Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.296
Peer-review started: June 11, 2020
First decision: November 14, 2020
Revised: November 23, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: January 16, 2021
Processing time: 210 Days and 18.1 Hours
Acute infections, including those due to Coronaviridae and other viruses, often stimulate a febrile response. A mild fever appears to improve outcome; it appears to diminish viral replication by several mechanisms, including virion entry into host cells and genome transcription, and improving host defence mechanisms against the pathogen. However, a fever may also damage host cellular and tissue function and increase metabolic demands. At temperatures at the lower end of the febrile range, the benefit of the fever appears to outweigh the detrimental effects. However, at higher temperatures, the outcome worsens, suggesting that the disadvantages of fever on the host predominate. A non-infective fever is associated with a worse outcome at lower temperatures, suggesting that hyperthermia carries less benefit in the absence of infection. This review discusses the risks and benefits of a fever on the host response, focusing on the effects of a fever on viral replication and host response, and the detrimental effect on the host.
Core Tip: Acute infections, including those due to coronavirus and other viruses, often stimulate a febrile response. A mild fever appears to improve outcome; it appears to diminish viral replication by several mechanisms, and improve host defence mechanisms against the pathogen. At higher temperatures, the outcome worsens, suggesting that the risks of fever on the host outweigh the benefit. A non-infective fever is associated with a worse outcome at lower temperatures. This paper discusses why a mild fever may be better than no, or very high fever.
- Citation: Belon L, Skidmore P, Mehra R, Walter E. Effect of a fever in viral infections — the ‘Goldilocks’ phenomenon? World J Clin Cases 2021; 9(2): 296-307
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.296
The presence of a fever in infection and its potential benefit has been known for over two millennia, with the Greek philosopher Parmenides suggesting: “Give me the power to produce fever and I'll cure all disease”[1].
In humans, normal core temperature varies between around 36.5 °C and 37.8 °C[2]. A raised temperature may be due to processes when the hypothalamus has a new regulatory set point causing the temperature to rise (for example, infection or inflammation), often called fever. Other incidences of a raised temperature may be due to heat gain in excess of heat loss (for example, in heat waves, after exertion or exercise, or illicit drug-taking), despite no change in the regulatory set point, a process often defined as hyperthermia[3]. Definitions of different degrees of fever also vary between different sources, but one consensus statement defines a fever in humans as equal to or greater than 38.3 °C[4], that is, 0.5 °C higher than the physiological range. Similarly, there is not a consensus on the definition of extreme hyperthermia, but there are significant effects above around 40 °C, suggesting that this temperature or higher poses particular risks.
A fever has numerous effects on the virus and on the human host. Whether a fever is useful or should be treated remains unclear. This review summarises the benefit and disadvantages of fever in acute viral infection, including what is known about pyrexia in coronavirus infection.
Fever is a common immunological response to bacterial and viral infections, and is conserved across the animal kingdom, suggesting that it might provide an evolutionary benefit[5].
A mildly raised temperature in patients with infection in the first 24 h following admission to the intensive care unit (ICU) is associated with a better outcome compared with normothermia or hyperthermia above 40 °C[6,7]. In a study of elderly patients with pneumonia, survival was significantly lower in patients who lacked fever (29%) when compared with patients who developed a febrile response (4%)[8]. A temperature greater than 38.2 °C is protective against invasive fungal infections in critically ill patients[9].
In contrast with a fever in response to sepsis, a non-pyrogenic fever does not appear to have any benefit. A temperature of 37.5 °C or greater at any point during an ICU admission trends towards a worse outcome, and becomes significant at temperatures greater than 38.5 °C[7].
Similarly, there is an increase in mortality for non-infectious neurological conditions (traumatic brain injury or stroke) in ICU at temperatures above 38.0 °C within the first 24 h, compared with normothermia; in patients with a brain infection, no significant increase in mortality is seen compared with normothermia until the temperature reaches above 40 °C[10].
However, fever may come at a cost: Fever during an ICU admission is associated with greater organ dysfunction, prolonged hospitalisation, and prolonged periods of mechanical ventilation[11,12]. A randomised controlled trial found that the return to normothermia from 38.5oC in critically ill, shocked patients resulted in significant vasopressor reduction by 50% at 12 h, improved early mortality outcomes, and higher rates of shock reversal[13].
In the treatment of acute febrile illness, antipyretics are often recommended. However, there is little evidence of benefit[14], and evidence that they may prolong acute varicella zoster infection without any amelioration of symptoms[15,16], prolong the duration of acute rhinovirus illness[17], and may worsen mortality[18]. In a recent systematic review, antipyretics suggested an increased mortality when used in influenza infections in animals[19].
In addition to humans, other warm-blooded animals[20], cold-blooded animals[21,22] and indeed plants[23] raise their temperature when acutely infected with a pathogen, and in animals at least[20-22], survival is often improved.
Viruses, from the Latin word virus, meaning poison, are tiny ubiquitous micro-organisms, usually a few hundred nanometers in size. A virion contains a single nucleic acid (RNA or DNA) core surrounded by a protein coat, and requires the cellular processes of animals, plants, and bacteria in order to replicate.
Viruses are often classified according to the Baltimore system[24], named after the professor of biology and Nobel laureate David Baltimore. Seven Baltimore groups are described, categorised by whether the viral genome is made of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), whether the genome is single- (ss) or double-stranded (ds), whether the sense of a single-stranded RNA genome is positive or negative, and whether reverse transcriptase (RT) is involved in replication (see Table 1). All of the viruses below may cause fever in acute infection, although the degree of fever generation depends on the virulence of the micro-organism and host response to the infection.
| Class | Type | Examples of virus | Examples of disease in humans |
| I | dsDNA virus | Adenoviruses | Bronchitis, pneumonia, gastroenteritis |
| Herpesviruses | Herpes, chickenpox | ||
| II | ssDNA virus | Parvoviruses | ‘Slapped cheek syndrome’, arthritis |
| III | dsRNA virus | Reoviruses | Gastroenteritis |
| IV | ssRNA virus | Coronaviruses | COVID-19, SARS, MERS |
| Togaviruses | German measles, encephalitis | ||
| V | ssRNA virus | Orthomyxoviruses | Influenza |
| Rhabdoviruses | Rabies | ||
| VI | ssRNA-RT virus | Retroviruses | AIDS, role in cancer |
| VII | dsDNA-RT virus | Hepadnaviruses | Hepatitis |
Coronaviruses are a group of related single-stranded, positive-strength large RNA viruses that cause diseases in mammals and birds. In humans, these viruses cause respiratory tract infections that can range from mild (for example, the common cold), to lethal [for example, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and SARS coronavirus 2 (SARS-CoV-2)]. They have characteristic club-shaped spikes that project from their surface, similar to the solar corona, from which their name derives.
That a febrile response to acute viraemia may be beneficial to host survival and inhibitory to viral replication and shedding has been known since at least the 1970s[25-27]; more recent work has improved the understanding of the mechanisms involved.
Viruses, including the coronaviruses, are acellular and cannot replicate without incorporating themselves into a host cell and relying on their host cells to provide the basic machinery to allow replication.
The first stage in the viral life-cycle is entry of the virion into the host cell. This often requires interaction with a specific receptor on the host cell surface, allowing fusion of the host cell and viral membranes, or endocytosis. In the case of SARS-CoV-2, this host cell receptor has been identified as the angiotensin-converting enzyme-2 (ACE2) protein[28]. Alterative mechanisms for some viruses to enter the host cells are suggested; for example, the African swine fever virus (ASFV) may enter host cells via micropinocytosis (non-receptor-mediated entry, involving non-selective endocytosis of fluid and solutes)[29]. ASFV infection in pigs often results in high fever in hosts.
Once inside the host cell, the viral genome can be transcribed and replicated. The viral capsid is first removed and degraded by viral enzymes or host enzymes, which releases the viral genomic nucleic acid. The method of replication of the viral genome varies between DNA and RNA viruses and between the structure of the genome. The process culminates in the synthesis of new viral proteins and genome, which are then assembled into new virions and released from the host cell.
The method of viral release from the host cell depends on the virion structure. Enveloped viruses are often released by budding, acquiring a phospholipid envelope from the host cell. Nonenveloped viruses exit an infected cell by lysis, resulting in destruction of the host cell.
The presence of a pyrexia appears to affect various stages of the replication process. Temperature appears to affect entry of the virion into cells via a number of mechanisms. Temperature has a direct effect on the fluidity of cell membranes, with early work suggesting that the increase in fluidity with increasing temperatures is a consequence of virion entry into the cell[30], with variable effects on viral entry. For example, membrane fluidity plays a key role in the accumulation of binding sites for human immunodeficiency virus 1 (HIV-1) cell entry; increases in temperature enhance HIV-1 adsorption and infectivity[31]. In contrast, increased membrane fluidity may be inhibitory to hepatitis C virion entry[32]. Temperature-dependent increases in fluidity appear to stimulate T-cell activation[33], suggesting enhanced viral detection and clearance. Whether the entry of SARS-CoV-2 into cells is affected by temperature-induced changes in host cell membrane fluidity remains unclear.
The influenza virus is internalised via receptor-mediated endocytosis, which requires an acidic environment for the endosome to trigger viral and endosomal membrane fusion[34,35]. High temperatures appear to increase endosomal pH, adversely affecting influenza virus entry and intracellular transportation[36].
Once inside the host cell, the efficacy of viral genome transcription and replication may be modulated by changes in temperature. For coronaviruses, as with other RNA viruses, replication is performed by an RNA-dependent RNA polymerase[37]. Features of this enzyme are remarkably conserved across RNA viruses[38], including in the recently described SARS-CoV-2 RNA polymerase[39], and thus temperature-dependent effects on its activity in other viruses may be analogous. Temperatures greater than 41 °C have been demonstrated to destabilise viral RNA polymerase in highly pathogenic avian influenza (HPAI), reducing its replicative ability[40]. While the viral messenger RNA transcription may be maintained at higher temperatures, genome replication is markedly reduced, due to reduced association of the polymerase with positive-sense RNA templates[41]. A raised temperature also appears to cause a reduction in the cytokine interleukin (IL)-6 levels after human cell infection with influenza virus[36]; IL-6 is required for a number of cytokine-mediated processes involved in viral replication[42].
However, certain influenza A strains may adapt to different host temperatures; reassortment of the RNA polymerase subunits has been shown to confer increased thermal stability[43,44], and this ability may allow the virus to cross species barriers.
The human immune system protects against invasive pathogens and is composed of innate and adaptive components. The innate system provides nonspecific defence mechanism (physical barriers and stimulation of inflammation and recruitment of white blood cells, complement and cytokines. The adaptive component is specific to a particular pathogen, mediated by antibodies and complement proteins. Almost every step of both components appears to be stimulated by generation of a fever[21].
Neutrophils are the first immune cell populations to be recruited to a site of infection, but their precise role has not yet been determined[45]. They appear to display a number of antiviral mechanisms, including phagocytosis and destruction of infected cells, blockage of viral genome replication, and production of antiviral cytokines[45]. Neutrophil production[46] and activity[21] are increased by the presence of a fever, and a fever appears to improve attraction and migration of neutrophils to the site of infection[47].
Similarly, fever stimulates the cytotoxicity of natural killer (NK) cells and increases migration to the site of infection[21]. While the precise role is again undefined, NK cells are likely to contribute to cytolytic killing of virus-infected cells, production of pro-inflammatory cytokines, and stimulation and recruitment of adaptive immune cells[48]. Elevated temperatures inhibit expression of MHC class I proteins and increase heat shock protein 70 (HSP70) production by tumour cells, both of which enhance NK cytotoxicity. Whether a similar response is seen in virus-infected cells is unclear[49].
Dendritic cells (DC) serve as antigen-presenting cells (APCs), by processing antigen material and presenting it on the cell surface to components of the adaptive immune system. Febrile temperatures have a variety of actions on DCs, including enhancing phagocytic activity, and increasing production of interferon-alpha (IFNα). Interferon-alpha appears to have a number of effects in acute viral infection, many inhibiting viral replication[50]. Expression of MHC class I and II molecules and co-stimulatory proteins, for example CD80 and CD86, is increased, enhancing T- and B-lymphocyte activity[21]. Migration of dendritic cells is also enhanced by a febrile temperature, with increased migration of epidermal DCs to the lymphatic system[51].
Lymphocyte trafficking is vital to ensure lymphocytes migrate to the required site of inflammation and through lymphoid tissue; it allows interaction between dendritic cells and lymphocytes, and ensures that there is exposure to antigen-presenting cells. Febrile temperatures enhance a number of mechanisms involved with effective migration, including binding of adhesion molecules and stimulation of endothelial trafficking molecules[21]. T-lymphocyte stimulation and cytotoxicity is enhanced by febrile temperatures[52,53].
Heat shock proteins (HSPs) are a family of proteins that are produced by cells in response to thermal stress and other stressors including exposure to cold, UV light toxins and hypoxia, and during wound healing or tissue remodelling. HSPs are one of the most phylogenetically conserved classes of proteins and are found in virtually all living organisms. They have a wide range of critical roles in maintaining cellular homeostasis and in protecting cells from stressful conditions, reflecting in large part their ability to facilitate folding of nascent protein or refolding of denatured protein.
In the context of viral infections, HSPs are stimulated both by viral-induced fever and by viral infection itself[54]. They have effects on both the pathogen and host; the exact interaction is still not well understood, and it is still debated whether the outcome may benefit the host or the pathogen. They have been variously described as having an enhancing[55-59] or deleterious[54,60] effect on the mechanisms of viral replication or activation. In particular, HSP70 has been implicated as a chaperone for the replication complex in influenza A infection[61], although conversely may inhibit the nuclear export of newly synthesised influenza ribonucleoprotein complexes and thus viral morphogenesis[60]. In fruit flies, the absence of heat shock transcription factor results in higher mortality in viral infection, suggesting that heat shock proteins may confer a benefit to survival in viral infection[62]. However, other organisms, such as salmonella, rely on stimulation of HSPs to enhance infectivity[63], suggesting the variable and pleotropic effects of HSPs in viral replication and host defence.
There is little understanding currently of the interaction between heat shock proteins and the Coronaviridae family, but recent work has suggested that the SARS-CoV uses host cellular endoplasmic reticulum as a site for the synthesising and processing of its viral proteins, and that it can induce the unfolded protein response (UPR)[64]. The UPR is a ubiquitous cellular stress response related to endoplasmic reticulum (ER) stress, which is activated in response to an accumulation of unfolded or misfolded proteins in the lumen of the ER. The UPR aims to restore normal function of the cell by a number of mechanisms, including halting protein translation, degrading misfolded proteins, and activating signaling pathways involved in protein folding. If restoration of function is not achieved, apoptosis may be triggered[65]. HSP90 appears to improve stability of associated intracellular protein kinases[66], with HSP90 inhibition accelerating cell lysis[67]. There is therefore a potential role for HSP90 inhibitors, known to have chemotoxic effects, as cytotoxic agents against coronavirus-infected cells[68].
A further effect of the febrile response is the effect on the efficacy of various medications. One study of 17 different antibiotics on 432 strains of bacteria at temperatures between 35 and 41.5 °C showed a progressive increase in antimicrobial activity as the temperature increased[69]. Little is known about the effect of temperature on the activity of antiviral pharmaceutical agents.
If there were no alternative benefit, conventional preconception would suggest that any departure from physiological homeostasis would be deleterious to an organism. Thermal dysregulation is no exception to this, and there appear to be injurious consequences observed in hyperthermia in humans. The deleterious effects of hyperthermia have been described similarly in a variety of causes, suggesting that it is the presence of a raised temperature, rather than the cause, that is primarily important. While much of the evidence comes from non-infective heat injury, biological plausibility suggests that similar effects also occur in infective fevers.
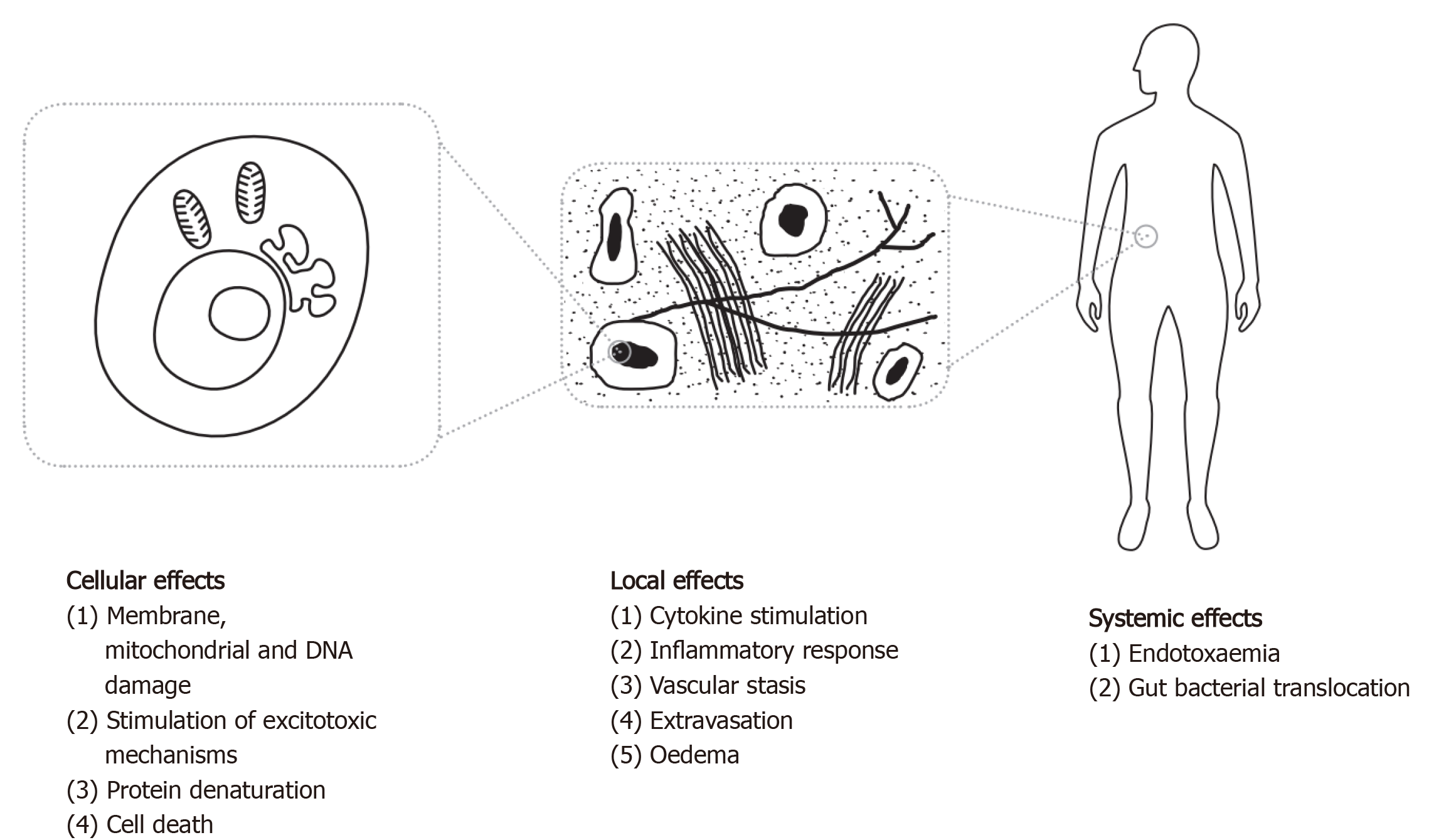
The mechanism of injury in hyperthermia, whether due to an infective or non-infective cause, appears to result from both direct heat stress on molecular, cellular and tissue-level structures (see Figure 1), and from local and systemic cytokine-driven responses. Pyrogenic cytokines such as IFNγ, IL-1b and IL-6, which are closely associated with the viral fever pathway[70], have been observed in mechanisms responsible for capillary leak, myocardial suppression, and systemic inflammation[71]. IL-1 and IL-6 knockout mice tend to manifest an attenuated febrile response[72,73]. Longer duration and higher magnitude of IL-6 peaks in heatstroke patients are associated with poor outcome irrespective of maximum core temperature[74], and are proportional to symptoms and the degree of fever in influenza[70]. However, the pleiotropic nature of these cytokines makes it difficult to accurately model a causal relationship in disease.
Hyperthermia results in disruption of transmembrane transport mechanisms, affecting ionic flux and gradient balance[75]. Impaired protein and nucleic acid synthesis is also observed[75], which may continue beyond re-establishment of normothermia[76]. At temperatures of 40 °C, nuclear processes are interrupted by damage to nuclear scaffolding structures[77]. Direct cellular death is observed above 41 °C, and the increase in rate of cell death rises significantly beyond this point[75,77].
Temperatures in excess of around 39-40 °C are associated with an increase in enteric epithelial permeability[78,79], and the translocation of enteric bacteria and endotoxins, improved when subsequently treated with antibiotics against enteric bacteria[80-82]. This is supported by evidence of bacterial infections and elevated circulating procalcitonin levels, a sensitive biomarker of sepsis, in more than 50% of heatstroke patients[83].
Neuroprotection conferred by the highly selective permeability of the blood-brain barrier (BBB) is increasingly lost at temperatures of greater than 38-39 °C with loss of structural integrity[84]. The changes in endothelial permeability of the BBB occur at similar temperatures to the endotoxaemic process postulated in the gastrointestinal (GI) tract, and this association may contribute to neurological impairment[85]. A prospective observational study of ICU patients describes intestinal permeability as a predictor of development of multiple organ dysfunction syndrome (MODS)[86].
Poor endothelial barrier function in hyperthermic states is also associated with cytokine-mediated extravasation of neutrophils and macromolecules from lung vasculature which, compounded with increased oxygen toxicity, aggravates acute lung injury in critical illness at temperatures above around 39 °C[47,87,88].
In addition to epithelial barrier dysfunction in the GI tract as described above, hyperpyrexia is associated with disruption of cellular structural mechanisms and exacerbation of oxidative stress in splanchnic tissue as a consequence of increased free radical production at the tissue level[89]. Intestinal ischaemia is observed, with circulatory diversion away from splanchnic tissues[78].
Neurological cytotoxicity and dysregulation are well documented in heat stress. There is evidence of higher cortical dysfunction, which may be chronic or permanent[85,90], and neuroradiological studies suggest a number of discrete pathologies, including oedema, ischaemia, haemorrhage, and encephalitic and accelerated degenerative changes[91,92]. Many parts of the central nervous system are vulnerable[91,92], but the Purkinje neurons are particularly susceptible[85,93]. Mammalian studies suggest that this particular thermosensitivity is related to the preferential expression of heme oxygenase-1 in the Purkinje cells[94].
Intravascular and haematological homeostasis is also disrupted by hyperthermia. Animal models demonstrate vasoplegia, venostasis and capillary hyperpermeability at sustained temperatures of greater than 40 °C[95]. There is evidence of coagulopathy in more than 40% of cases, compounded by impaired platelet aggregation above 38 °C[96,97]. Hepatic dysfunction and hepatocellular structural damage is often observed alongside coagulopathic states in hyperthermia[98-100].
Acute kidney injury, sometimes sufficiently severe to require renal replacement therapy, has been described across heat stress conditions of various aetiologies[96,101,102], the magnitude of which correlates with mortality[101]. Similar to dysfunction in other organs, renal capillary bed dilatation, vascular stasis, and interstitial haemorrhage are seen[95]. This may be further exacerbated by stimulation of the renin-aldosterone-angiotensin system which in turn reduces renovascular flow at temperatures as low as 38 °C[103].
The association between pyrexia and hypermetabolism is well established; however, there are little data on the effect on patient outcome. A calorimetric study on 204 critically ill patients found fever to be the most significant single determinant of hypermetabolism of those observed, above even the presence of SIRS[104]. Another study determined that cooling patients from 39.5 °C to 37 °C resulted in a reduction in oxygen uptake, carbon dioxide production, cardiac output, and oxygen extraction fraction of approximately 20%[105]. A one-degree increase above 37 °C results in a 10% increase in metabolic rate[106]. The ability of the organism to cope with this increased demand is likely to depend on the metabolic reserve of an individual patient, but may be significant.
Current epidemiological and biological evidence suggests that viral replication is reduced and that human host defence mechanisms are enhanced at degrees of mild fever, compared with normothermia. However, at higher degrees of fever, (around 39-40 °C), mortality again increases, suggesting that any benefit at mildly elevated temperatures is outweighed by the damage the fever causes on the host. This ‘U’-shaped curve, demonstrating improved outcomes at mild febrile temperatures, does not appear to be replicated in non-pathogenic states, suggesting that the damage that the fever exerts on the host is not matched by any perceived benefit, and that this permissive pyrexia may only be true with an infective aetiology.
What is less clear is the effect that modulating the temperature has on clinical outcomes, or the optimal temperature. The latter is likely to be impacted by the physiological reserve of the patient and their ability to tolerate the increase in metabolic demands that a fever requires.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Song G, Sun H, Zhang W S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Duffell E. Curative power of fever. Lancet. 2001;358:1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Ogoina D. Fever, fever patterns and diseases called 'fever'--a review. J Infect Public Health. 2011;4:108-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H; American College of Critical Care Medicine; Infectious Diseases Society of America. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Young PJ, Saxena M. Fever management in intensive care patients with infections. Crit Care. 2014;18:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Young PJ, Saxena M, Beasley R, Bellomo R, Bailey M, Pilcher D, Finfer S, Harrison D, Myburgh J, Rowan K. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, Tada K, Tanaka K, Ietsugu K, Uehara K, Dote K, Tajimi K, Morita K, Matsuo K, Hoshino K, Hosokawa K, Lee KH, Lee KM, Takatori M, Nishimura M, Sanui M, Ito M, Egi M, Honda N, Okayama N, Shime N, Tsuruta R, Nogami S, Yoon SH, Fujitani S, Koh SO, Takeda S, Saito S, Hong SJ, Yamamoto T, Yokoyama T, Yamaguchi T, Nishiyama T, Igarashi T, Kakihana Y, Koh Y; Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care. 2012;16:R33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Ahkee S, Srinath L, Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. 1997;90:296-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O; AmarCand Study Group. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit Care Med. 2009;37:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 10. | Saxena M, Young P, Pilcher D, Bailey M, Harrison D, Bellomo R, Finfer S, Beasley R, Hyam J, Menon D, Rowan K, Myburgh J. Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med. 2015;41:823-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Netzer G, Dowdy DW, Harrington T, Chandolu S, Dinglas VD, Shah NG, Colantuoni E, Mendez-Tellez PA, Shanholtz C, Hasday JD, Needham DM. Fever is associated with delayed ventilator liberation in acute lung injury. Ann Am Thorac Soc. 2013;10:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, Dellamonica J, Bouadma L, Cook F, Beji O, Brun-Buisson C, Lemaire F, Brochard L. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012;185:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (2)] |
| 14. | Ludwig J, McWhinnie H. Antipyretic drugs in patients with fever and infection: literature review. Br J Nurs. 2019;28:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox? J Pediatr. 1989;114:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Jefferies S, Braithwaite I, Walker S, Weatherall M, Jennings L, Luck M, Barrett K, Siebers R, Blackmore T, Beasley R, Perrin K; Pi Study Group. Randomized controlled trial of the effect of regular paracetamol on influenza infection. Respirology. 2016;21:370-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Earn DJ, Andrews PW, Bolker BM. Population-level effects of suppressing fever. Proc Biol Sci. 2014;281:20132570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. J R Soc Med. 2010;103:403-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Kurosawa S, Kobune F, Okuyama K, Sugiura A. Effects of antipyretics in rinderpest virus infection in rabbits. J Infect Dis. 1987;155:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 730] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 22. | Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. 1976;193:237-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Yarwood CE. Heat of Respiration of Injured and Diseased Leaves. Phytopathology. 1953;43:675-681. |
| 24. | Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971;35:235-241. [PubMed] |
| 25. | Overton HA, Sweet C, Coates DM, Smith H. Molecular studies of the differential replication at pyrexial temperatures of two influenza viruses differing in virulence for ferrets. Virus Res. 1986;5:235-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Husseini RH, Sweet C, Collie MH, Smith H. Elevation of nasal viral levels by suppression of fever in ferrets infected with influenza viruses of differing virulence. J Infect Dis. 1982;145:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Bell JF, Moore GJ. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infect Immun. 1974;10:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14271] [Article Influence: 2854.2] [Reference Citation Analysis (0)] |
| 29. | Gaudreault NN, Madden DW, Wilson WC, Trujillo JD, Richt JA. African Swine Fever Virus: An Emerging DNA Arbovirus. Front Vet Sci 2020; 7: 215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 30. | Levanon A, Kohn A, Inbar M. Increase in lipid fluidity of cellular membranes induced by adsorption of RNA and DNA virions. J Virol. 1977;22:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Harada S, Akaike T, Yusa K, Maeda Y. Adsorption and infectivity of human immunodeficiency virus type 1 are modified by the fluidity of the plasma membrane for multiple-site binding. Microbiol Immunol. 2004;48:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Chamoun-Emanuelli AM, Pecheur EI, Simeon RL, Huang D, Cremer PS, Chen Z. Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes. Antimicrob Agents Chemother. 2013;57:2571-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Gombos I, Vígh L. Membrane fluidity in the center of fever-enhanced immunity. Cell Cycle. 2015;14:3014-3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C betaII in influenza virus entry via late endosomes. J Virol. 2003;77:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1498] [Cited by in RCA: 1550] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 36. | Yamaya M, Nishimura H, Lusamba Kalonji N, Deng X, Momma H, Shimotai Y, Nagatomi R. Effects of high temperature on pandemic and seasonal human influenza viral replication and infection-induced damage in primary human tracheal epithelial cell cultures. Heliyon. 2019;5:e01149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1013] [Cited by in RCA: 1131] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 38. | McDonald SM. RNA synthetic mechanisms employed by diverse families of RNA viruses. Wiley Interdiscip Rev RNA. 2013;4:351-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, Wang T, Sun Q, Ming Z, Zhang L, Ge J, Zheng L, Zhang Y, Wang H, Zhu Y, Zhu C, Hu T, Hua T, Zhang B, Yang X, Li J, Yang H, Liu Z, Xu W, Guddat LW, Wang Q, Lou Z, Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 933] [Cited by in RCA: 1082] [Article Influence: 216.4] [Reference Citation Analysis (0)] |
| 40. | Scholtissek C, Rott R. Effect of temperature on the multiplication of an Influenza virus. J Gen Virol. 1969;5:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Dalton RM, Mullin AE, Amorim MJ, Medcalf E, Tiley LS, Digard P. Temperature sensitive influenza A virus genome replication results from low thermal stability of polymerase-cRNA complexes. Virol J. 2006;3:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The Role of Interleukin 6 During Viral Infections. Front Microbiol. 2019;10:1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 43. | Kashiwagi T, Hara K, Nakazono Y, Hamada N, Watanabe H. Artificial hybrids of influenza A virus RNA polymerase reveal PA subunit modulates its thermal sensitivity. PLoS One. 2010;5:e15140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Bradel-Tretheway BG, Kelley Z, Chakraborty-Sett S, Takimoto T, Kim B, Dewhurst S. The human H5N1 influenza A virus polymerase complex is active in vitro over a broad range of temperatures, in contrast to the WSN complex, and this property can be attributed to the PB2 subunit. J Gen Virol. 2008;89:2923-2932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Galani IE, Andreakos E. Neutrophils in viral infections: Current concepts and caveats. J Leukoc Biol. 2015;98:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 46. | Capitano ML, Nemeth MJ, Mace TA, Salisbury-Ruf C, Segal BH, McCarthy PL, Repasky EA. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner. Blood. 2012;120:2600-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He JR, Rice P, Frank M, Goldblum SE, Viscardi RM. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162:2005-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Waggoner SN, Reighard SD, Gyurova IE, Cranert SA, Mahl SE, Karmele EP, McNally JP, Moran MT, Brooks TR, Yaqoob F, Rydyznski CE. Roles of natural killer cells in antiviral immunity. Curr Opin Virol. 2016;16:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3- large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood. 1995;86:1374-1382. [PubMed] |
| 50. | Teijaro JR. Type I interferons in viral control and immune regulation. Curr Opin Virol. 2016;16:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 51. | Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int J Hyperthermia. 2003;19:520-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Zynda ER, Grimm MJ, Yuan M, Zhong L, Mace TA, Capitano M, Ostberg JR, Lee KP, Pralle A, Repasky EA. A role for the thermal environment in defining co-stimulation requirements for CD4(+) T cell activation. Cell Cycle. 2015;14:2340-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia. 2012;28:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Santoro MG, Amici C, Rossi A. Role of Heat Shock Proteins in Viral Infection. In: Pockley A, Calderwood S, Santoro M (eds). Prokaryotic and Eukaryotic Heat Shock Proteins in Infectious Disease. Springer, Dordrecht. 2010: 51-84. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Tanguy Le Gac N, Boehmer PE. Activation of the herpes simplex virus type-1 origin-binding protein (UL9) by heat shock proteins. J Biol Chem. 2002;277:5660-5666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Roesch F, Meziane O, Kula A, Nisole S, Porrot F, Anderson I, Mammano F, Fassati A, Marcello A, Benkirane M, Schwartz O. Hyperthermia stimulates HIV-1 replication. PLoS Pathog. 2012;8:e1002792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Pujhari S, Brustolin M, Macias VM, Nissly RH, Nomura M, Kuchipudi SV, Rasgon JL. Heat shock protein 70 (Hsp70) mediates Zika virus entry, replication, and egress from host cells. Emerg Microbes Infect. 2019;8:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Ye J, Chen Z, Zhang B, Miao H, Zohaib A, Xu Q, Chen H, Cao S. Heat shock protein 70 is associated with replicase complex of Japanese encephalitis virus and positively regulates viral genome replication. PLoS One. 2013;8:e75188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Lahaye X, Vidy A, Fouquet B, Blondel D. Hsp70 protein positively regulates rabies virus infection. J Virol. 2012;86:4743-4751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Hirayama E, Atagi H, Hiraki A, Kim J. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J Virol. 2004;78:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Manzoor R, Kuroda K, Yoshida R, Tsuda Y, Fujikura D, Miyamoto H, Kajihara M, Kida H, Takada A. Heat shock protein 70 modulates influenza A virus polymerase activity. J Biol Chem. 2014;289:7599-7614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Merkling SH, Overheul GJ, van Mierlo JT, Arends D, Gilissen C, van Rij RP. The heat shock response restricts virus infection in Drosophila. Sci Rep. 2015;5:12758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Tang SW, Abubakar S, Devi S, Puthucheary S, Pang T. Induction and characterization of heat shock proteins of Salmonella typhi and their reactivity with sera from patients with typhoid fever. Infect Immun. 1997;65:2983-2986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Chan CP, Siu KL, Chin KT, Yuen KY, Zheng B, Jin DY. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2006;80:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Fribley A, Zhang K, Kaufman RJ. Regulation of apoptosis by the unfolded protein response. Methods Mol Biol. 2009;559:191-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506-8513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 67. | Sreedhar AS, Mihály K, Pató B, Schnaider T, Steták A, Kis-Petik K, Fidy J, Simonics T, Maraz A, Csermely P. Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J Biol Chem. 2003;278:35231-35240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Sultan I, Howard S, Tbakhi A. Drug repositioning suggests a role for the heat shock protein 90 inhibitor Geldanamycin in treating COVID-19 infection. [preprint]. Research Square 2020. . [DOI] [Full Text] |
| 69. | Mackowiak PA, Marling-Cason M, Cohen RL. Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis. 1982;145:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | Mackowiak PA. Brief history of antipyretic therapy. Clin Infect Dis. 2000;31 Suppl 5:S154-S156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269-R277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR, Zheng H. IL-6 and IL-1 beta in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci. 1998;856:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Hashim IA, Al-Zeer A, Al-Shohaib S, Al-Ahwal M, Shenkin A. Cytokine changes in patients with heatstroke during pilgrimage to Makkah. Mediators Inflamm. 1997;6:135-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia. 2003;19:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 76. | Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1021] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 77. | Roti Roti JL. Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events. Int J Hyperthermia. 2008;24:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 78. | Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol. 2001;280:H509-H521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 324] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 79. | Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev. 2004;32:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Bynum G, Brown J, Dubose D, Marsili M, Leav I, Pistole TG, Hamlet M, LeMaire M, Caleb B. Increased survival in experimental dog heatstroke after reduction of gut flora. Aviat Space Environ Med. 1979;50:816-819. [PubMed] |
| 81. | Gathiram P, Wells MT, Brock-Utne JG, Wessels BC, Gaffin SL. Prevention of endotoxaemia by non-absorbable antibiotics in heat stress. J Clin Pathol. 1987;40:1364-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Walter E, Gibson O. The efficacy of antibiotics in reducing morbidity and mortality from heatstroke - A systematic review. J Therm Biol. 2020;88:102509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Hausfater P, Hurtado M, Pease S, Juillien G, Lvovschi VE, Salehabadi S, Lidove O, Wolff M, Bernard M, Chollet-Martin S, Riou B. Is procalcitonin a marker of critical illness in heatstroke? Intensive Care Med. 2008;34:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161:926-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 85. | Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 86. | Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 87. | Tulapurkar ME, Almutairy EA, Shah NG, He JR, Puche AC, Shapiro P, Singh IS, Hasday JD. Febrile-range hyperthermia modifies endothelial and neutrophilic functions to promote extravasation. Am J Respir Cell Mol Biol. 2012;46:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Shah NG, Tulapurkar ME, Damarla M, Singh IS, Goldblum SE, Shapiro P, Hasday JD. Febrile-range hyperthermia augments reversible TNF-α-induced hyperpermeability in human microvascular lung endothelial cells. Int J Hyperthermia. 2012;28:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol. 1999;276:G1195-G1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Bazille C, Megarbane B, Bensimhon D, Lavergne-Slove A, Baglin AC, Loirat P, Woimant F, Mikol J, Gray F. Brain damage after heat stroke. J Neuropathol Exp Neurol. 2005;64:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Li J, Zhang X, Wang B, Geng J. MRI findings in human brain after heat stroke. J Nat Sci. 2015;1:e42. |
| 92. | Zhang XY, Li J. Susceptibility-weighted imaging in heat stroke. PLoS One. 2014;9:e105247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | MALAMUD N, HAYMAKER W, CUSTER RP. Heat stroke; a clinico-pathologic study of 125 fatal cases. Mil Surg. 1946;99:397-449. [PubMed] |
| 94. | Li CW, Lin YF, Liu TT, Wang JY. Heme oxygenase-1 aggravates heat stress-induced neuronal injury and decreases autophagy in cerebellar Purkinje cells of rats. Exp Biol Med (Maywood). 2013;238:744-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Vlad M, Ionescu N, Ispas AT, Giuvărăşteanu I, Ungureanu E, Stoica C. Morphological changes during acute experimental short-term hyperthermia. Rom J Morphol Embryol. 2010;51:739-744. [PubMed] |
| 96. | Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 260] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 97. | Etulain J, Lapponi MJ, Patrucchi SJ, Romaniuk MA, Benzadón R, Klement GL, Negrotto S, Schattner M. Hyperthermia inhibits platelet hemostatic functions and selectively regulates the release of alpha-granule proteins. J Thromb Haemost. 2011;9:1562-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Chem. 1997;43:1182-1187. [PubMed] |
| 99. | Jin Q, Chen E, Jiang J, Lu Y. Acute hepatic failure as a leading manifestation in exertional heat stroke. Case Rep Crit Care. 2012;2012:295867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Diehl KA, Crawford E, Shinko PD, Tallman RD Jr, Oglesbee MJ. Alterations in hemostasis associated with hyperthermia in a canine model. Am J Hematol. 2000;64:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Pease S, Bouadma L, Kermarrec N, Schortgen F, Régnier B, Wolff M. Early organ dysfunction course, cooling time and outcome in classic heatstroke. Intensive Care Med. 2009;35:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Crit Care. 2016;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 103. | Badoer E. Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am J Physiol Renal Physiol. 2010;298:F839-F846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Frankenfield DC, Smith JS Jr, Cooney RN, Blosser SA, Sarson GY. Relative association of fever and injury with hypermetabolism in critically ill patients. Injury. 1997;28:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt GA, Wood LD. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Kluger MJ. Is fever beneficial? Yale J Biol Med. 1986;59:89-95. [PubMed] |