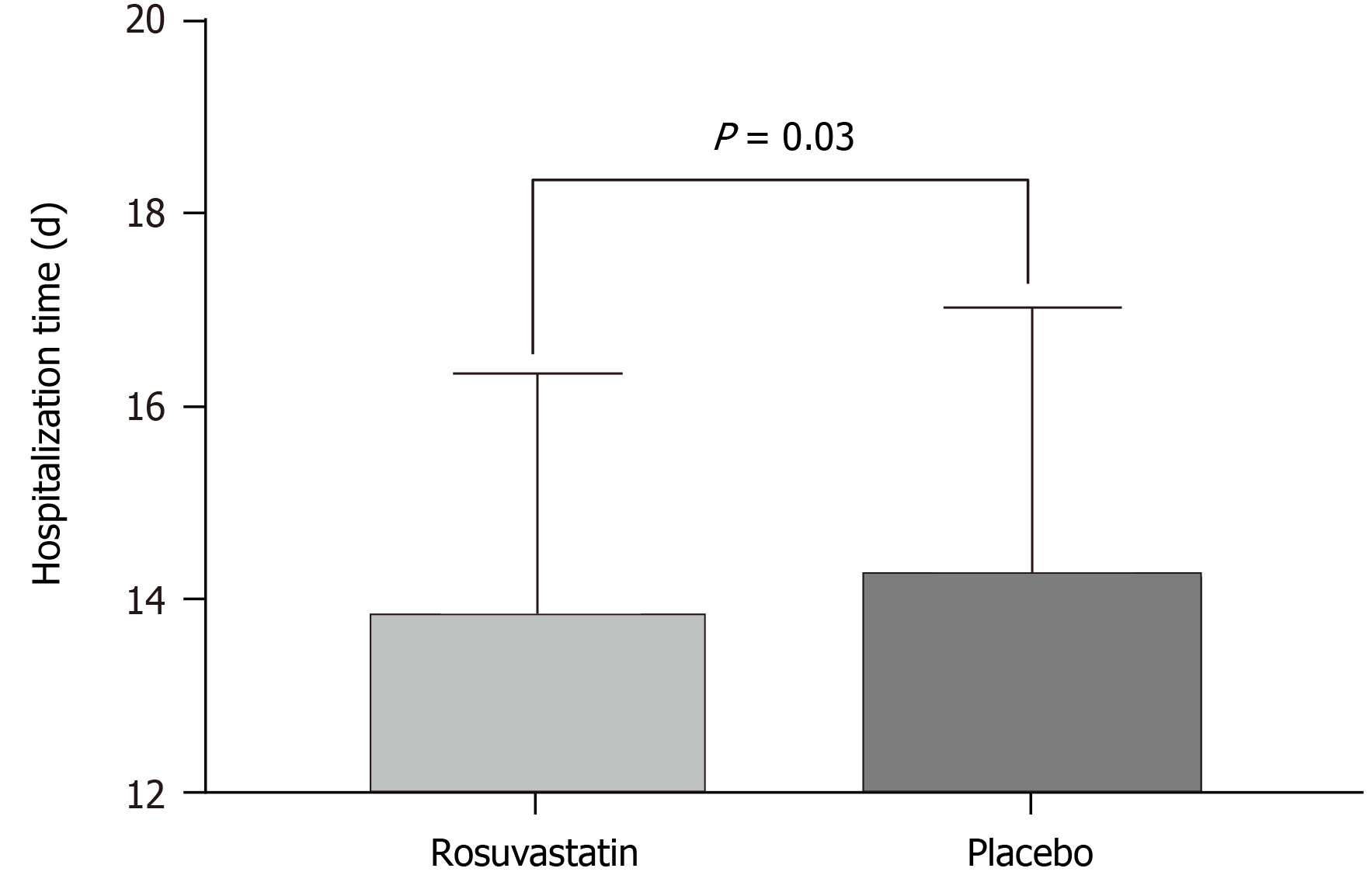
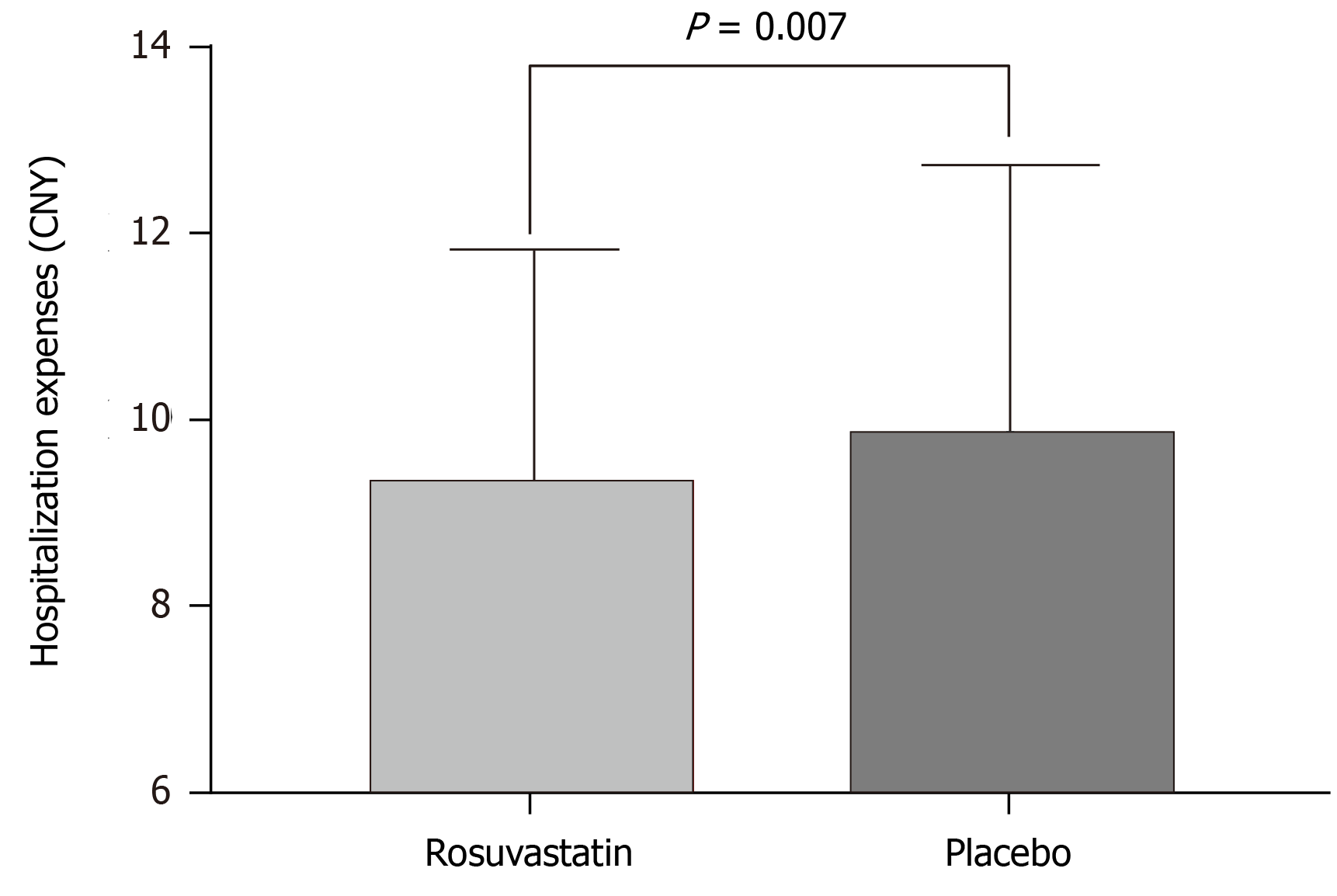
Many previous studies have reported that delirium increases 30-d all-cause mortality, hospitalization time, and hospitalization expenses. Thus, the present study also evaluated the differences between the two groups in 30-d all-cause mortality, hospitalization time, and hospitalization expenses. Regarding 30-d all-cause mortality, 24 (6.1%) of 391 patients in the rosuvastatin group and 35 (8.7%) of 395 in the placebo group died (OR = 0.67, 95%CI: 0.39-1.2, P = 0.147; Figure 4). Hospitalization time was significantly increased in the placebo group compared to that in the rosuvastatin group (13.8 ± 2.5 vs 14.2 ± 2.8, P = 0.03; Figure 5). Similarly, hospitalization expenses of the placebo group were significantly higher than those of the rosuvastatin group (9.3 ± 2.5 vs 9.8 ± 2.9, P = 0.007; Figure 6).
We evaluated drug-related complications, including abnormal liver enzyme levels and rhabdomyolysis. The occurrence of postoperative abnormal liver enzyme levels was not significantly different between the two groups (9.0% vs 7.1%, OR = 1.307, 95%CI: 0.787-2.169, P = 0.30; Table 2). The occurrence of rhabdomyolysis in the placebo group and rosuvastatin group showed no differences (0.73% vs 0.24%, OR = 3.020, 95%CI: 0.31-29.2, P = 0.37; Table 2). Although no statistically significant differences in abnormal liver enzyme levels or rhabdomyolysis were observed between the two groups, the incidence of postoperative complications in the rosuvastatin group was higher than that in the placebo group, which requires attention to avoid serious complications.
| Variable | Place group | Rosuvastatin group | P value |
| Number of patients | 411 | 410 | |
| Abnormal liver enzymes | 29 (7.1) | 37 (9.0) | 0.30 |
| Rhabdomyolysis | 1 (0.24) | 3 (0.73) | 0.37 |
As shown in the present study, rosuvastatin significantly decreased postoperative delirium in elderly surgical patients under general anesthesia. Meanwhile, rosuvastatin also reduced the length of the hospital stay and hospitalization expenses. Based on our data, rosuvastatin did not affect 30-d all-cause mortality. The incidences of postoperative complications, especially drug-related complications, including abnormal liver enzyme levels and rhabdomyolysis, were similar.
Our study reported that the incidence of postoperative delirium was 13.5% in placebo-treated patients and 5.6% in the rosuvastatin group, and the results were similar to those of a large number of previous studies[12,17,20,21]. Liu et al[22] performed a retrospective cohort study that included 361 elderly patients and reported that 19.9% of noncardiac surgery individuals developed postoperative delirium after surgery. Kant et al[23] also conducted a study on 413 patients and reported that the incidence of postoperative delirium was as high as 17%, and preoperative brain magnetic resonance imaging features may indicate a predisposition for developing delirium after major surgery. Humeidan et al[24] reported that the incidence of delirium was 23.0% in control participants, and the delirium rate in the intervention group was 14.4%. Thus, the incidence of postoperative delirium after general anesthesia is extremely high, and it has seriously affected the operative effect and long-term outcome. In addition, postoperative delirium is associated with high morbidity and mortality rates, worse operative effects, and worse long-term outcomes[2,25]. A previous meta-analysis reported that postoperative delirium increases the risk of a person experiencing poorer outcomes[26]. Additionally, postoperative delirium also leads to a longer hospitalization time and higher hospitalization expenses. Therefore, medication has now become the main treatment.
Many factors may lead to postoperative delirium, and delirium is usually a multifactorial disease[2,27]. Chen et al[27] reported that major surgery, drugs (sedatives or hypnotics), trauma (especially traumatic brain injury), coma, and sleep deprivation are the most important precipitating factors for delirium by performing a literature review and summarized the potential mechanisms of delirium, including systemic neuroinflammation, neurotransmitters, cerebral hypoperfusion, microth
According to Yu et al[28], simvastatin has the potential to be employed as a therapy for depression associated with neuroinflammation by suppressing the activation of microglia and decreasing the expression of proinflammatory cytokines in the hippocampus. Statins reduce the glutamate concentration by upregulating nitric oxide synthase. Jiang et al[29] also found that statins inhibit 5-HT and 5-HTT expression in patients with chronic obstructive pulmonary disease and pulmonary artery hypertension; then, these drugs may ameliorate delirium by regulating neurotransmitters. Many previous studies have indicated that statins alleviate cerebral vasospasm and cerebral perfusion, prevent endothelial dysfunction, and ameliorate neuronal apoptosis and brain injury[7-11]. Thus, statins may prevent postoperative delirium, and reasonable hypotheses on potential mechanisms have been proposed.
Redelmeier et al[30] first reported a large sample retrospective cohort study which enrolled 284158 consecutive patients aged > 65 years who were admitted for elective surgery, and found that statins may increase the risk of postoperative delirium among elderly patients. Katznelson et al[31] reported another study of 1059 patients and indicated that preoperative statins may reduce the odds of postoperative delirium after cardiac surgery with cardiopulmonary bypass. Trezzi et al[32] reported a retrospective cohort analysis enrolling 12741 patients who underwent cardiac surgery, and the results showed no significant difference between the statin group and the nonstatin group after the cardiac operation. Oh et al[17] retrospectively reviewed a large cohort of patients who underwent total knee replacement under spinal anesthesia, and continuous perioperative statin use was shown to reduce the risk of delirium after total knee arthroplasty under spinal anesthesia. However, all of these studies were retrospective cohort studies and ultimately did not report uniform and satisfactory results. For postoperative delirium, high evidence-based medicine is not available, and more prospective randomized controlled studies are needed. In recent studies, most large prospective randomized controlled studies have explored the effect of early administration of statins on the prevention and treatment of delirium in critically ill patients or patients undergoing mechanical ventilation[15,19,21,33]. Thus, no related large-sample prospective randomized controlled trials have explored the role of perioperative statins in preventing postoperative delirium in elderly patients who received general anesthesia.
The present study is the first to show that perioperative rosuvastatin treatment potentially reduced the incidence of delirium in patients after an elective operation under general anesthesia and decreased the length of stay in the ICU and hospital costs. Additionally, rosuvastatin did not increase drug-related complications. The study was masked and enrolled 821 patients; most patients completed the follow-up. Another strength of this trial was the double-blind, randomized, and placebo-controlled design (the study was registered at http://www.chictr.org.cn, number: ChiCTR-IPR-17011984). This study has several limitations that need to be improved. Additional clinical factors should be examined, such as sleep quality and pain. The follow-up time in this study was too short and long-term follow-up is required; additionally, the effects on long-term follow-up[34] are also unknown. This study administered a single dose of statins (40 mg/d), and the conventional low dose may not work well.
In the present study, the administration of perioperative rosuvastatin may reduce the incidence of delirium in elderly patients after an elective operation under general anesthesia and decrease the length of stay in the ICU and hospital costs. No benefit in terms of the clinical outcome or 30-d all-cause mortality was observed after perioperative rosuvastatin treatment. The effects of longer-term or larger doses of rosuvastatin remain unclear. The effects of longer-term follow-up are also unknown. Further investigation of elderly patients undergoing an elective operation under general anesthesia and treated with different doses of rosuvastatin is needed to fully understand the potential usefulness of rosuvastatin for preventing postoperative delirium.
Our previous basic research study found that statins exert pleiotropic effects, including anti-inflammatory and antioxidative stress effects, inhibition of platelet aggregation, and promotion of neuroprotection. The anti-delirium role of statins was not clearly determined in recent studies.
Delirium is a severe clinical syndrome characterized by a temporary organic mental disorder, which may induce long-term cognitive impairment, death/disability, and increased length of hospital stay and costs. An increasing number of basic research studies indicate that statins play an important role in postoperative delirium. Our preliminary clinical experiment also revealed that perioperative rosuvastatin had potential value as a treatment for postoperative delirium.
This randomized, double-blind, and placebo-controlled trial explored the anti-delirium effect of perioperative rosuvastatin and compared the clinical efficacy and economic efficiency, as well as the safety of perioperative rosuvastatin, in elderly patients who underwent surgery under general anesthesia.
This randomized, double-blind, and placebo-controlled trial was conducted in a single center and enrolled patients aged more than 60 years who underwent an elective operation under general anesthesia. The patients were randomly assigned to receive either rosuvastatin (40 mg/d) or placebo.
The final analysis included 411 patients in the placebo group and 410 patients in the rosuvastatin group. The incidence of postoperative delirium was significantly lower in the rosuvastatin group than in the placebo group (P < 0.05). No significant difference in 30-d all-cause mortality was observed between the two groups (P > 0.05). Rosuvastatin decreased the hospitalization time and hospitalization expenses (P < 0.057). No significant differences in abnormal liver enzyme levels or rhabdomyolysis were observed between the two groups (P > 0.05).
Perioperative rosuvastatin treatment potentially reduces the incidence of delirium after an elective operation under general anesthesia, without a higher incidence of drug-related complications.
In the future, a large prospective randomized investigation will definitively address the effect of rosuvastatin on postoperative delirium in elderly patients undergoing an elective operation under general anesthesia.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hori T S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO Jr, Jones RN, Marcantonio ER, Inouye SK. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 2. | Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 2313] [Article Influence: 210.3] [Reference Citation Analysis (0)] |
| 3. | Redelmeier DA, Manzoor F, Thiruchelvam D. Association Between Statin Use and Risk of Dementia After a Concussion. JAMA Neurol. 2019;76:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ, Khan B, Pisani MA, Hyzy RC, Schmidt GA, Schweickert WD, Hite RD, Bowton DL, Masica AL, Thompson JL, Chandrasekhar R, Pun BT, Strength C, Boehm LM, Jackson JC, Pandharipande PP, Brummel NE, Hughes CG, Patel MB, Stollings JL, Bernard GR, Dittus RS, Ely EW; MIND-USA Investigators. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med. 2018;379:2506-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 5. | van den Boogaard M, Slooter AJC, Brüggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW, Van der Voort PHJ, Houterman S, van der Hoeven JG, Pickkers P; REDUCE Study Investigators; van der Woude MCE, Besselink A, Hofstra LS, Spronk PE, van den Bergh W, Donker DW, Fuchs M, Karakus A, Koeman M, van Duijnhoven M, Hannink G. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. JAMA. 2018;319:680-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM, Downey RJ, Yulico H, Noh GJ, Lee YH, Waszynski CM, Arya VK, Pagel PS, Hudetz JA, Muench MR, Fritz BA, Waberski W, Inouye SK, Mashour GA; PODCAST Research Group. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Chen JH, Yang LK, Chen L, Wang YH, Wu Y, Jiang BJ, Zhu J, Li PP. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of AQP4 expression in rabbits. Int J Mol Med. 2016;37:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Chen J, Li M, Zhu X, Chen L, Yang S, Zhang C, Wu T, Feng X, Wang Y, Chen Q. Atorvastatin reduces cerebral vasospasm and infarction after aneurysmal subarachnoid hemorrhage in elderly Chinese adults. Aging (Albany NY). 2020;12:2939-2951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Chen JH, Wu T, Xia WY, Shi ZH, Zhang CL, Chen L, Chen QX, Wang YH. An early neuroprotective effect of atorvastatin against subarachnoid hemorrhage. Neural Regen Res. 2020;15:1947-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Chen JH, Wu T, Yang LK, Chen L, Zhu J, Li PP, Hu X, Wang YH. Protective effects of atorvastatin on cerebral vessel autoregulation in an experimental rabbit model of subarachnoid hemorrhage. Mol Med Rep. 2018;17:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Chen J, Zhang C, Yan T, Yang L, Wang Y, Shi Z, Li M, Chen Q. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of pyroptosis and neuroinflammation. J Cell Physiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Lee DS, Lee MY, Park CM, Kim DI, Kim YW, Park YJ. Preoperative statins are associated with a reduced risk of postoperative delirium following vascular surgery. PLoS One. 2018;13:e0192841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Martínez-Comendador J, Alvarez JR, Sierra J, Teijeira E, Adrio B. Preoperative statin therapy in cardiac surgery is more effective in patients who display preoperative activation of the inflammatory system. Tex Heart Inst J. 2013;40:42-49. [PubMed] |
| 14. | Zeng ZW, Zhang YN, Lin WX, Zhang WQ, Luo R. A meta-analysis of pharmacological neuroprotection in noncardiac surgery: focus on statins, lidocaine, ketamine, and magnesium sulfate. Eur Rev Med Pharmacol Sci. 2018;22:1798-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, Morris PE, Mendez-Tellez PA, Ely EW, Hopkins RO. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 16. | Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 533] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 17. | Oh TK, Park HY, Shin HJ, Jeon YT, Do SH, Hwang JW. The Role of Perioperative Statin Use in the Prevention of Delirium After Total Knee Replacement Under Spinal Anesthesia. J Arthroplasty 2018; 33: 3666-3671. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Xuan Y, Fan R, Chen JH, Wang YH, Wu JY, Yang JJ, Luo YC. Effects of dexmedetomidine for postoperative delirium after joint replacement in elderly patients: a randomized, double-blind, and placebo-controlled trial. Int J Clin Exp Med. 2018;11:13147-13157. |
| 19. | Page VJ, Davis D, Zhao XB, Norton S, Casarin A, Brown T, Ely EW, McAuley DF. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med. 2014;189:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Burry L, Hutton B, Williamson DR, Mehta S, Adhikari NK, Cheng W, Ely EW, Egerod I, Fergusson DA, Rose L. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9:CD011749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Page VJ, Casarin A, Ely EW, Zhao XB, McDowell C, Murphy L, McAuley DF. Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2017;5:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Liu H, Dai M, Guan H, Gao X, Zhou Y, Sun X, Zhou J, Hu X, Li X, Song Y, Han Y, Cao J. Preoperative Prognostic Nutritional Index Value is Related to Postoperative Delirium in Elderly Patients After Noncardiac Surgery: A Retrospective Cohort Study. Risk Manag Healthc Policy. 2021;14:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Kant IMJ, de Bresser J, van Montfort SJT, Mutsaerts HJMM, Witkamp TD, Buijsrogge M, Spies C, Hendrikse J, Slooter AJC. Preoperative brain MRI features and occurrence of postoperative delirium. J Psychosom Res. 2021;140:110301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Humeidan ML, Reyes JC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E, Zuleta-Alarcon A, Otey A, Abdel-Rasoul M, Bergese SD. Effect of Cognitive Prehabilitation on the Incidence of Postoperative Delirium Among Older Adults Undergoing Major Noncardiac Surgery: The Neurobics Randomized Clinical Trial. JAMA Surg. 2021;156:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA. 2017;318:1161-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2017;29:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 27. | Chen J, Wang Y, Hu X, Li M, Xiong K, Zhang Z, Chen Q. The role of statins in the management of delirium: Recent advances. CNS Neurol Disord Drug Targets. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Yu XB, Zhang HN, Dai Y, Zhou ZY, Xu RA, Hu LF, Zhang CH, Xu HQ, An YQ, Tang CR, Lin GY. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord. 2019;245:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Jiang X, Yuan L, Li P, Wang J, Wang P, Zhang L, Sun B, Sun W. Effect of Simvastatin on 5-HT and 5-HTT in a Rat Model of Pulmonary Artery Hypertension. Cell Physiol Biochem. 2015;37:1712-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Redelmeier DA, Thiruchelvam D, Daneman N. Delirium after elective surgery among elderly patients taking statins. CMAJ. 2008;179:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Katznelson R, Djaiani GN, Borger MA, Friedman Z, Abbey SE, Fedorko L, Karski J, Mitsakakis N, Carroll J, Beattie WS. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Trezzi M, Blackstone EH, Sun Z, Li L, Sabik JF 3rd, Lytle BW, Gordon SM, Koch CG. Statin therapy is associated with fewer infections after cardiac operations. Ann Thorac Surg. 2013;95:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Morandi A, Hughes CG, Thompson JL, Pandharipande PP, Shintani AK, Vasilevskis EE, Han JH, Jackson JC, Laskowitz DT, Bernard GR, Ely EW, Girard TD. Statins and delirium during critical illness: a multicenter, prospective cohort study. Crit Care Med. 2014;42:1899-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |