Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5822
Peer-review started: March 21, 2021
First decision: April 29, 2021
Revised: May 10, 2021
Accepted: May 26, 2021
Article in press: May 26, 2021
Published online: July 26, 2021
Processing time: 121 Days and 14.1 Hours
Tissue acquisition from subepithelial lesions is often attempted by endoscopic ultrasound (EUS)-sampling as conventional endoscopic biopsy usually fails to reach deeper layers of the gastrointestinal wall.
To investigate the utilisation, safety and diagnostic yield of an intensified “bite-on-bite” tunnel biopsy technique.
In this retrospective cohort study, all patients presenting with subepithelial masses in the upper gastrointestinal tract from March 2013 to July 2019 were included. Data were analysed for size and location of the subepithelial mass, use of intensified tunnel biopsy protocol (more than 10 double bite-on-bite biopsies) or superficial conventional biopsies, histology and imaging results, occurrence of readmission and adverse events after endoscopy.
Two hundred and twenty-nine patients with subepithelial lesions were included. Superficial conventional biopsies were taken in 117 patients and were diagnostic only in one lipoma (0.9 %). Tunnel biopsies taken in 112/229 (48.9%) patients were significantly more likely to provide histological diagnosis (53.6%; P < 0.001). For lesions ≥ 10mm the diagnostic yield of tunnel biopsies further increased to 41/67 (61.2%). No immediate or delayed complications were reported. Only 8 of the 51 endoscopists (15.7%) regularly attempted tunnel biopsies.
Tunnel biopsy is a simple, safe and efficient but underutilised diagnostic modality for tissue acquisition in subepithelial masses. It should be routinely attempted at the initial endoscopy.
Core Tip: Subepithelal lesions are found relatively often on cross sectional imaging or during endoscopy. Conventional endoscopic biopsies will not reveal the histological diagnosis in most cases. Endoscopic ultrasound (EUS) with fine needle aspiration or biopsy allows the diagnosis in 50%-90% but requires specialist skills and equipment and is expensive. Tunnel biopsies taken in simple bite-on-bite technique allow histological diagnosis in more than 50% of subepithelial lesions. If tunnel biopsies are taken at the index endoscopy, further follow-up endoscopies and EUS investigations can be avoided in many cases.
- Citation: Koutsoumpas A, Perera R, Melton A, Kuker J, Ghosh T, Braden B. Tunneled biopsy is an underutilised, simple, safe and efficient method for tissue acquisition from subepithelial tumours. World J Clin Cases 2021; 9(21): 5822-5829
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5822.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5822
Subepithelial masses within the gastrointestinal wall are relatively often encountered as protuberances with normal overlying mucosa during endoscopy of the upper gastrointestinal tract or are detected incidentally by cross sectional imaging. As these lesions are covered by the epithelium and located in the submucosa or muscular layers, conventional biopsy forceps techniques rarely provide a histological diagnosis.
Endoscopic ultrasound (EUS) can further characterise the subepithelial lesions (SEL) by its echogenicity, shape, vascularity or layer of origin but often it will not be possible to provide a definitive diagnosis based only on endosonographic criteria. EUS can also differentiate mural masses from extrinsic indentation by surrounding organs or can identify non-tumorous structures such as varices and cysts which might mimic a subepithelial solid mass.
The differential diagnosis of subepithelial lesions in the wall of the upper gastrointestinal tract encompasses benign, premalignant and malignant entities and therefore might require completely different management depending on the histology. Lipoma and leiomyoma have almost no malignant potential and pancreatic rests or duplication cysts will also not need any further treatment. However, neuroendocrine tumours or gastrointestinal stroma tumours (GIST) would require observation or resection due to the malignant potential. More rarely, metastasis to the stomach can also present as a subepithelial lesion[1].
Appropriate management of subepithelial masses will often require tissue acquisition and that can be challenging for the endoscopist. EUS fine needle aspiration (EUS-FNA) has a relatively low diagnostic yield, especially in lesions of less than 1 cm[2-4]. In a meta-analysis including 987 attempts using EUS-FNA and –FNB (fine needle biopsy), the diagnostic rate of EUS-guided needle sampling was only 59.9%[4].
In this retrospective single center study, we investigated the utilisation, diagnostic yield and safety of an intensified bite-on-bite tunnel biopsy technique using conventional biopsy forceps compared to the conventional superficial biopsy method.
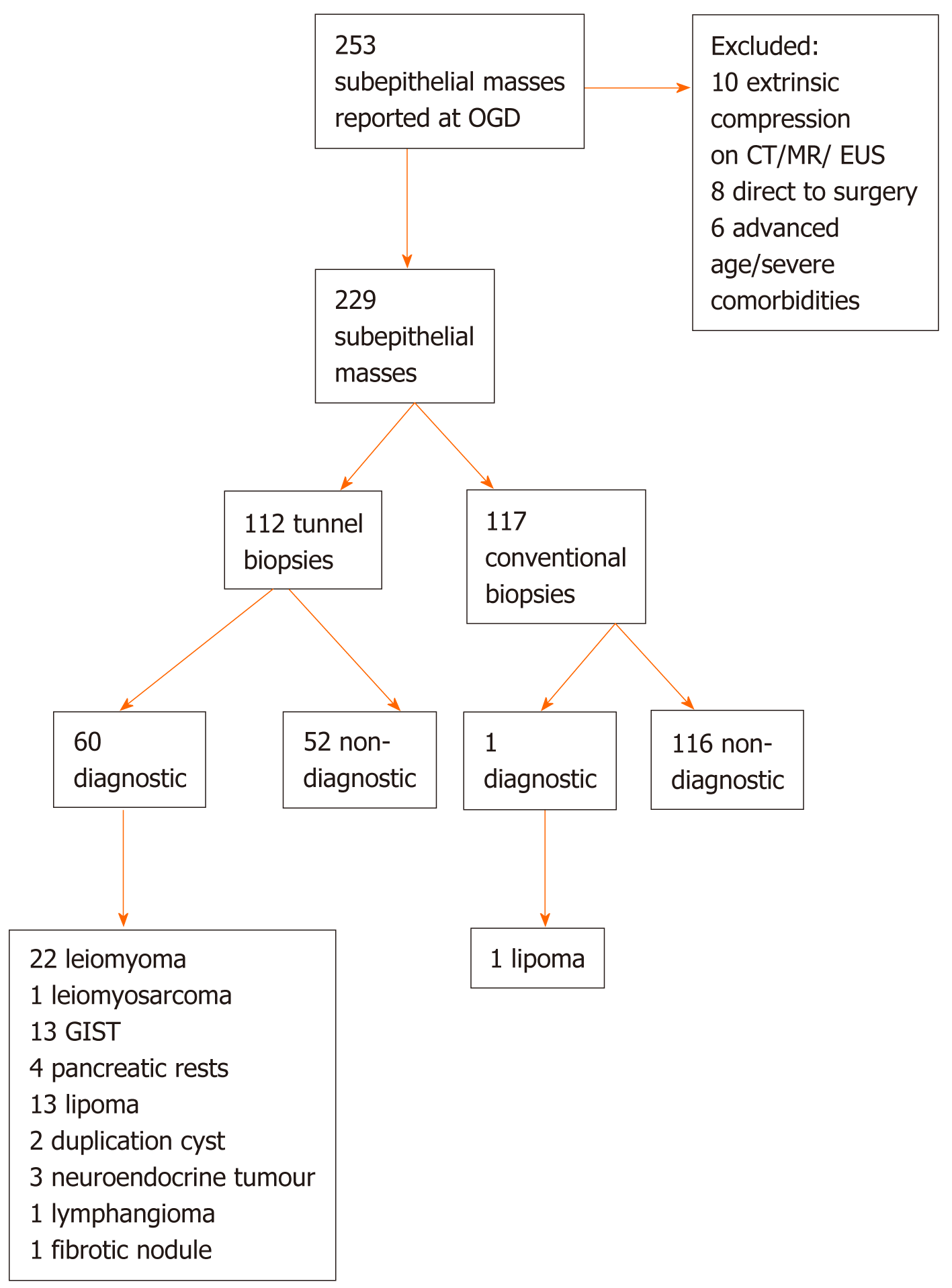
We searched our Endoscopy reporting database (Endobase, Olympus, Tokyo, Japan) from March 2013 to July 2019 for patients who underwent OGD and who were found to have a subepithelial lesion in the upper gastrointestinal tract (Figure 1).
Data were analysed from the endoscopy and pathology reports regarding whether these patients had tunnel biopsies, superficial conventional biopsies, endoscopic ultrasound with fine-needle aspiration or fine needle biopsy or surgical resection with histology. Medical records were reviewed for clinical events after the gastroscopy with biopsy such as readmission, haematemesis, melaena or significant drop in hae
Histology and cytology reports were retrieved from the patient files and electronic patient records.
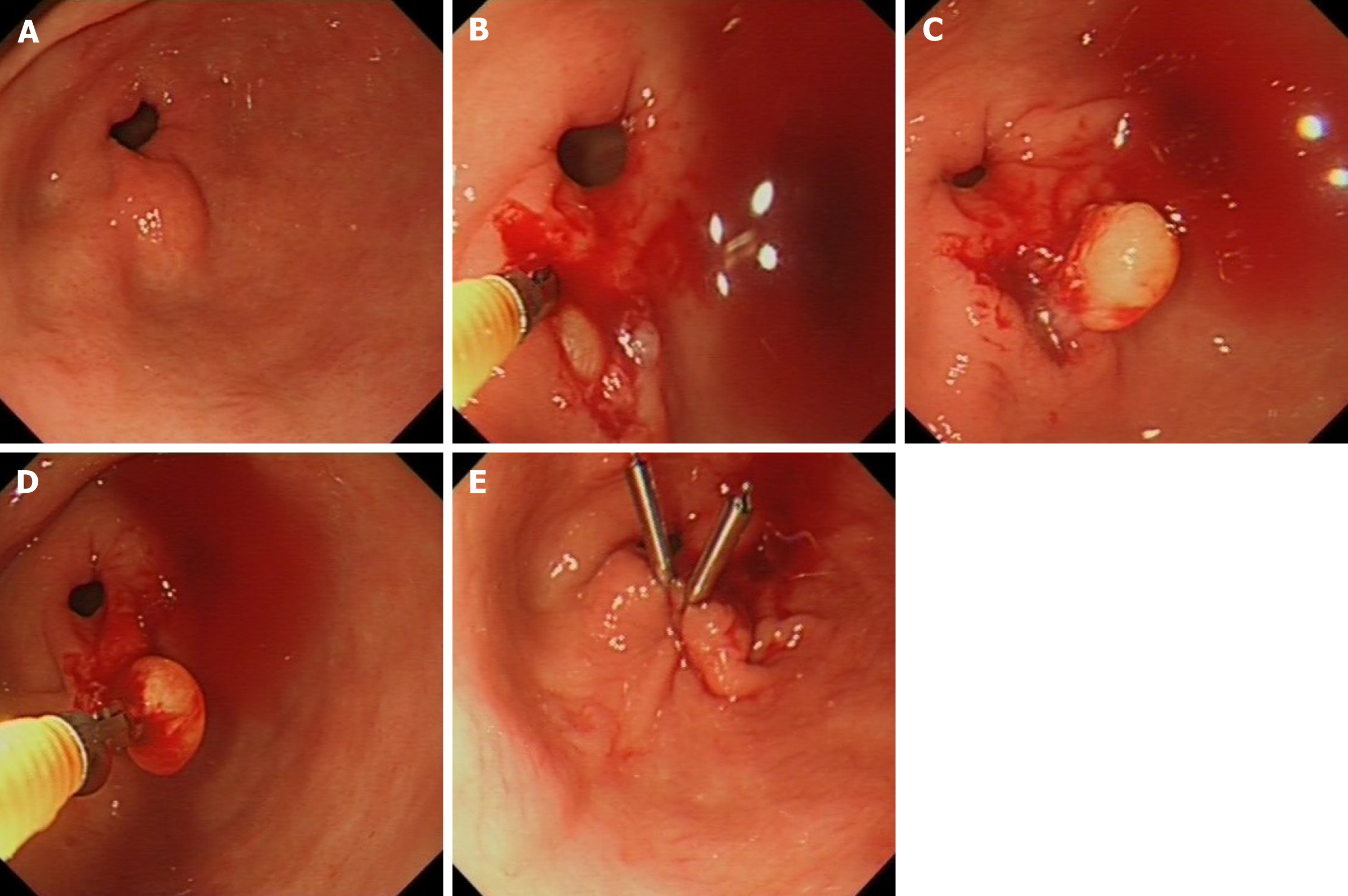
In this study, tunnel biopsy according to the intensified protocol is defined as taking at least 10 passes of double biopsies from the identical spot targeting the subepithelial lesion using conventional sized biopsy forceps (Radial JawTM 4, large capacity with needle, Single-Use Biopsy Forceps., Boston Scientific, ElCoyol, Costa Rica). After creating a defect in the mucosa with the first biopsies, the closed biopsy forceps is then advanced into the submucosal space and only opened there to obtain deeper sampling. Each bite is directed in the same direction as the previous to reach deeper layers within the lesion (Figure 2). If there is persisting bleeding from the biopsy tunnel after obtaining the biopsy samples the mucosal defect is closed by clip application.
The observational retrospective nature of the study was established with the Health Research Authority and Trust RD department. According to the Health Research Authority, this type of study does not require approval from a research ethics committee. The study was therefore registered locally in accordance with Trust clinical governance guidelines; the study follows the principles of the declaration of Helsinki.
All authors had access to the study data and have reviewed and approved the final manuscript.
For quantitative data, mean, standard deviation (SD) and range are presented or median and interquartile range. Qualitative data are expressed in absolute and relative frequencies with 95% confidence intervals. Microsoft Excel (Microsoft Cooperation, Redmond, WA, United States) was used for data handling, MedCalc 11.2.1.0 (MedCalc Software, Ostend, Belgium) for data analysis. Differences between groups were compared using the two-tailed Fisher’s exact test. A P value < 0.05 was considered significant.
Two hundred and fifty-three patients were found to have at least one subepithelial lesion at oesophagogastroduodenoscopy (OGD) or endoscopic ultrasound EUS.
Ten patients with obvious extrinsic compression by an extramural mass or cyst on CT/MR/EUS mimicking a subepithelial mass were excluded. In six patients > 85 years and severe comorbidities the reported subepithelial mass was considered not relevant and therefore not biopsied at the time of endoscopy and not followed. 8 patients with bleeding complications from gastric tumours in keeping with GIST were directly referred for surgical resection.
The mean age of the 229 patients included was 64 ± 15 years (interquartile range 54-75 years). 123 (53.7%) were female. The mean size of the subepithelial lesion was 18 ± 16 mm (interquartile range 8-25 mm). Thirty subepithelial masses were located in the oesophagus, 30 in the gastric fundus, 50 in the gastric body, 81 in the antrum, 8 in the duodenal bulb and 30 in the extrabulbar duodenum.
Superficial conventional biopsies were taken in 117 patients and were diagnostic revealing lipoma in one patient (0.9 %).
Tunnel biopsies according to the intensified protocol with at least 10 double biopsy passes were taken in 112 of 229 patients (48.9%) and could provide conclusive histology from the subepithelial lesion in 60/112 (53.6%; 95% confidence interval 43.9%-63.0%). Two patients underwent tunnel biopsies twice, i.e. 114 histological samples from tunnel biopsies were obtained.
Tunnel biopsies were taken from subepithelial lesions of the oesophagus (n = 19), fundus (n = 16), gastric body (n = 24), antrum (n = 39), and duodenum (n = 14). Tunnel biopsies were diagnostic in 60/112 (53.6%), revealing 22 Leiomyomas, 1 Leiomyosarcoma, 13 Lipomas, 4 pancreatic rests, 13 GISTs. 1 fibrotic nodule, 1 Lymph
| Histology | Oesophagus (n = 19) | Stomachproximal (n = 40) | Stomachdistal (n = 39) | Duodenum (n = 14) | Total (n = 112) |
| Lipoma | 0 | 0 | 8 | 5 | 13 |
| Leiomyoma | 12 | 8 | 2 | 0 | 22 |
| Leiomyosarcoma | 0 | 1 | 0 | 0 | 1 |
| Pancreatic rest | 0 | 0 | 3 | 1 | 4 |
| GIST | 1 | 10 | 1 | 1 | 13 |
| Metastasis | 0 | 0 | 0 | 0 | 0 |
| Duplication cyst | 2 | 0 | 0 | 0 | 2 |
| Neuroendocrine tumour | 0 | 0 | 0 | 3 | 3 |
| Fibrotic nodule | 0 | 1 | 0 | 0 | 1 |
| Lymphangioma | 0 | 0 | 0 | 1 | 1 |
| Non-diagnostic | 4 | 20 | 25 | 3 | 52 |
| Diagnostic yield | 15/19 (80.0%) | 20/40 (50.0%) | 14/39 (35.9%) | 11/14 (78.6%) | 60/112 (53.6%) |
For subepithelial lesions measuring more than 10 mm in diameter (67 Lesions), the diagnostic yield of tunnel biopsies further increased to 41/67 (61.2 %; 95% confidence interval 48.5%-72.9%); while smaller lesions ≤ 10 mm had successful diagnostic tunnel biopsies allowing characterising histology in 19/45 (42.2 %; 95% confidence interval 27.7%- 57.8%; P = 0.05).
Immediate or delayed complications after biopsies were not observed after conventional or after tunnel biopsies. In 22 patients, clip application was performed for prolonged bleeding from the biopsy tunnel or to prevent delayed haemorrhage.
Tunnel biopsies had a significantly higher diagnostic yield compared to conventional biopsies (P < 0.0001), however, this bite-on-bite sampling technique was routinely utilised for diagnostic characterisation of subepithelial lesions by only 8 endoscopists (15.7%), while all other 51 endoscopists in this study used conventional biopsy techniques.
One hundred and six patients also underwent endoscopic ultrasound for further characterisation of the submucosal lesion. In 43 patients EUS-guided fine needle aspiration or fine needle biopsy was performed which was diagnostic in 28/43 (65.1 %; 95% confidence interval 49.1%-79.0%).
The additional time to take 10 paired tunnel biopsies had been measured in 20 patients and ranged between 3 and 5 min (mean 4 min and 4 s).
The diagnostic approach to small subepithelial masses in the upper gastrointestinal tract varies widely between endoscopists. Subepithelial lesions in the upper gastrointestinal tract are often referred for EUS for further characterisation and tissue acquisition. However, EUS is a time consuming, resource-intense procedure, often offered only in specialised centres. The needle for fine needle aspiration or biopsy amounts to substantial additional costs. Furthermore, the diagnostic yield of EUS with fine needle aspiration or biopsy allowing a definitive final diagnosis can also be disappointingly low (45-75%)[4-7], mainly in small lesions. Fine needle aspiration samples usually do not provide sufficient core tissue for immunostaining. Introduction of new fine needle biopsy needles increase the diagnostic yield for tissue cores suitable for histology, immunostaining and sequencing[8].
On the other hand, the risk of progression in incidental small subepithelial tumours in the upper gastrointestinal tract is low[9] and serial follow-up endoscopies might be sufficient. The European Society of Gastrointestinal Endoscopy (ESGE) discourages the use of EUS for lesions < 2 cm and recommends endoscopic follow-up in small subepi
Obtaining histology from tunnel biopsies during the initial endoscopy, when the subepithelial mass is detected, can shorten the patient’s pathway and obviate the need for EUS examination or endoscopic follow-up. If histology clearly identifies the aetiology and confirms benign disease of the subepithelial mass, e.g., a pancreatic rest, leiomyoma or lipoma, further EUS or endoscopy follow up can be avoided.
In our large study on subepithelial masses in the upper gastrointestinal tract, tunnel biopsies in an intensified bite-on-bite technique with at least 10 double biopsies directed to the same spot had a significantly higher diagnostic yield (53.6%) compared to conventional biopsy techniques (0.9 %; P < 0.0001).
The diagnostic success rate by bite-on-bite tunnel biopsy technique has already been reported by Buscaglia et al[5] (58.9%) and Ji et al[11] (38%) but this endoscopic technique is generally underutilised as shown in our retrospective study; only 15 % of the endoscopists involved in our study routinely used bite-on-bite sampling technique to obtain histology samples from subepithelial masses. By simple means, perseverance and the correct tunnelling technique, tissue diagnosis can be achieved in about half of encountered subepithelial masses. Main principles of the technique is that a mucosal defect is created by the first biopsies; in subsequent biopsies the closed biopsy forceps is introduced through the mucosal defect into the submucosal space and then the branches are opened followed by a gentle pressure onto the forceps while the biopsy is taken and the branches are closed again.
In contrast to the 34.9% bleeding rate reported in the study by Buscaglia et al[5] using Jumbo biopsy forceps, we did not observe post-procedural bleedings after tunnel biopsies taken with conventional biopsy forceps. This might be due to the fact that the strict tunnel technique with very targeted conventional biopsy forceps created a tract which could easily be closed by clips in case of prolonged bleeding which persisted during the time of the endoscopy. In 22 patients the mucosal defect was prophylactically closed using clips.
The intensified protocol with a higher number of double passes during the tunnel biopsies might increase the diagnostic outcome as it allows deeper penetration into the gastrointestinal wall. In a previous study with only four double passes the diagnostic yield was only 17%[12].
Other more aggressive endoscopic un-roofing techniques have been described to obtain tissue from subepithelial tumours. Forceps biopsies can be taken after un-roofing by needle knife incision[13], ligation-resection[14], snare resection[15] or endoscopic submucosal dissection[16,17]. We would suggest that such techniques that would require higher endoscopic interventional skills are reserved for cases in which simple tunnel biopsies have failed.
Tumours originating from the muscularis mucosae or submucosa are more likely to be accessible by the bite-on-bite technique, while exophytic growing tumours from the muscularis propria will unlikely be reached.
Limitations of our single centre, controlled study are its retrospective nature and the fact that subsequent EUS has been performed only in a fraction of patients. Therefore, we cannot exclude that the proportion of subepithelial lesions located in the submucosa or in deep muscular layers differed between the groups with conventional and tunnel biopsies. However, patients have not been selected for certain endoscopists or biopsy techniques. The clinical setting in this study matched the situation of the index endoscopy when a subepithelial mass is encountered during endoscopy.
The bite-on-bite technique is simple and does not require any extra equipment as it is performed using the conventional forceps biopsy. The procedure time is increased only about 4 min by taking the additional biopsies.
The argument often encountered that tunnel biopsies might render later endoscopic or surgical treatment more difficult, could not be confirmed in our group of patients receiving resections as part of their clinical management.
In our study, simple tunnel biopsy technique provided the definitive diagnosis in about half of the cases, thereby avoiding EUS-FNA or more aggressive endoscopic techniques; follow-up endoscopies in those patients with subepithelial masses without malignant potential such as lipoma or pancreatic rests could be saved. Economical reasons as well as patient convenience favour attempting tissue acquisition at the first endoscopy.
Tunnel biopsy in bite-on-bite technique is a simple, safe and efficient diagnostic modality for tissue acquisition in subepithelial lesions of the upper gastrointestinal tract. This technique should be routinely attempted if a subepithelial lesion is detected at the index endoscopy as it prolongs the examination only for a few minutes and can often avoid the need for further EUS and fine needle aspiration/EUS biopsy or follow up endoscopies.
Subepithelial tumours of the gastrointestinal tract can be benign, pre-malignant or malignant. Most small tumours are benign. Lipoma, leiomyoma, pancreatic rests or duplications cyst will usually not need further follow-up. Gastrointestinal stromal tumours or neuroendocrine tumours will require resection or surveillance. Metastasis to the gastrointestinal wall can rarely also present as subepithelial lesion.
Histology acquisition from subepithelial tumours is challenging as conventional endoscopic biopsies do usually not reach deeper than the mucosal layer. Subepithelial tumours often present a diagnostic dilemma.
The authors investigated the use, the safety and the diagnostic success of performing tunnel biopsies from subepithelial tumours to obtain histology.
Tunnel biopsy was defined as repeating at least 10 double pass biopsies targeting the identical spot on the subepithelial mass with conventional biopsy forceps. All patients with subepithelial tumours reported at oesophagogastroduodenoscopy presenting within the 6 year study period were included and data were analysed regarding size and location of the tumour, histology, radiological findings, re-admissions and adverse events.
Only in about half of the 229 encountered subepithelial tumours tunnel biopsies were attempted. However, when tunnel biopsies were performed, they were diagnostic in 53.6%. Adverse events were not observed.
Performing tunnel biopsies from subepithelial tumours during endoscopy prolongs the procedure only a few minutes but can save endoscopic ultrasound-guided sampling or the need for follow-up in about 50%.
Further randomized studies with cost-analysis should assess the diagnostic yield of tunnel biopsies performed at the index endoscopy compared with endoscopic ultrasound-guided sampling.
The authors would like to thank the team of the Endoscopy Unit and the department of Pathology at the University Hospitals Oxford for their excellent support.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwon KA S-Editor: Ma YJ L-Editor: A P-Editor: Zhang YL
| 1. | Oda, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, Yoshida S, Shimoda T. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K; German EUS Club. Endoscopic ultrasonography. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [PubMed] |
| 4. | Zhang XC, Li QL, Yu YF, Yao LQ, Xu MD, Zhang YQ, Zhong YS, Chen WF, Zhou PH. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016;30:2431-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV, Kim MK. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Schlag C, Menzel C, Götzberger M, Nennstiel S, Klare P, Wagenpfeil S, Schmid RM, Weirich G, von Delius S. Endoscopic ultrasound-guided tissue sampling of small subepithelial tumors of the upper gastrointestinal tract with a 22-gauge core biopsy needle. Endosc Int Open. 2017;5:E165-E171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | El Chafic AH, Loren D, Siddiqui A, Mounzer R, Cosgrove N, Kowalski T. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2017;86:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Song JH, Kim SG, Chung SJ, Kang HY, Yang SY, Kim YS. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015;47:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono PG, Bastos P, Carrara S, Czakó L, Fernández-Esparrach G, Fockens P, Ginès À, Havre RF, Hassan C, Vilmann P, van Hooft JE, Polkowski M. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49:695-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Ji JS, Lee BI, Choi KY, Kim BW, Choi H, Huh M, Chung WC, Chae HS, Chung IS. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Shimamura Y, Hwang J, Cirocco M, May GR, Mosko J, Teshima CW. Efficacy of single-incision needle-knife biopsy for sampling subepithelial lesions. Endosc Int Open. 2017;5:E5-E10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Binmoeller KF, Shah JN, Bhat YM, Kane SD. Suck-ligate-unroof-biopsy by using a detachable 20-mm loop for the diagnosis and therapy of small subepithelial tumors (with video). Gastrointest Endosc. 2014;79:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Lee CK, Chung IK, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Lee HL, Kwon OW, Lee KN, Jun DW, Eun CS, Lee OY, Jeon YC, Han DS, Yoon BC, Choi HS, Hahm JS, Paik SS. Endoscopic histologic diagnosis of gastric GI submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc. 2011;74:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Tae HJ, Lee HL, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS. Deep biopsy via endoscopic submucosal dissection in upper gastrointestinal subepithelial tumors: a prospective study. Endoscopy. 2014;46:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |