Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4789
Peer-review started: January 12, 2021
First decision: March 14, 2021
Revised: March 28, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: June 26, 2021
Collision carcinoma is a rare histological pattern, and includes two or more different types of tumors coexisting in the same organ as one neoplasm. Different to the combined type, the two adjacent tumors of collision carcinoma are histologically distinct. Collision carcinoma may occur from any origin or organ, including the cecum, liver, cervix, thyroid, stomach, kidney, and esophagus. In the rectum, adenocarcinoma is the most common pathological type, the combined type is rare, and collision tumors are even rarer. To date, only a limited number of collision carcinoma cases originating from the rectum have been reported. Due to the scarcity of rectal collision carcinoma, more cases need to be reported to fully understand the clinico-pathological features and biological behavior of the tumor.
Here we report a 40-year-old female who presented with the chief complaints of persistent changes in bowel habits and hematochezia for 10 d. She underwent Miles' operation which revealed a collision carcinoma of the rectum, showing a “side by side” pattern, composed of a high grade neuroendocrine carcinoma, (small cell carcinoma) and moderately differentiated adenocarcinoma, based on its clinico-pathological features and biological behavior. The patient remained disease-free at 12 mo follow-up. We also focused on the related literature and expert opinion.
Collision carcinoma is a rare tumor with ambiguous biological behavior. Greater attention should be paid to its clinico-pathologic diagnosis. Regular and adequate follow-up is essential to help rule out metastasis and assess the prognosis.
Core Tip: Combined small cell carcinoma is a rare type of tumor, which has an additional component that consists of any histological type of non-small cell carcinoma, usually adenocarcinoma, squamous cell carcinoma, or large cell carcinoma. Collision carcinoma is even rarer because of the strict diagnostic criteria. To the best of our knowledge, the present case represents the tenth case of primary collision carcinoma of the rectum, consisting of neuroendocrine carcinoma and adenocarcinoma. Moreover, more cases are necessary to understand the prognosis and treatment of collision carcinoma.
- Citation: Zhao X, Zhang G, Li CH. Collision carcinoma of the rectum involving neuroendocrine carcinoma and adenocarcinoma: A case report. World J Clin Cases 2021; 9(18): 4789-4796
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4789.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4789
Rectal cancer is the most common type of gastrointestinal cancer, and adenocarcinoma is the most common pathological type. Collision tumor refers to a tumor, composed of two components, which are difficult to distinguish from each other[1]. It represents a tumor, which includes two adjacent tumors but these tumors are histologically distinct. Collision carcinoma is a rare malignancy of the rectum, due to the low incidence of this tumor in the literature, its pathogenesis, treatment and prognosis are ambiguous. Simultaneous high grade neuroendocrine carcinoma and moderately differentiated adenocarcinoma is a very rare pathology. Some gene mutations have been recognized to cause adenocarcinoma of the rectum; however, no explicit gene mutation has been reported to cause collision carcinoma[2]. Treatment protocols for rectal collision carcinoma are usually complex because of the presence of different histologic types; thus, individualized treatment strategies are required[3]. In this report, we describe a case of neuroendocrine carcinoma and adenocarcinoma (NEC-ACC) of the rectum and discuss its clinico-pathological features in order to provide a reference and improve our understanding of collision carcinoma.
A 40-year-old female presented to the outpatient clinic of Gastrointestinal Surgery in our hospital, with the chief complaints of persistent changes in bowel habits and hematochezia for 10 d in January 2019.
The patient complained of a history of hematochezia and considerable changes in her daily bowel habits for 10 d.
The patient had no previous medical history.
Personal and family history was not contributory.
Rectal palpation demonstrated the presence of a hard painful mass on the wall of the rectum 2 cm proximal to the dentate line in the chest-knee position.
Routine laboratory tests were unremarkable.
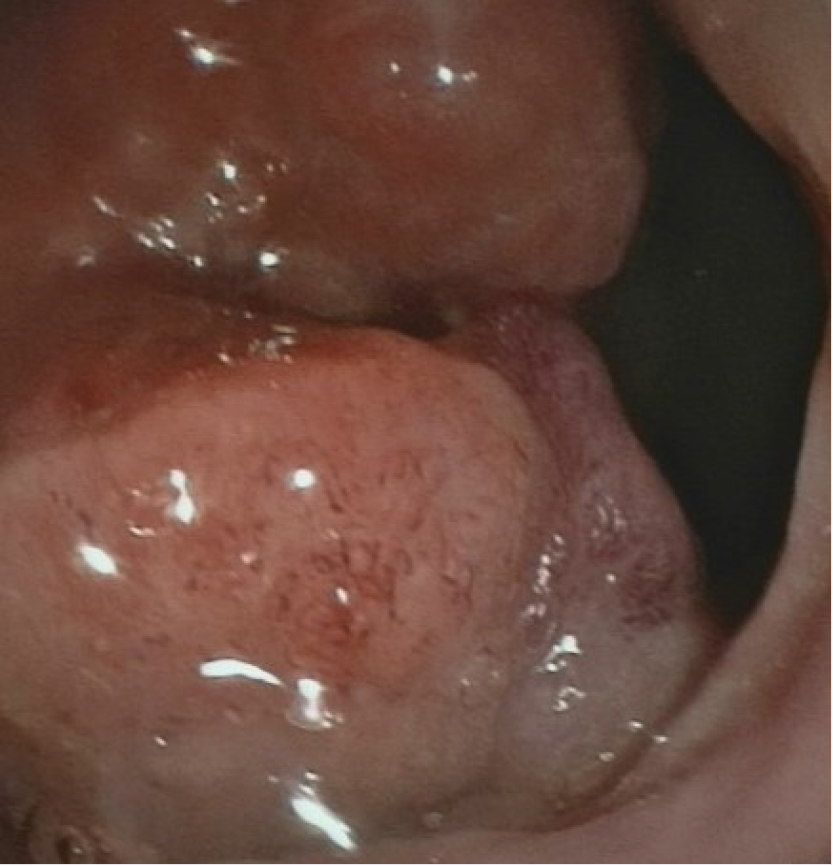
The patient underwent total colonoscopy which confirmed the presence of an ulcerated lesion located in the rectum 6 cm proximal to the anal verge (Figure 1). B ultrasonography was advised which indicated the presence of a mass 21.1 mm × 10.0 mm in size.
Biopsy was performed during colonoscopy and histology showed adenocarcinoma.
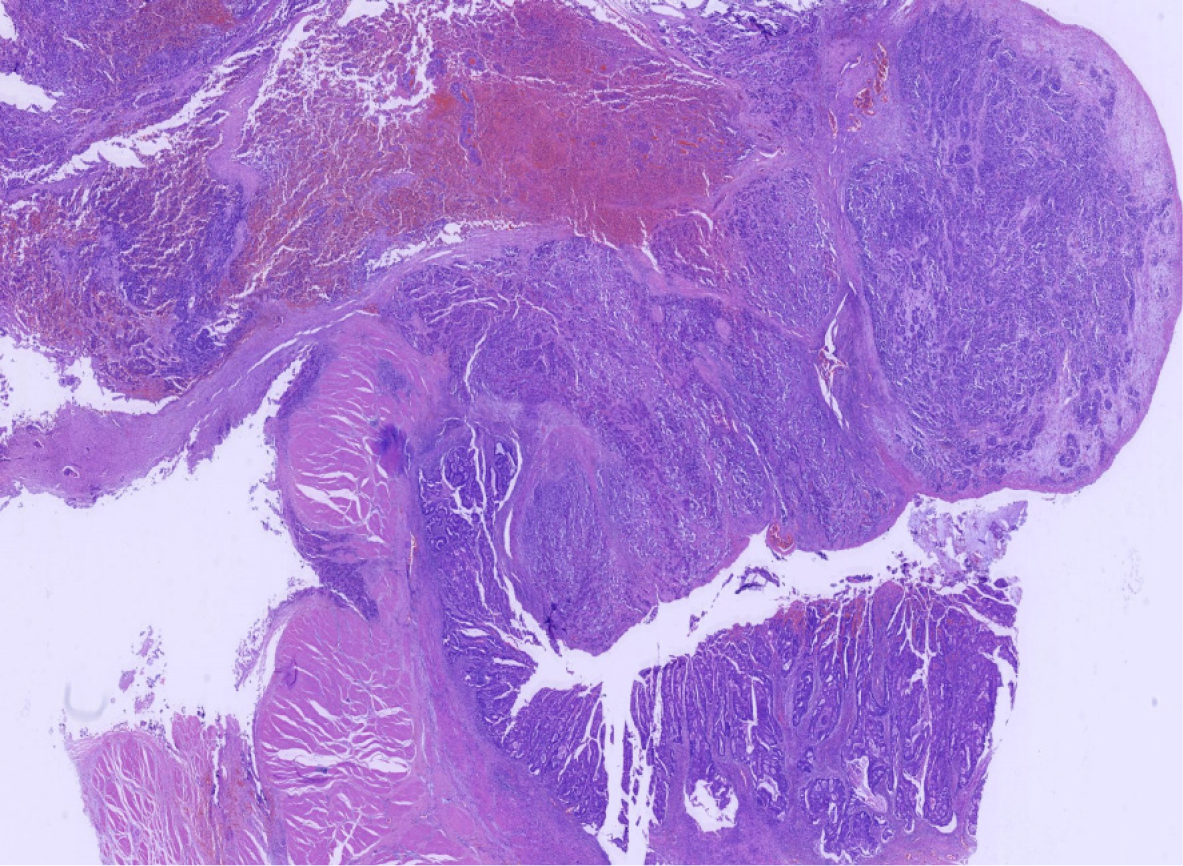
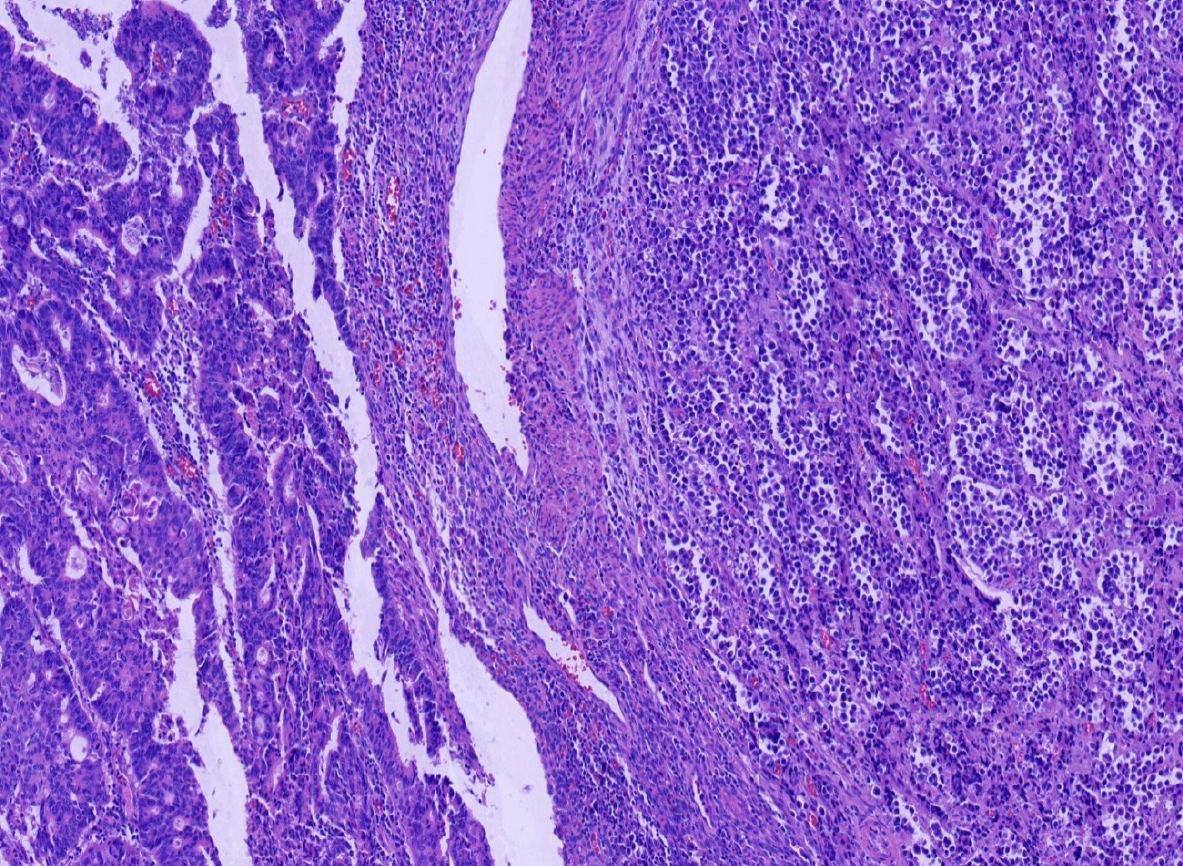
Miles' operation was performed immediately. The specimen was routinely fixed in 10% buffered formalin for 12 h. Macroscopically, the rectal segment with overlying fatty tissue measured 12 cm in length. A neoplastic lesion measuring 2.0 cm × 2.0 cm × 1.0 cm in maximum dimensions was detected 4 cm proximal to the anal verge. The rectal mucosa was gray in color and several necrotic and hemorrhagic areas were observed. Most of the surface was solid. The neoplastic lesion extended around the circumference of the rectum. Two components of the tumor were not identified on gross observation. The specimen was embedded in paraffin and cut into 4-μm-thick sections. Hematoxylin and eosin staining was performed, and the sections were examined by microscopic examination in the Pathology Department of the Affiliated Hospital of Chengde Medical College. Histologically, two coexisting distinct tumor types were noted in separate areas and without cross-growth, separated from each other by fibrous septae. In low-power view, the ratio of neuroendocrine carcinoma to adenocarcinoma was 3: 1 (Figure 2). The fact that they intermixed minimally at the interface was consistent with the diagnosis of a true "collision tumor" (Figure 3). The collision tumor had invaded the muscularis mucosae into the muscularis and fibrous tunica. One component was a typical moderately differentiated adenocarcinoma. The other component was high grade neuroendocrine carcinoma, which displayed an organoid structure with prevalent large trabeculae, and a rosette-like and palisading pattern. Necrosis was found in the center of solid nests. The tumor cells were small with atypical mitoses.
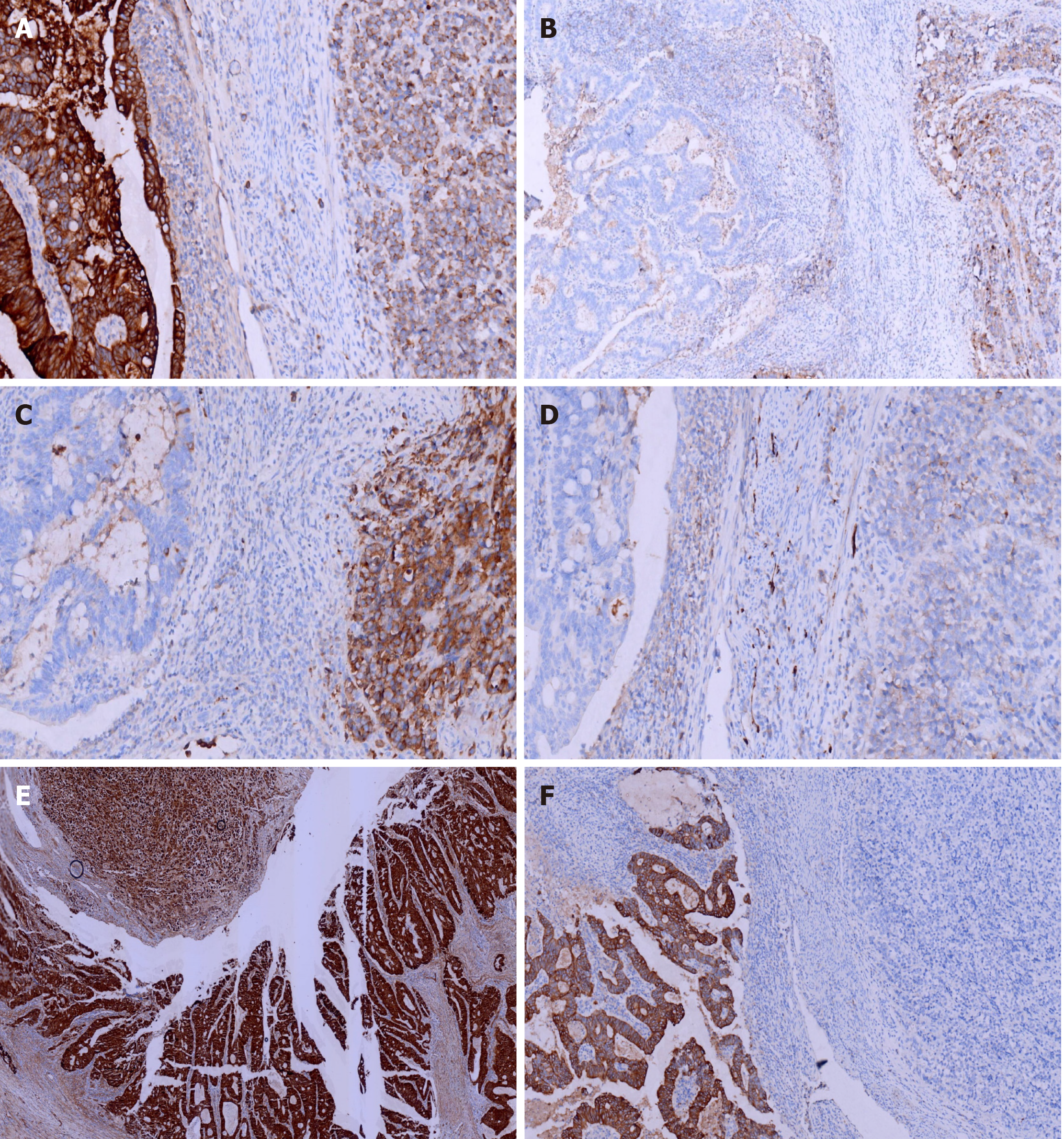
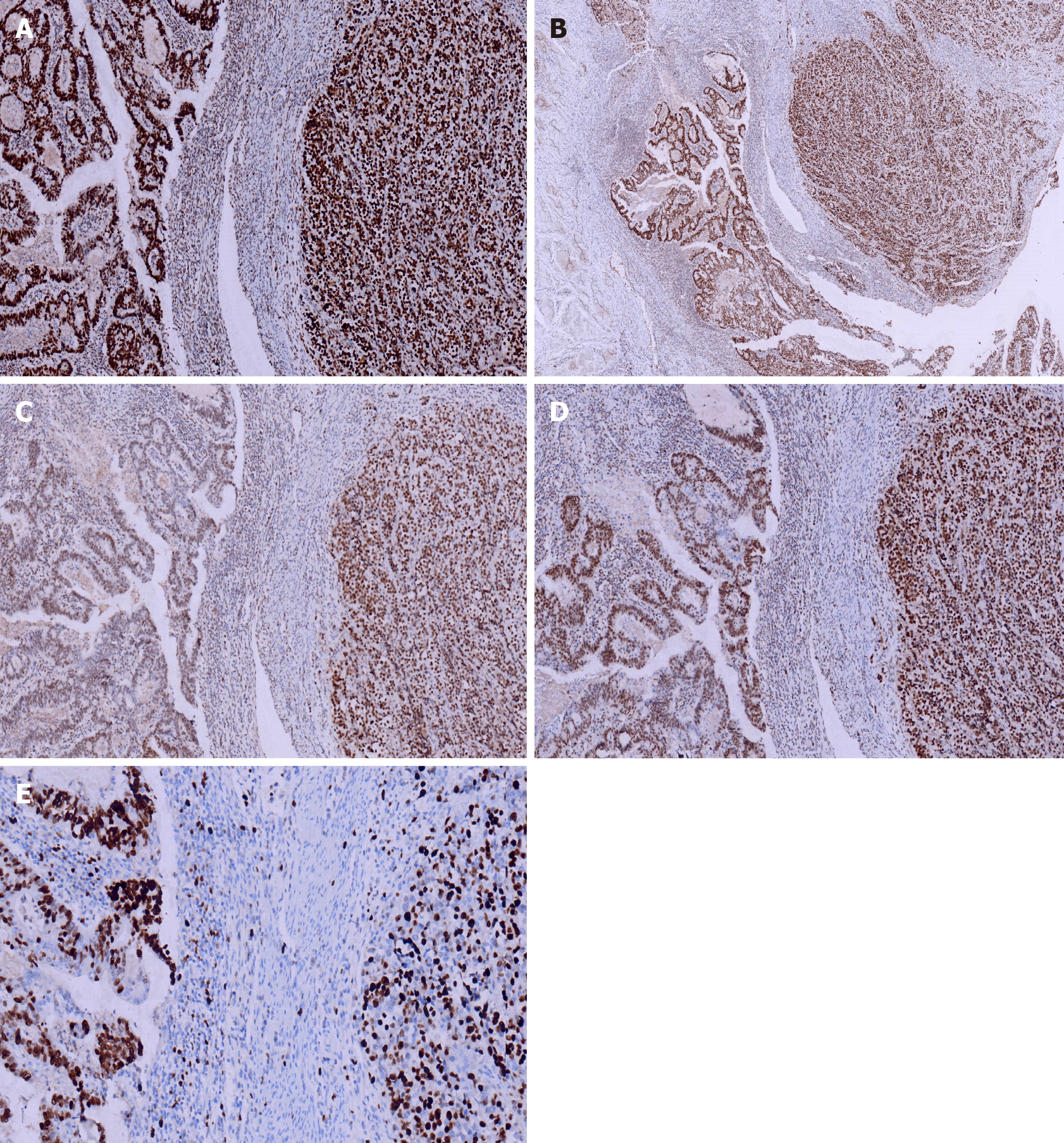
Immunohistochemical staining of cytokeratin (CK), chromogranin A (CgA), synaptophysin (Syn), and CD56, β-Catenin, CK7, CK20, CD10, MSH2, MSH6, PMS2, and MLH1was performed. All monoclonal antibodies used were purchased from OriGene Technologies, Inc. The adenocarcinoma cells were strongly positive for CK, β-Catenin (in the cell membrane), CK20, MSH2, MSH6, PMS2, MLH1, and the Ki-67 proliferation index was 60%. These cells were negative for CgA, Syn, CD56, CK7, and CD10. The neuroendocrine carcinoma cells were weekly positive for CK, positive for CgA, Syn, CD56, and β-Catenin (in the cell nucleus), MSH2, MSH6, PMS2, MLH1, and the Ki-67 proliferation index was 40%. These cells were negative for CK7, CK20 and CD10 (Figures 4 and 5).
The histologic features and immunohistochemical profile supported the diagnosis of collision carcinoma of the rectum consisting of neuroendocrine carcinoma (small cell carcinoma) and moderately differentiated adenocarcinoma. No metastatic lymph nodes were found. Based on the pathological information obtained from the histopathology specimens, the tumor, node and metastasis stage of the rectal cancer was classified as pT3N0M0 stage IIA.
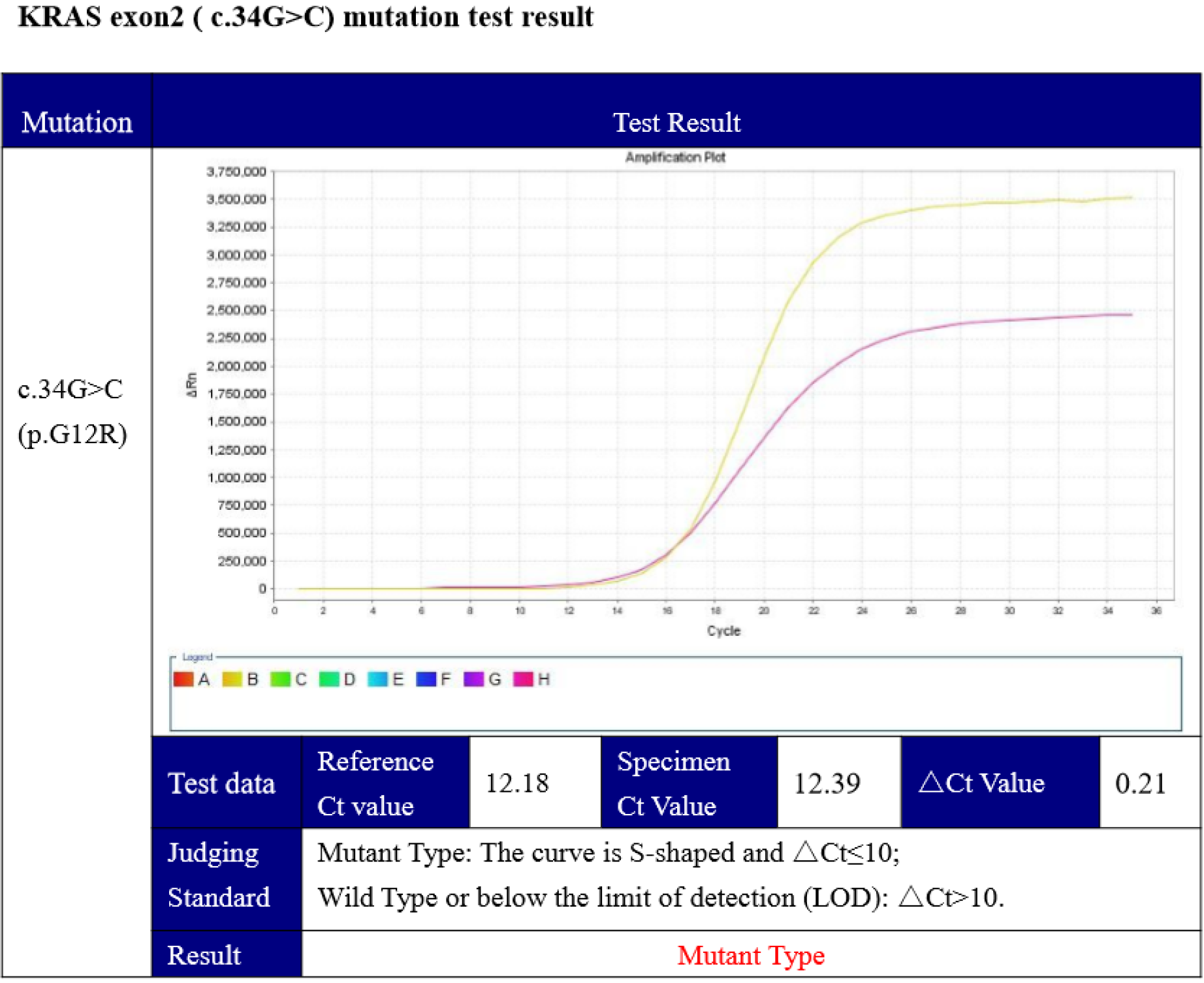
Furthermore, mutation of theBRAFV600Eoncogeneand KRAS was identified by genetic testing (competitive blocker quantitative polymerase chain reaction), which showed a KRAS (c.34G>C) mutation (Figure 6).
Following surgery, the patient underwent routinechemotherapy.
Post-operation, the patient had a favorable recovery without complications. Routine chemotherapy and regular follow up were undertaken after the operation. Hepatic and adrenal gland metastases were detected 12 mo after surgery. In March, 2021, the patient is still alive with tumor.
Colorectal cancer is the second most common carcinoma in women and the third most common in men worldwide according to the 2018 statistics[4]. The highest incidence is seen in developed countries. In recent years, the incidence has increased rapidly and younger patients in developing countries have been identified[5]. The most important risk factor is genetic predisposition depending on the type of mutation, in addition, red meat, alcohol, smoking and excess body fat are also risk factors. Dietary fiber and physical activity may decrease the incidence of colorectal cancer[6].
Approximately 90% of rectal cancers are adenocarcinomas, and NOS (non-specified) is the most common subtype. In addition, several histopathological subtypes can be distinguished, such as mucinous adenocarcinoma, signet–ring cell carcinoma, medullary carcinoma and serrated adenocarcinoma. Neuroendocrine neoplasms of the rectum are rectal epithelial neoplasms with neuroendocrine differentiation, including well-differentiated neuroendocrine tumors, poorly-differentiated neuroendocrine carcinomas (NECs), and mixed neuroendocrine-non-neuroendocrine neoplasms, for example mixed adeno-neuroendocrine carcinoma (MANEC)[7], which accounts for approximately 3% to 9.6% of all rectal carcinomas. According to the boundary line of the mixed component, MANECs are classified into composite and collision carcinomas.
There are two types of tumors in collision carcinoma, of different histological types and adjacent to each other. This tumor type is different from the combined or mixed tumors which are independent and never intermix leading to the “one upon another” and “side by side” pattern. Collision carcinomas originating from the rectum are uncommon, with a small number of cases reported in the literature. To the best of our knowledge, the mixtures in collision carcinomas include lymphoma and adenocarcinoma, squamous cell carcinoma and adenocarcinoma, and so on[8]. Here we report a case of collision carcinoma of the rectum, which showed the “side by side” pattern, and was composed of high grade NEC (small cell carcinoma) and moderately differentiated ACC, based on its clinico-pathological features, biological behavior, related literature and expert opinion.
The clinical biological behavior of these tumors is unclear, because of their rarity and histological heterogeneity. Reports on MANECs of the digestive system are limited, and the biological behavior and clinico-pathologic features obtained from publications on MANECs derived from the rectum are limited and most are from isolated case reports. The mean age of patients with mixed tumors of the rectum is 65 years[9], and gender predominance is not reported in the literature. The incidence of mixed tumors may be higher, as many patients may be misdiagnosed or never diagnosed. Most MANECs are clinically silent, sometimes with bleeding and pain or widespread metastases[10].
The pathogenesis of these tumors is unclear and debated, multiple hypotheses have been suggested as to the origin of collision tumors: (1) Some researchers[11] suggest that the components of the mixed tumor arise from different precursor cells independently, as metachronous or synchronous carcinoma. During their development, they may contact each other by accident; (2) A number of other researchers[12] regard a type of pluripotent stem cell as the progenitor of collision tumor, which has bidirectional differentiation under the action of carcinogenesis; (3) Another hypothesis is similar to the above assumption, that neuroendocrine neoplasms develop from an epithelial phenotype, which is supported by molecular and genetic aberrations; and (4) The last hypothesis suggests that they occur contiguously. The microenvironment is altered by the first neoplasm, leading to the development of an adjacent second neoplasm. As far as we know[13], endocrine cells are derived from multipotent stem cells in the gastrointestinal tract, which supports the second and third assertions.
MANECs are mostly composed of a poorly differentiated neuroendocrine carcinoma and an adenocarcinoma. Low grade neuroendocrine tumors are rare. The tumor often has a background of long-term inflammation. In rare cases, adenomas can be found together with neuroendocrine carcinoma. During our literature search, Roh et al[14] reported a rare collision tumor of the rectum that was composed of a rectal adenocarcinoma within a metastatic gastric adenocarcinoma. The two different components could not be identified macroscopically. Microscopically, most of the tumor was composed of poorly differentiated adenocarcinoma, while well-differentiated tubular adenocarcinoma was the smaller component. The results of immunohistochemical staining confirmed the two components of the tumor. The poorly differentiated adenocarcinoma was positive for MUC2 but negative for CK7, whereas the tumor cells of the well-differentiated tubular adenocarcinoma were positive for CK7 but negative for MUC2. In consideration of this proportion, it was suggested that the microenvironment was changed by the metastatic gastric adenocarcinoma leading to the development of rectal carcinoma. Choi et al[15] presented a case of synchronous rectal small cell carcinoma and adenocarcinoma in a 45-year-old man. Lymph node metastasis and lymphovascular invasion were found, and the clinical stage was T3N2b. The patient received surgery and chemotherapy, developed local recurrence, and died of multiple metastatic diseases.
Collision carcinoma of the rectum consisting of neuroendocrine carcinoma and adenocarcinoma is rare, and the pathogenesis, features and prognosis are still unclear, and there is still a lack of effective clinical evidence. We report this case in the hope of increasing awareness of this enigmatic tumor and avoiding diagnostic pitfalls.
Collision carcinoma (neuroendocrine carcinoma and adenocarcinoma) is one of the most uncommon lesions encountered by surgeons and pathologists. Due to their rarity, it is easy to miss the correct pathological diagnosis, and the tumor is usually misdiagnosed as neuroendocrine carcinoma or adenocarcinoma. Herein, we report a patient with collision carcinoma derived from stage IIA (pT3N0M0) rectal cancer, the difference in treatment between collision carcinoma and neuroendocrine carcinoma or adenocarcinoma is undefined. Understanding collision carcinoma is important for accurate diagnosis and appropriate management.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Murakami T S-Editor: Zhang H L-Editor: A P-Editor: Wang LL
| 1. | Kanthan R, Tharmaradinam S, Asif T, Ahmed S, Kanthan SC. Mixed epithelial endocrine neoplasms of the colon and rectum - An evolution over time: A systematic review. World J Gastroenterol. 2020;26:5181-5206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Schizas D, Michalinos A, Alexandrou P, Moris D, Baliou E, Tsilimigras D, Throupis T, Liakakos T. A unique tripartite collision tumor of the esophagus: A case report. Medicine (Baltimore). 2017;96:e8784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Minaya-Bravo AM, Garcia Mahillo JC, Mendoza Moreno F, NoguelaresFraguas F, Granell J. Large cell neuroendocrine - Adenocarcinona mixed tumour of colon: Collision tumour with peculiar behaviour. What do we know about these tumours? Ann Med Surg (Lond). 2015;4:399-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Qian T, Gao F, Chen MZ, Meng FH, Li XJ, Liu YJ, Yin HB. Collision tumor of the esophagus: report of a case with mixed squamous cell carcinoma and gastrointestinal stromal tumor. Int J ClinExpPathol. 2014;7:1206-1211. [PubMed] [Cited in This Article: ] |
| 5. | Yu Q, Chen YL, Zhou SH, Chen Z, Bao YY, Yang HJ, Yao HT, Ruan LX. Collision carcinoma of squamous cell carcinoma and small cell neuroendocrine carcinoma of the larynx: A case report and review of the literature. World J Clin Cases. 2019;7:242-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 6. | Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, Sperti C. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J SurgOncol. 2017;15:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Shu P, Li R, Xie D, He Y, Wang X, Li Q. Clinical profile and treatment outcome of collision carcinoma in cervix. Medicine (Baltimore). 2020;99:e19131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Peng L, Schwarz RE. Collision tumor in form of primary adenocarcinoma and neuroendocrine carcinoma of the duodenum. Rare Tumors. 2012;4:e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Oh KS, Bahmad HF, Febres-Aldana CA, Safdie FM, Sriganeshan V. Collision tumors of the lung: A case report of urothelial carcinoma metastasizing to renal cell carcinoma with heterotopic ossification. Respir Med Case Rep. 2020;31:101297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kim VM, Goicochea L, Fang SH. Case Report: Collision Tumour of Colon Leiomyosarcoma and Adenocarcinoma. J ClinDiagn Res. 2016;10:PD03-PD04. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Miyamoto R, Kikuchi K, Uchida A, Ozawa M, Kemmochi A, Sano N, Tadano S, Inagawa S, Adachi S, Yamamoto M. Collision tumor consisting of a colorectal adenocarcinoma and dissemination of a gastric adenocarcinoma. SAGE Open Med Case Rep. 2018;6:2050313X17751839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Izumi H, Furukawa D, Yazawa N, Masuoka Y, Yamada M, Tobita K, Kawashima Y, Ogawa M, Kawaguchi Y, Hirabayashi K, Nakagohri T. A case study of a collision tumor composed of cancers of the bile duct and pancreas. Surg Case Rep. 2015;1:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kadowaki Y, Nishimura T, Komoto S, Yuasa T, Tamura R, Okamoto T, Ishido N. Gastroduodenal intussusception caused by a gastric collision tumor consisting of adenocarcinoma and neuroendocrine carcinoma. Case Rep Gastroenterol. 2014;8:89-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Roh YH, Lee HW, Kim MC, Lee KW, Roh MS. Collision tumor of the rectum: a case report of metastatic gastric adenocarcinoma plus primary rectal adenocarcinoma. World J Gastroenterol. 2006;12:5569-5572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Choi PW. Synchronous small cell carcinoma and adenocarcinoma of the rectum. J Surg Case Rep. 2017;2017:rjx069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |